Abstract
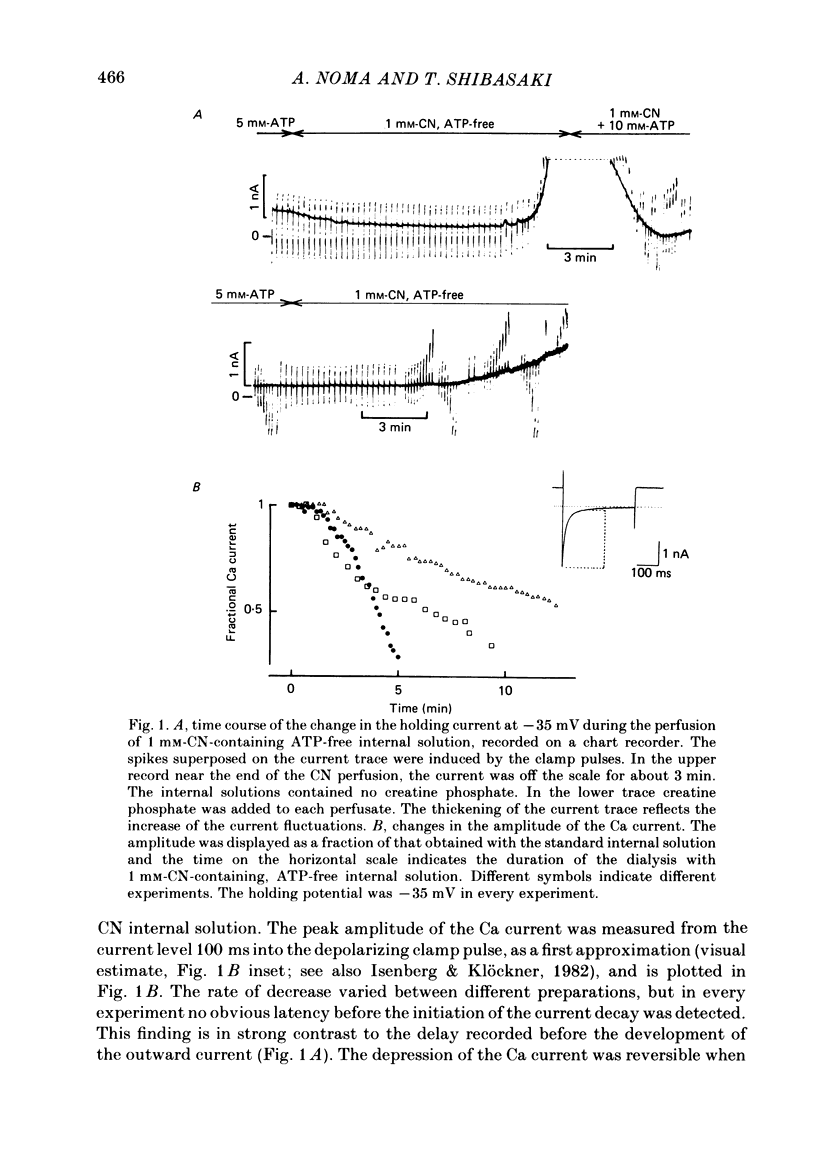
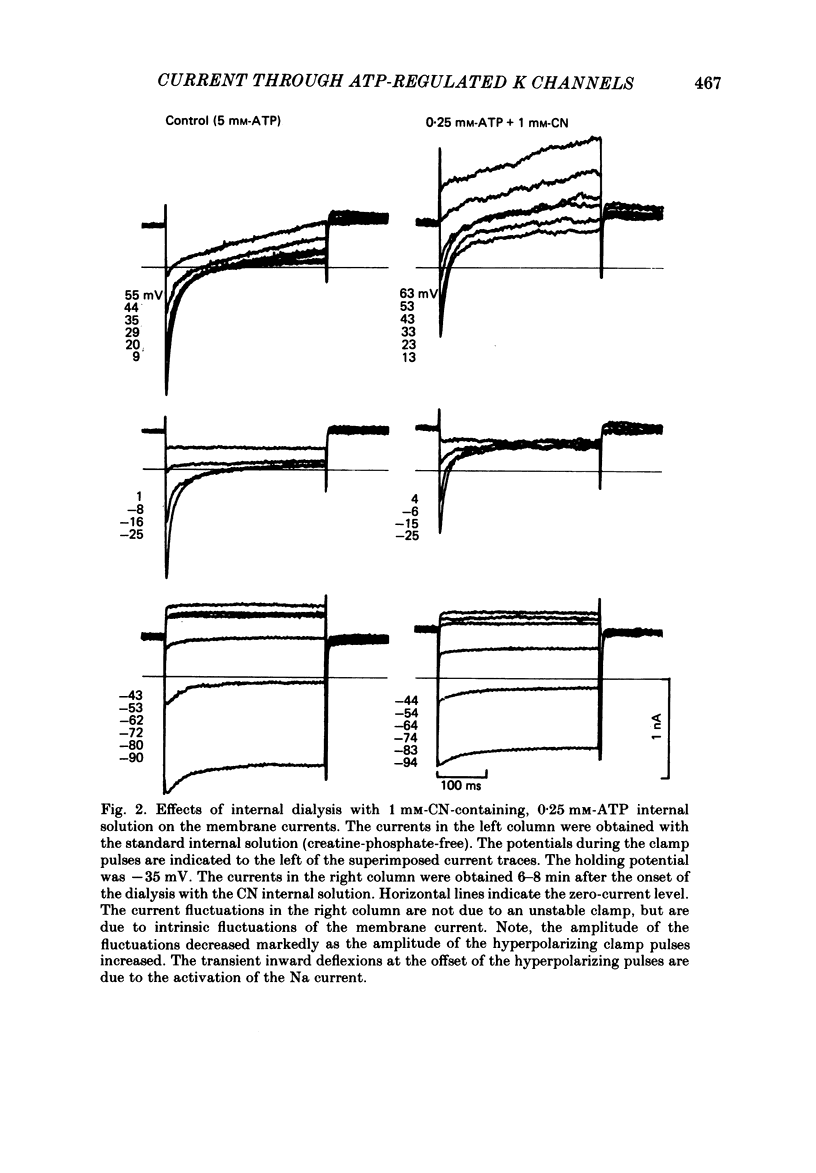
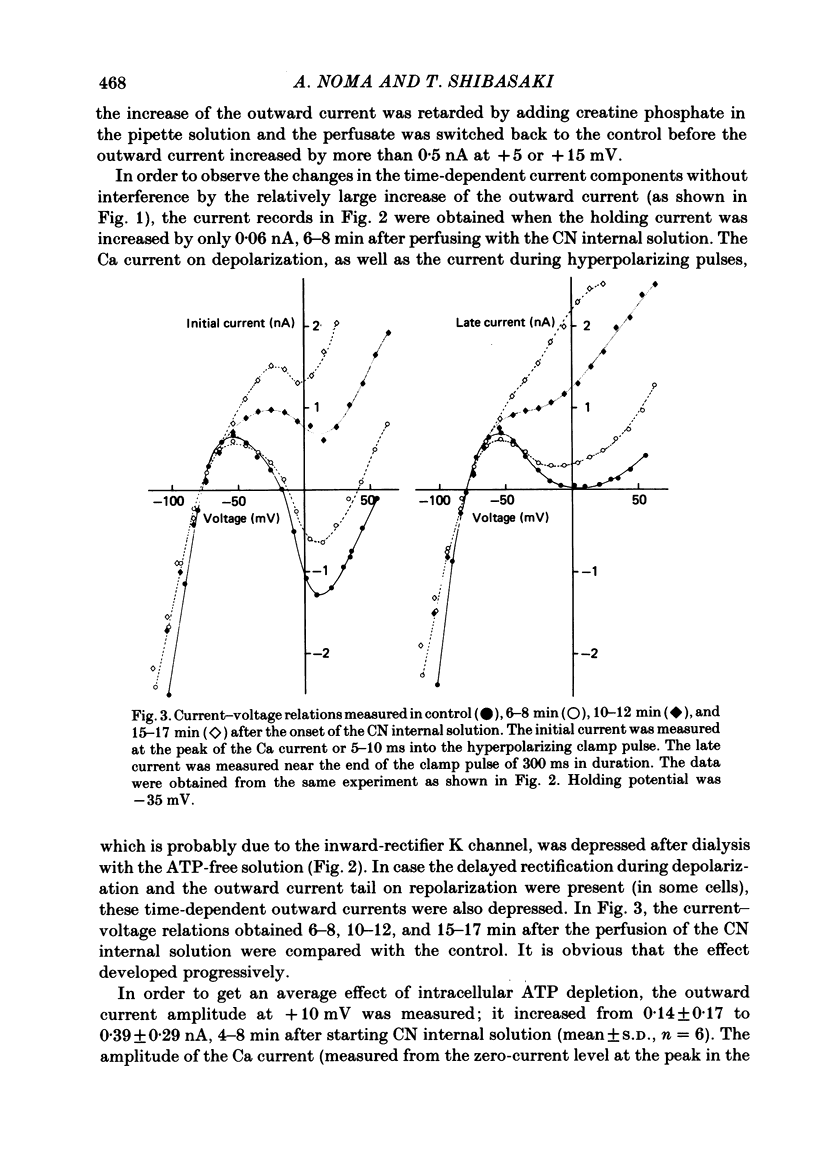
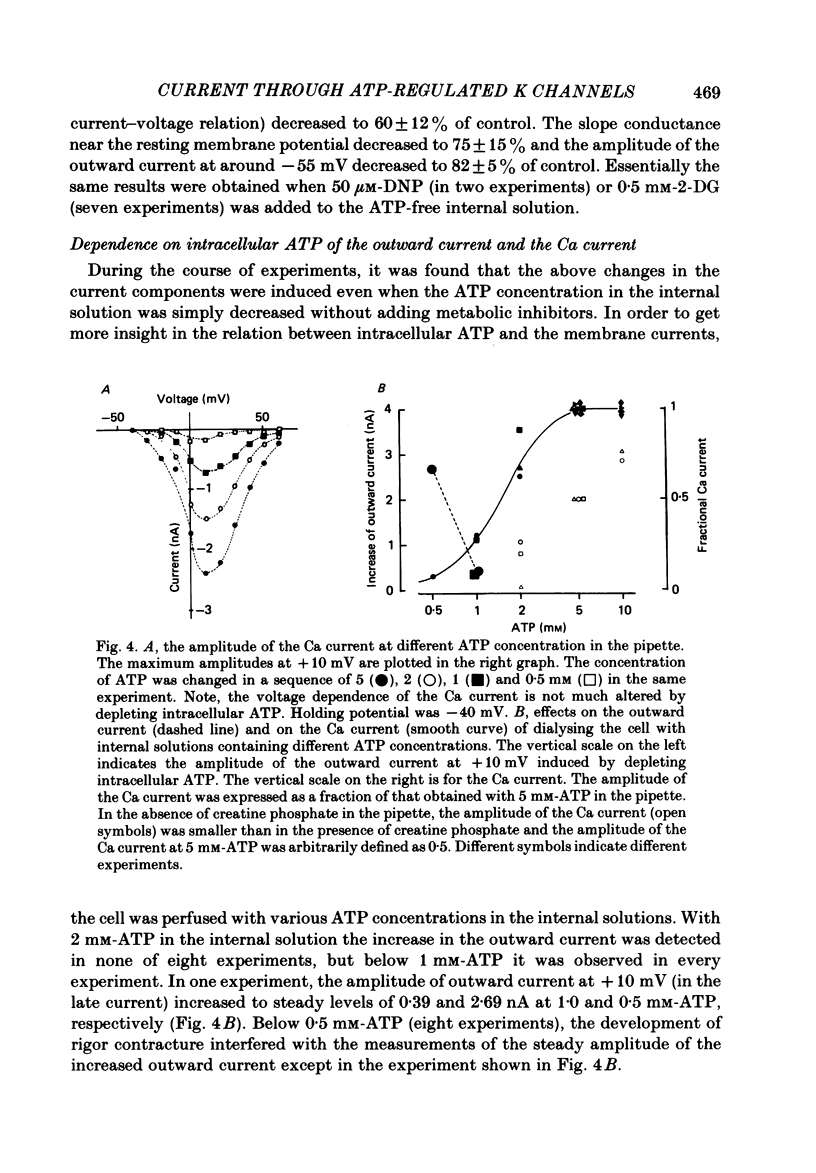
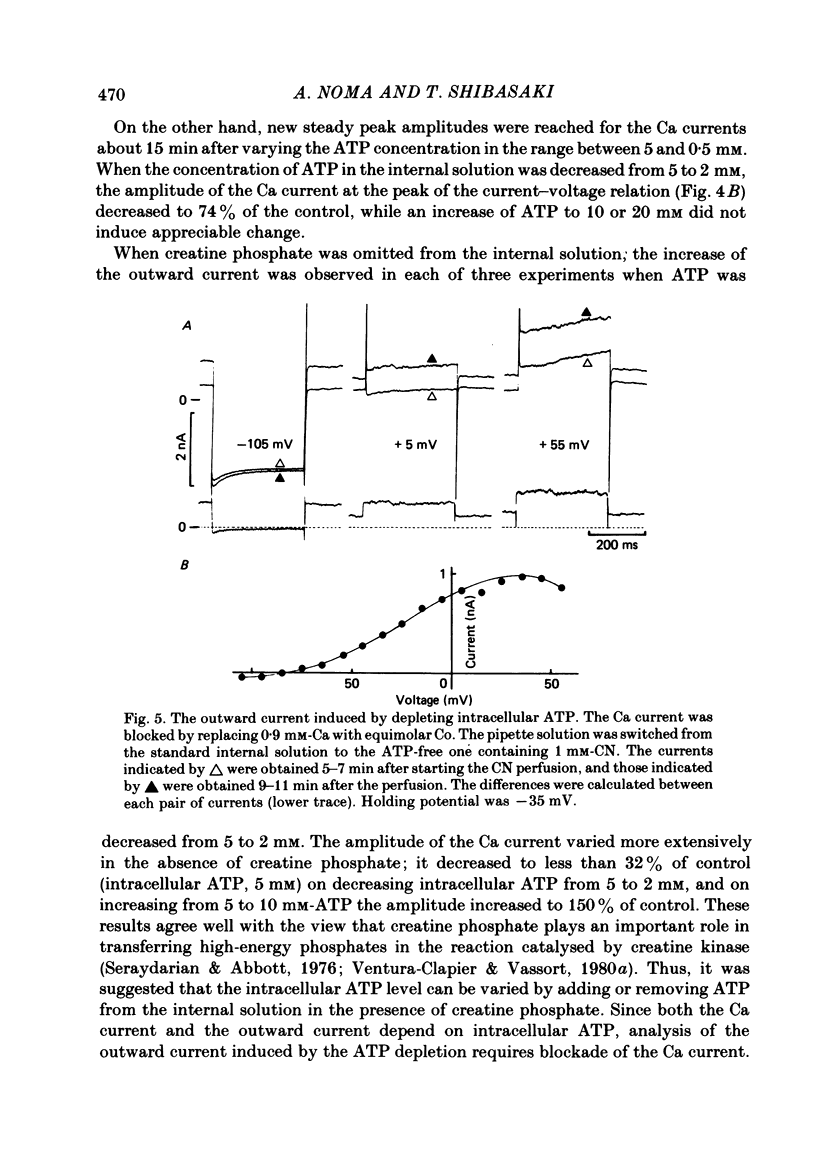
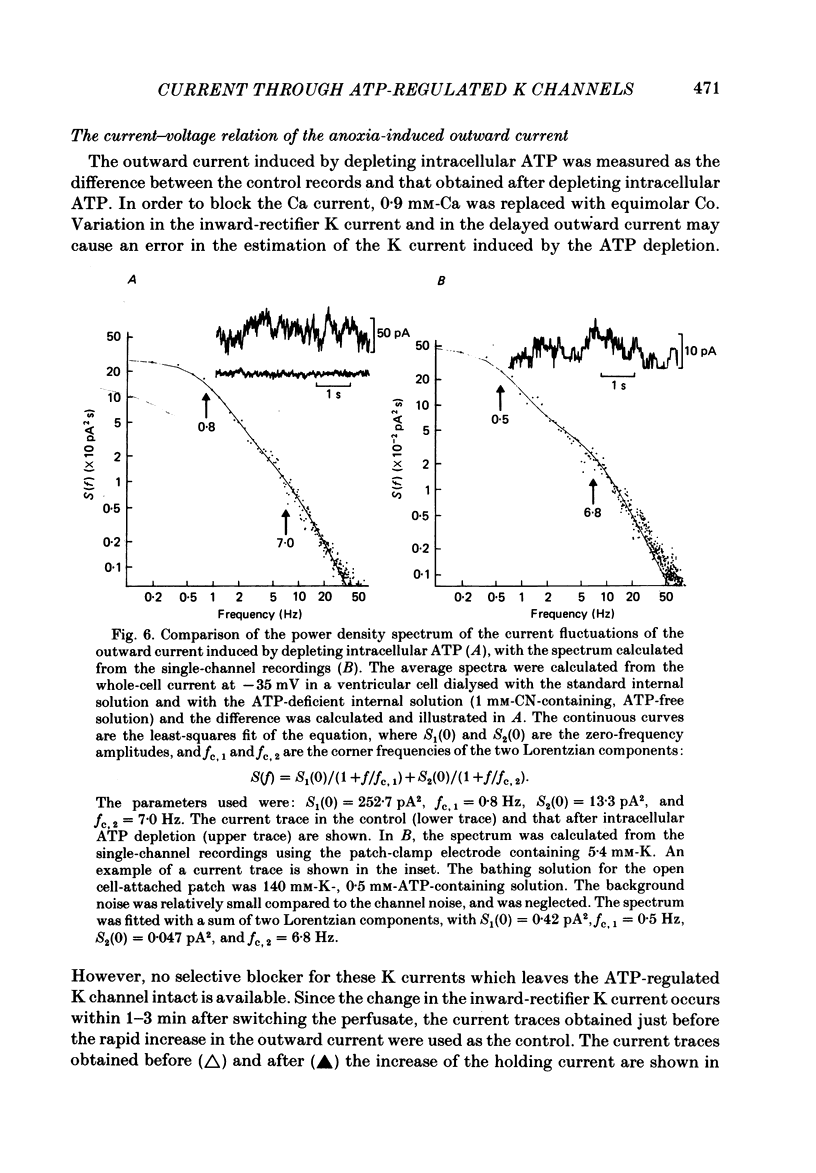
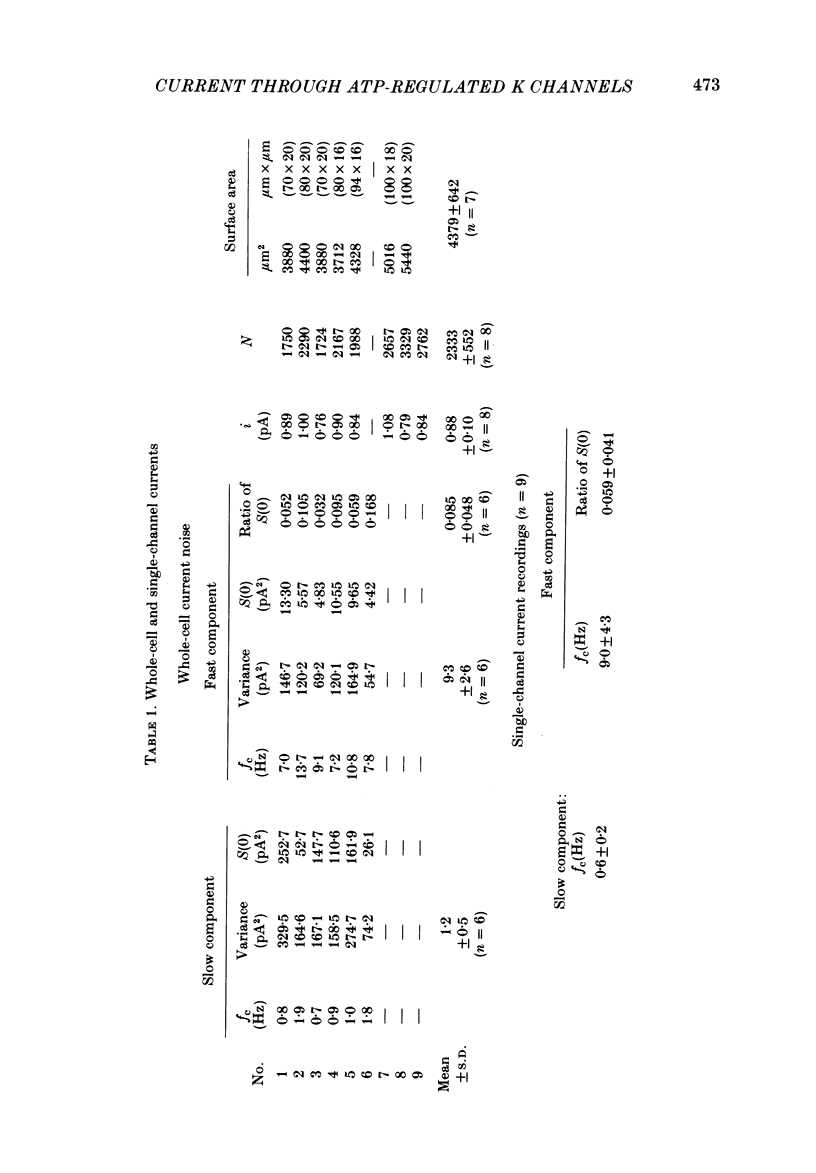
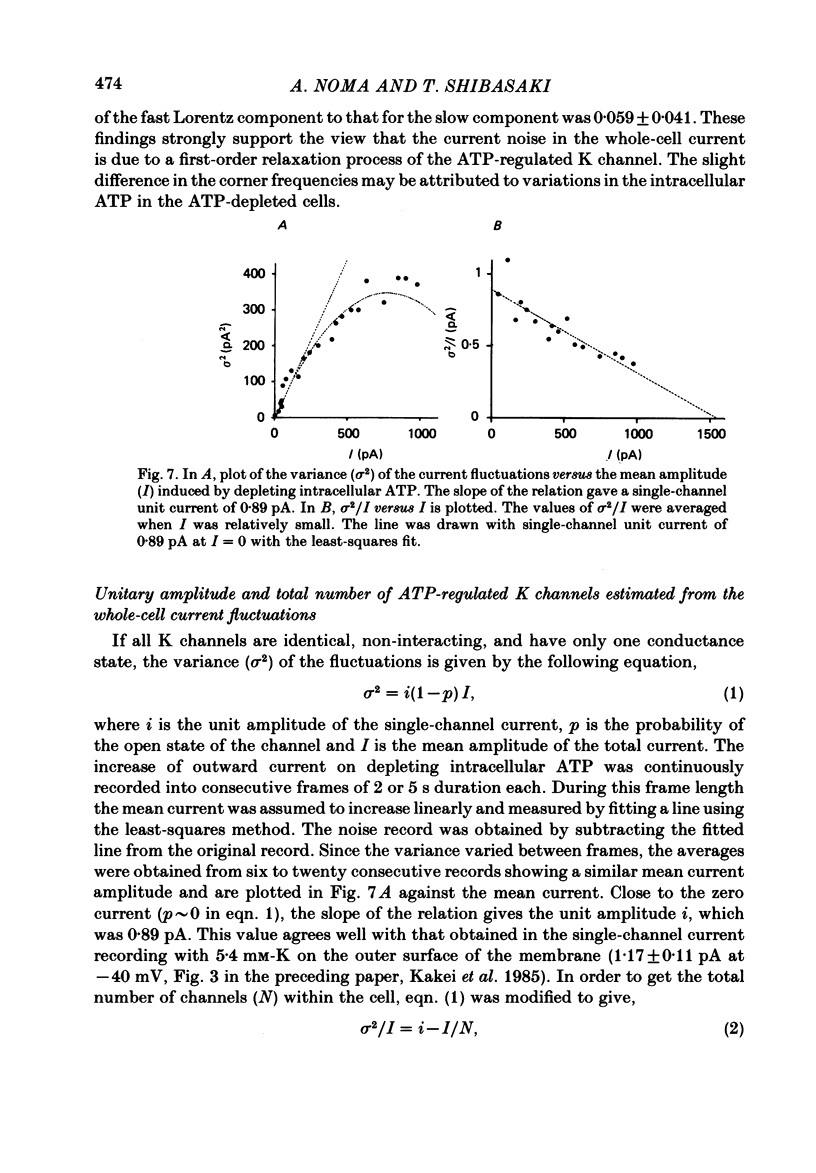
The question whether activation of the ATP-regulated K channel is responsible for macroscopic anoxia-induced outward currents was examined in ventricular cells isolated enzymatically from guinea-pig heart. Gigaseal patch-clamp electrodes were used for a whole-cell voltage clamp. Membrane currents were compared in the same cell while the cell interior was dialysed by perfusing the electrode with different solutions. When the cell was dialysed with various ATP-deficient (less than or equal to 2 mM) internal solutions, the Ca current decreased in a dose-dependent manner to less than 10% of control at 0.5 mM-ATP. A slight (ca. 25%) decrease of the slope conductance for hyperpolarizing current was observed. When a delayed rectification on depolarization followed by a marked outward current tail on repolarization was present under control conditions, this time-dependent outward current was also depressed. An increase in a time-independent outward current was observed accompanied by marked current fluctuations. The outward current showed a reversal potential near the K equilibrium potential, inward rectification, and no relaxation on voltage jumps. The power density spectrum of the current fluctuations showed a pattern similar to the spectrum calculated from the single-channel currents of ATP-regulated K channels. The amplitude of the single-channel current, estimated from the fluctuations, was almost equal to that of the single-channel current. The total number of channels within one cell was estimated as 2000-3000. It is concluded that the ATP-regulated K channels are responsible for the increase in the outward current and the shortening of the action potential duration under various anoxic conditions.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M., Cohen C. J., McDonald T. F. Heterogeneity of intracellular potassium activity and membrane potential in hypoxic guinea pig ventricle. Circ Res. 1981 Nov;49(5):1181–1189. doi: 10.1161/01.res.49.5.1181. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L. Cardiac energetics. Physiol Rev. 1978 Jan;58(1):174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- Grinwald P. M., Hearse D. J., Segal M. B. A possible mechanism of glycolytic impairment after adenosine triphosphate deplection in the perfused rat heart. J Physiol. 1980 Apr;301:337–347. doi: 10.1113/jphysiol.1980.sp013209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjarnason S., Mathes P., Ravens K. G. Functional compartmentation of ATP and creatine phosphate in heart muscle. J Mol Cell Cardiol. 1970 Sep;1(3):325–339. doi: 10.1016/0022-2828(70)90009-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls steady state potassium conductance. Pflugers Arch. 1977 Oct 19;371(1-2):71–76. doi: 10.1007/BF00580774. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls the potassium permeability via the conductance components gK1 and gK2. Pflugers Arch. 1977 Oct 19;371(1-2):77–85. doi: 10.1007/BF00580775. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Vereecke J., van der Heyden G., Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983 Jun 1;397(4):251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Kübler M. The influence of metabolic inhibitors upon the transmembrane slow inward current in the mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(2-3):265–274. doi: 10.1007/BF00510555. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Mnich Z., Maier G. Alterations of the excitation process of the sinoatrial pacemaker cell in the presence of anoxia and metabolic inhibitors. J Mol Cell Cardiol. 1977 Jun;9(6):477–488. doi: 10.1016/s0022-2828(77)80027-8. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Effects of intracellular calcium injection on steady state membrane currents in isolated single ventricular cells. Pflugers Arch. 1983 Apr;397(1):81–83. doi: 10.1007/BF00585176. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. DNP-induced dissipation of ATP in anoxic ventricular muscle. J Physiol. 1973 Mar;229(3):583–599. doi: 10.1113/jphysiol.1973.sp010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. Metabolism and the electrical activity of anoxic ventricular muscle. J Physiol. 1973 Mar;229(3):559–582. doi: 10.1113/jphysiol.1973.sp010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Nargeot J., Challice C. E., Tan K. S., Garnier D. Influence of metabolic inhibition by NaCN on Electrical and mechanical activities of frog atrial fibers: studies using current and voltage clamp. J Mol Cell Cardiol. 1978 May;10(5):469–485. doi: 10.1016/0022-2828(78)90368-1. [DOI] [PubMed] [Google Scholar]
- Nargeot J. Current clamp and voltage clamp study of the inhibitory action of DNP on membrane electrical properties of frog auricular heart muscle. J Physiol (Paris) 1976;72(2):171–180. [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E., Demers J. M. Slow inward and outward currents of rat ventricular fibers under anoxia. J Physiol (Paris) 1978;74(1):31–35. [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. A., Sperelakis N. The demonstration of energy dependence of the isoproterenol-induced transcellular Ca2+ current in isolated perfused guinea pig hearts--an explanation for mechanical failure of ischemic myocardium. J Surg Res. 1974 Apr;16(4):389–403. doi: 10.1016/0022-4804(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Seraydarian M. W., Abbott B. C. The role of the creatine--phosphorylcreatine system in muscle. J Mol Cell Cardiol. 1976 Oct;8(10):741–746. doi: 10.1016/0022-2828(76)90081-x. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Noma A., Irisawa H. Modification of the cardiac action potential by intracellular injection of adenosine triphosphate and related substances in guinea pig single ventricular cells. Circ Res. 1983 Aug;53(2):131–139. doi: 10.1161/01.res.53.2.131. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Vassort G. The hypodynamic state of the frog heart. Further evidence for a phosphocreatine-creatine pathway. J Physiol (Paris) 1980 Nov;76(6):583–589. [PubMed] [Google Scholar]
- Vleugels A., Vereecke J., Carmeliet E. Ionic currents during hypoxia in voltage-clamped cat ventricular muscle. Circ Res. 1980 Oct;47(4):501–508. doi: 10.1161/01.res.47.4.501. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]



