Significance
DR5, also known as TNFRSF10B, can potently restrict the infection of herpesviruses, including Kaposi sarcoma–associated herpesvirus (KSHV), Epstein–Barr virus, herpes simplex virus type 1, and herpes simplex virus type 2. We found that DR5 inhibits KSHV lytic replication by activating the extrinsic apoptotic pathway. Our study also demonstrated that three amino acids of DR5 have undergone positive selection in primates and reverse mutation of one amino acid (A62D) alleviates its antiviral activity. KSHV has evolved multiple strategies to escape innate immunity. Specifically, to counteract the DR5-induced antiviral effect, KSHV utilizes its K5 protein to degrade DR5 during infection. These findings highlight the identification of host restriction factor that affects different herpesviruses infection and highlight the complexity of virus–host interaction.
Keywords: DR5, KSHV, Herpesvirus, restriction factor
Abstract
Restriction factors are dominant proteins that target different essential steps of the viral life cycle; thus, these proteins provide an early line of defense against viruses. Here, we found that the internalization of DR5, an important receptor of the extrinsic apoptotic pathway, initiates apoptosis to inhibit Kaposi sarcoma–associated herpesvirus (KSHV) lytic replication. An evolutionary analysis of the DR5 sequence demonstrated that three amino acids underwent positive selection in primates. Notably, one of these positive selection sites, A62, is essential for the antiviral function of DR5 and is important for the binding of DR5 to its ligand, TNF-related apoptosis-inducing ligand. Moreover, DR5 exhibits broad antiviral activity against and inhibits various herpesviruses, including Epstein–Barr virus, herpes simplex virus type 1, and herpes simplex virus type 2. As a countermeasure, the KSHV K5 protein interacts with DR5 and promotes DR5 degradation through the lysosomal and proteasomal degradation pathways; lysine 245 of DR5 is essential for K5-induced DR5 degradation. These findings demonstrate that DR5 is a restriction factor for human herpesviruses.
Human herpesviruses are a family of double-stranded DNA viruses associated with a variety of human diseases; this family contains eight structurally similar viruses that can be classified into three subfamilies. Kaposi sarcoma–associated herpesvirus (KSHV) belongs to the γ-herpesvirus subfamily and is etiologically associated with various malignant tumors, including Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman’s disease (MCD) (1–3). Like other herpesviruses, KSHV has a biphasic life cycle consisting of periods of latency and lytic replication. During latent infection, only a few viral genes, including latency-associated nuclear antigen (LANA), viral cyclin (vCyclin), viral Fas-associated protein with death domain (FADD)-like interleukin-1β-converting enzyme (FLICE)-like inhibitory protein (vFLIP), Kaposins, and microRNAs, are expressed, and no infectious virions are produced (4). When latency is disrupted, the KSHV life cycle switches from latency to a lytic replication stage, in which the virus expresses most of its genes and produces infectious virions (5). Viral lytic replication exposes the virus to detection by the host antiviral response through various pathways and processes, such as the IFN signaling pathway, inflammasome activation, apoptosis, autophagy, and ER stress.
Apoptosis is an important antiviral defense mechanism that can eliminate infected or damaged cells. Apoptosis occurs when specific cell-surface receptors, such as tumor necrosis factor receptor (TNFR), Fas-ligand (FASL) receptor, and death receptors, bind their ligands (the extrinsic apoptotic pathway) or when B-cell lymphoma 2 (Bcl-2) family proapoptotic proteins cause permeabilization of the mitochondrial outer membrane (the intrinsic apoptotic pathway) (6, 7). In the extrinsic apoptotic pathway, the activation of these receptors leads to the formation of a protein complex composed of the death receptor adaptor proteins TNFR-associated death domain (TRADD) and/or Fas-associated death domain (FADD), activating the intracellular protease caspase-8 (7). In turn, caspase-8 then cleaves the effectors caspase-3 and caspase-7, which mediate apoptosis by cleaving numerous cellular proteins, including poly(ADP-ribose) polymerase (PARP-1) and inhibitor of caspase-activated DNase (ICAD) (8). However, in the intrinsic apoptotic pathway, mitochondrial outer membrane permeabilization (MOMP) is induced by the Bax or Bak protein. In response to apoptotic stimuli, Bax or Bak translocates from the cytoplasm to the mitochondria, disrupting the integrity of the outer membrane and triggering the release of cytochrome C. This release of cytochrome c facilitates the oligomerization of apoptotic protease-activating factor 1 (APAF-1) and the formation of a heptameric complex known as the apoptosome (9). Subsequently, caspase-9 is recruited to the apoptosome, leading to caspase-9 activation and the subsequent cleavage of caspase-3 and caspase-7, ultimately resulting in cell death (6).
KSHV encodes various antiapoptotic factors that mitigate cellular apoptotic pathways. LANA, the major latent protein, directly binds the tumor suppressor p53 and inhibits p53-induced apoptosis (10). vFLIP, a homolog of the cellular FLIP, can be recruited to the death-inducing signaling complex (DISC) and blocks caspase-8 signaling (11). vBCL-2, a viral homolog of the cellular Bcl-2 protein, inhibits apoptosis by blocking proapoptotic BH3 domain-containing proteins (12). vIRF1 inhibits p53-induced apoptosis through its interaction with p53 and the upstream kinase ATM (13). K1 inhibits apoptosis by inducing the release of growth factors, in turn activating the PI3K-AKT pathway (14). K7 is structurally related to survivin-DeltaEx3 and protects cells from apoptosis (15). Cellular apoptosis modulated by p53 and PI3K-AKT is part of the intrinsic apoptotic pathway. However, the role of the extrinsic apoptotic pathway in KSHV infection has been less studied.
In this study, we examined the expression of the receptors FasR, TNFR1, DR3, DR4, and DR5 in the extrinsic apoptotic pathway in KSHV-positive Tet-RTA-BCBL1 and BC3 cells and found that DR5 expression was significantly upregulated during KSHV lytic replication. DR5 functions as an interferon-stimulated gene (ISG) that inhibits KSHV lytic replication. Mechanistically, the suppression of KSHV lytic replication by DR5 can be attributed to the activation of an alternative apoptotic pathway termed lysosome-dependent apoptosis, which is triggered by DR5. Importantly, we found that three amino acids of DR5 have undergone positive selection in primates and that reverse mutation of one of these amino acids (A62D) alters the binding of DR5 to its ligand, TNF-related apoptosis-inducing ligand (TRAIL), which in turn alters the antiviral function of DR5 during KSHV infection. Notably, we also found that DR5 inhibits Epstein–Barr virus (EBV), herpes simplex virus type 1 (HSV-1), and herpes simplex virus type 2 (HSV-2) infection. Conversely, as a countermeasure to DR5-mediated inhibition, the KSHV K5 protein interacts with DR5, facilitating the ubiquitination and subsequent degradation of DR5. Taken together, these findings reveal the involvement of the extrinsic apoptosis pathway in KSHV lytic replication and confirm that DR5 is a host restriction factor for herpesviruses.
Results
DR5 Inhibits KSHV Replication.
To determine which receptors in the extrinsic apoptotic pathway are involved in KSHV lytic replication, we detected the expression of FasR, TNFR1, DR3, DR4, and DR5 in KSHV-positive Tet-RTA-BCBL1 and BC3 cells, as well as in KSHV-negative iSLK.Puro cells. In KSHV-positive cells, after induction by doxycycline (Dox) or tetradecanoyl phorbol acetate (TPA) and sodium butyrate (NaB), KSHV enters lytic replication leading to varying degrees of upregulation in the mRNA expression of TNFR1, DR3, and DR5. Notably, the most significant increase was observed in the expression of DR5 (SI Appendix, Fig. S1 A and C). DR5 exists in two isoforms: a long form around 48 kDa and a short form nearly 40 kDa. Both isoforms are ubiquitously expressed in human tissues and cell lines. The long form generally predominates, but the proportion of the two isoforms varies depending on the tissue type (16, 17). In terms of activating apoptosis, there were no significant functional differences between the two forms of DR5 when DR5s were greatly overexpressed (16). Intriguingly, the protein levels of DR5 showed a distinct pattern characterized by initial downregulation followed by subsequent upregulation (SI Appendix, Fig. S1 B and D–F). In contrast, the protein levels of other receptors remain largely unchanged, except for an upregulation in DR4 expression (SI Appendix, Fig. S2 A and B). In iSLK.Puro cells, Dox-induced RTA expression led to a significant reduction in DR5 mRNA levels and a slight decrease in DR5 protein levels (SI Appendix, Fig. S2 C–F).
To explore the role of DR5 in KSHV lytic replication, we initially generated a stable cell line in iSLK.RGB cells expressing DR5 (Fig. 1A). iSLK.RGB cells contain a Dox-inducible KSHV reporter virus that expresses three fluorescent protein cassettes marking latent (red), immediate early (green), and late viral gene expression (blue). Subsequently, we investigated the impact of DR5 on KSHV replication. After Dox-induction, we found that overexpression of DR5 markedly reduced the viral loads of KSHV in supernatants (Fig. 1B). Finally, we also detected the expression of viral genes during different life cycles and found that overexpression of DR5 inhibits latent and lytic viral gene expression, but not the host 18S rRNA (Fig. 1C). In contrast, downregulation of DR5 using specific siRNAs in iSLK.RGB and BCBL1 cells (Fig. 1D and SI Appendix, Fig. S3A), or sgRNAs in iSLK.RGB cells (SI Appendix, Fig. S3C) led to a significant increase in viral loads compared to control cells, demonstrating that downregulation of DR5 enhanced KSHV replication (Fig. 1E and SI Appendix, Fig. S3 B and D). Furthermore, the mRNA levels of viral genes showed a notable increase in the cells where DR5 was knocked down or knocked out (Fig. 1F and SI Appendix, Fig. S3E). These findings collectively demonstrated that DR5 is an antiviral gene that functionally suppressed KSHV lytic replication. Given that the expression of DR4 is altered in both KSHV-positive cells and iSLK.Puro cells, we used specific DR4 siRNAs to downregulate DR4 expression in iSLK.RGB cells. Our findings revealed that DR4 knockdown did not affect KSHV lytic replication (SI Appendix, Fig. S2 G and H).
Fig. 1.

DR5 inhibits KSHV replication. (A–C) Overexpression of DR5 inhibits KSHV replication. (A) DR5 expression was evaluated via qPCR and immunoblotting. (B) Extracellular viral loads after DNase treatment were quantified via qPCR. (C) Viral gene expression was analyzed via qPCR. (D–F) Knockdown of DR5 promotes KSHV lytic replication. (D) si-NC or si-DR5 was transfected into iSLK.RGB cells. After 24 h, the cells were collected, and the expression of DR5 was detected via qPCR and immunoblotting. (E) iSLK.RGB cells expressing DR5 siRNA were treated with Dox. After 48 h, the viral loads in the supernatants were quantified via qPCR. (F) Viral gene expression was detected via qPCR. Representative results from three biological replicates are presented. The error bars indicate the SDs. The data were analyzed with Student’s multiple t tests (ns, no significant difference; *P < 0.05; **P < 0.01; ****P < 0.0001).
DR5 Inhibits KSHV Replication Through the Induction of Apoptosis.
As DR5 is an important receptor in the extrinsic apoptotic pathway, we next investigated the effect of DR5-induced apoptosis on KSHV lytic replication. First, DR5-induced caspase-3/7 activation was assessed during KSHV lytic replication. As shown in Fig. 2 A and B, caspase-3/7 activity increased twofold or decreased by 25 to 40% with increased or decreased expression of DR5, respectively, indicating that DR5-related apoptosis may play an important role in KSHV lytic replication. To further confirm that DR5-induced apoptosis regulates KSHV lytic replication, we used Z-IETD-FMK, a caspase-3/8 inhibitor, and Z-DEVD-FMK, a caspase-3-specific inhibitor, to treat DR5-expressing cells and control cells. We subsequently measured the extracellular viral loads after DNase treatment. As shown in Fig. 2C, DR5 overexpression decreased the viral titer by 60%; however, the caspase-8 and caspase-3 inhibitors almost completely abolished the DR5-induced inhibition of KSHV lytic replication, which indicated that DR5-mediated inhibition of KSHV replication is dependent on the apoptosis pathway.
Fig. 2.

DR5 inhibits KSHV replication through the induction of apoptosis. (A and B) DR5-expressing cells, DR5-knockdown cells, and control cells were treated with Dox for 24 h and 48 h. Then, caspase-Glo 3/7 reagents were added to the culture medium, and the luminescence of each sample was measured with a luminometer according to the manufacturer’s instructions. (C) DR5-expressing cells and control cells were treated with DMSO or inhibitors of caspase-3/8 and caspase-3, after which the viral loads in the supernatants were detected via qPCR. (D) DR5-expressing cells and control cells were treated with Dox for 24 h to induce KSHV reactivation. Then, the cells were incubated with DMSO, chloroquine, or bafilomycin A1 for another 10 h, and the viral loads in the supernatants were determined by qPCR. (E–G) Viral loads in the supernatants, the percentage of GFP-expressing HEK293T cells infected with viral supernatants and viral gene expression were detected as described above. (H) DR5-expressing cells, DR5-L311/312A mutant-expressing cells, and control cells were treated with Dox for 24 h and 48 h. Then, caspase 3/7 activity was measured with a luminometer according to the manufacturer’s instructions. Representative results from three biological replicates are presented. The error bars indicate the SDs. The data were analyzed with Student’s t tests (ns, no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
DR5 may trigger apoptosis through the classic apoptotic pathway and/or an alternative lysosomal pathway (18). Upon stimulation with TRAIL, DR5 recruits the adaptor protein FADD, which then recruits and activates caspase-8 and caspase-10. These activated initiator caspases directly trigger the activation of the effector caspases caspase-3/6/7, leading to cell death (19). Furthermore, DR5 can also be internalized through endocytosis and transported via vesicles to lysosomes, which become permeabilized, release cathepsin B, and subsequently promote cell apoptosis (20). To determine whether DR5 suppresses KSHV lytic replication by activating the DR5 internalization-induced apoptotic pathway, DR5-expressing cells and control cells were treated with the lysosome inhibitors chloroquine and bafilomycin A1. The viral loads in the supernatants were subsequently quantified. As shown in Fig. 2D, both chloroquine and bafilomycin A1 abolished the inhibitory effect of DR5 on KSHV replication, indicating that DR5 internalization is vital for the DR5-induced inhibition of KSHV lytic replication. Furthermore, we mutated the dileucine motif at amino acids 311 and 312 of DR5, which is necessary for DR5 internalization (20). We found that the DR5-L311/312A mutant had almost no inhibitory effect on KSHV replication (Fig. 2 E–G). This result suggests that the internalization of DR5 is essential for the ability of DR5 to inhibit KSHV replication. We subsequently examined apoptosis induced by the DR5-L311/312A mutant in the KSHV life cycle. As shown in Fig. 2H, the caspase-3/7 activity of the DR5-L311/312A mutant group was lower than that of the DR5-expressing group and approached the level of the control group. These results indicate that DR5 internalization is necessary for DR5-induced apoptosis during KSHV lytic replication. To eliminate the interference caused by red and green fluorescence in iSLK.RGB cells during Annexin-V-FITC and PI detection, we developed DR5-expressing, DR5-L311/312A mutant-expressing, and DR5-knockdown cell lines in SLK cells. Subsequently, these cells were infected with 30 copies of KSHV DNA, and conducted an Annexin-PI assay. Annexin V and PI staining revealed that DR5-induced apoptosis plays an important role in the KSHV life cycle and that DR5 internalization is necessary for DR5-induced apoptosis (SI Appendix, Figs. S4 and S5). Taken together, these results suggest that the inhibitory effect of DR5 on KSHV replication is achieved through DR5 internalization-induced apoptosis.
DR5 Is an ISG.
These results indicated that DR5 can inhibit KSHV lytic replication, suggesting the potential role of DR5 as a restriction factor. One defining characteristic of restriction factors is their close association with the innate immune response; notably, the expression of these factors is often significantly upregulated by interferons (21). Studies have shown that IFN-α can increase the expression of DR5, rendering specific human hepatoma cell lines, HuH-7 and Hep3B cells, sensitive to TRAIL-induced apoptosis (22). We also observed that DR5 is upregulated in KSHV-positive PEL cells during KSHV lytic replication and that DR5 can significantly inhibit KSHV replication. Therefore, we speculated that DR5 may be an ISG. To test this hypothesis, we first analyzed the sequence upstream of the DR5 transcriptional start site (TSS) and identified several signal transducer and activator of transcription (STAT) motifs and interferon-stimulated response element (ISRE) motifs (Fig. 3A). Next, we treated the human leukemia monocytic cell line THP-1 with interferons, which are extensively used to study interferon signaling pathways, and measured the expression of DR5 via qPCR and western blotting. The expression of ISG15 was used as a positive control (SI Appendix, Fig. S6 A–C). As depicted in Fig. 3 B–D, DR5 mRNA expression was significantly increased two- to eightfold after interferon treatment compared with that in the untreated group. Correspondingly, the protein levels of DR5 exhibited a similar increase (Fig. 3 E–G). To further validate these findings, we cloned DR5 promoter sequence into the pGL3-basic vector (DR5-promoter) and generated a derivative construct by deleting the STAT/ISRE motifs (DR5-delete-promoter). Subsequently, these constructs, along with a Renilla plasmid, were transfected into HEK293T cells. After 18 h, the cells were treated with various types of interferons (IFNs) for another 6 h. The cells were then lysed, and the luciferase activities were measured. The results demonstrated that IFNs robustly activated DR5-promoter, whereas deletion of the STAT/ISRE motifs abolished this activation (SI Appendix, Fig. S6 D–F). In summary, these results suggest that DR5 is an ISG.
Fig. 3.

DR5 is an ISG. (A) Analysis of the STAT motif and ISRE motif upstream of the DR5 TSS. The red squares indicate the STAT motif, and the green squares indicate the ISRE motif. TSS indicates the transcription start site, and CDS indicates the coding sequence. (B–D) THP-1 cells were treated with IFNα 2/IFN β/IFN for different durations, and the expression of DR5 was determined via qPCR. (E–G) THP-1 cells were treated with IFNα 2/IFN β/IFN for different durations, and the expression of DR5 was determined via western blotting. Representative results from three biological replicates are presented. The error bars indicate the SDs. The data were analyzed with Student’s t tests (***P < 0.001, ****P < 0.0001).
DR5 Has Evolved Under Positive Selection in Primates.
Another defining feature of restriction factors is that they often undergo rapid evolution (21). Generally, mutations in a population are maintained only when they confer a selective advantage. If a host species is subjected to multiple rounds of pathogen pressure, altered variants of host restriction factors that are no longer susceptible to the pathogen are selected. Our previous findings suggest that DR5 is involved in regulating KSHV lytic replication. Therefore, we wanted to determine whether DR5 underwent positive selection during primate evolution to alter its susceptibility to KSHV. To trace the evolutionary history of DR5, we compared the DR5 sequences of 28 primate species that represent 40 million years of evolution (23). The sequences of these genes were downloaded from publicly available datasets (Dataset S1). Phylogenetic analysis of these gene sequences revealed that the gene trees derived from nucleotide alignments could be divided into three groups, namely, Old World monkeys, New World monkeys, and apes, which is in accordance with the accepted species tree from Perelman and colleagues (24) (SI Appendix, Fig. S7A). To assess whether DR5 has experienced diversifying selection during primate evolution, we performed two types of positive selection analysis. First, we used the single-likelihood ancestor counting (SLAC) method, which uses a combination of maximum likelihood (ML) and counting approaches to infer nonsynonymous substitution rates on a per-site basis for a given coding alignment and corresponding phylogeny (25). This method assumes that the selection pressure for each site is constant throughout the entire phylogeny. By using this method, we found that most codons in DR5 are extremely conserved, with over 81% having a ratio of nonsynonymous to synonymous nucleotide (dN/dS) substitution lower than 1. However, a few sites were identified as having evolved under significant positive selection; this was especially true for four sites: 187, 202, 362, and 399 (P < 0.15) (SI Appendix, Fig. S7 B and C). Second, we used the mixed-effects model of evolution (MEME) method, which employs a mixed-effects ML approach to detect sites evolving under positive selection under a proportion of branches (25). Through MEME analysis, we also identified four sites that experienced positive selection: 202, 306, 362, and 399 (P < 0.01) (SI Appendix, Fig. S7 B and C). By combining the results obtained with these two methods, we identified three sites under significant positive selection: 202, 362, and 399. We subsequently compared the DR5 sequences of Old World monkeys, New World monkeys, and apes to further analyze the evolutionary trends of these three codons. We found that site 202 evolved from aspartic acid (Asp-GAT) to valine (Val-GTT) to alanine (Ala-GCT), site 362 evolved from leucine (Leu-TTA/CTC/CTA) to serine (Ser-TCA) to proline (Phe-CCA/TTC) to alanine (Ala-GCA), and site 399 evolved from histidine (His-CAT) to arginine (Arg-CGT) (SI Appendix, Fig. S7D). Therefore, although most DR5 protein sequences are conserved in primates, a few sites have experienced significant positive selection and exhibit substantial genetic plasticity. The characteristics of these genetic variations in DR5 could be reminiscent of an evolutionary arms race with pathogenic viruses.
The A62D Mutation in the Ligand-Binding Domain of DR5, Which Occurred Under Positive Selection, Mitigates Apoptosis.
To determine whether these positive selection sites are responsible for restricting KSHV replication, we first analyzed the relative positions of these three positive selection sites in Homo sapiens. We found that in H. sapiens, site 202 corresponds to site 62, site 362 corresponds to site 221, and site 399 corresponds to site 257. In apes, site 62 corresponds to an alanine (Ala-GCT), while in New World monkeys, it corresponds to a valine (Val-GTT), and in Old World monkeys, it corresponds to an aspartic acid (Asp-GAT). Furthermore, in apes, site 221 corresponds to an alanine (Ala-GCA); in New World monkeys, it corresponds to both an alanine (Ala-GCA) and a proline (Phe-CCA/TTC), whereas in Old World monkeys, it predominantly corresponds to a leucine (Leu-TTA/CTC/CTA). Finally, in apes, site 257 corresponds to an arginine (Arg-CGT), whereas in New World monkeys and Old World monkeys, it corresponds to a histidine (His-CAT) and an arginine (Arg-CGT), respectively. Therefore, we mutated the amino acids at these specific sites in H. sapiens DR5 to match the amino acids at the corresponding sites in DR5 from Old World monkeys and obtained the mutants A62D, A221L, and R257H. Stable cell lines were subsequently generated from the iSLK.RGB cell line and used to evaluate the roles of the mutants in KSHV replication. During KSHV lytic replication, RTA protein expression was lower in DR5-, DR5-A221L-, and DR5-R257H-expressing cells than in control cells (Fig. 4A). Conversely, an increase in RTA protein expression was observed in the DR5-A62D group compared with the wild-type DR5 group, suggesting that the A62D mutation may eliminate the antiviral activity of DR5 (Fig. 4A). Furthermore, we examined the viral loads released into the supernatants to confirm the effects of these positive selection sites on KSHV replication. Compared with those in the control group, notable reductions in the viral load were observed upon the expression of wild-type DR5 or the DR5-A221L or DR5-R257H mutant. Notably, there was no significant difference in the degree to which wild-type DR5 and the DR5-A221L and DR5-R257H mutants decreased the viral load. However, compared with wild-type DR5, the A62D mutant significantly increased the viral load, indicating the crucial role of this site in the antiviral function of DR5. In contrast, the mutations DR5-A221L and DR5-R257H did not affect antiviral activity, suggesting that these sites may have other undiscovered functions (Fig. 4 B and C).
Fig. 4.

The A62D mutation of DR5 attenuates the inhibitory effect of DR5 on KSHV replication due to the reduced ability of DR5 to bind the ligand TRAIL. (A–C) The A62D mutation of DR5 attenuates the inhibitory effect of DR5 on KSHV replication. (A) DR5 expression was evaluated by immunoblotting to validate the successful establishment of iSLK.RGB cell lines with stable expression of DR5 and DR5 mutants. (B) DNase-treated extracellular viral loads were quantified via qPCR. (C) Viral gene expression was analyzed via qPCR. (D and E) The reduced inhibitory effect of the A62D mutant of DR5 on KSHV replication occurred due to a decrease in the ability of DR5 to bind the ligand TRAIL. (D) The ligand TRAIL (1 µg/mL) was coated on a 96-well plate, followed by the incubation of various concentrations of wild-type His-DR5 or His-DR5-A62 mutant with the TRAIL for 2 h at room temperature. Subsequently, anti-His-mouse antibody was added and incubated for 1.5 h, followed by incubation with anti-HRP-mouse antibody for 1 h. A substrate solution was then added to each well and incubated for 20 min at room temperature. The reaction was stopped by adding a stop solution, and the optical density of each well was measured using a microplate reader set to 450 nm. (E) Wild-type DR5-, DR5-A62D-, DR5-A221L-, and DR5-R257H-expressing cells and control cells were constructed from HEK293 cells. These cells were subsequently infected with 30 copies of KSHV DNA for 8 h, after which caspase 3/7 activity was measured with a luminometer according to the manufacturer’s instructions. Representative results from three biological replicates are presented. The error bars indicate the SDs. The data were analyzed with Student’s multiple t tests (ns, no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
DR5 is a cell-surface receptor that specifically binds TRAIL and induces apoptosis. DR5, a type I transmembrane protein, comprises a cysteine-rich extracellular domain, a transmembrane domain, and an intracellular domain that contains a death domain (DD) (26, 27). The domains of DR5 are shown in a schematic in SI Appendix, Fig. S8A. The first cystine of the extracellular structural domain contains a preligand assembly structural domain (PLAD), which promotes the oligomerization of DR5 to form trimers and thus enhances the binding of TRAIL and DR5 (28). Through sequence comparison, we found that the positive selection site A62 is located at the first cystine of the extracellular domain of DR5, which is necessary for the binding of TRAIL to DR5. Therefore, we hypothesized that the attenuated antiviral function of the DR5-A62D mutant might be due to attenuated binding between TRAIL and DR5. To test this hypothesis, we investigated the interaction between TRAIL and wild-type DR5 and between TRAIL and the DR5-A62D mutant. The extracellular domains of wild-type DR5 and the A62D mutant were expressed and purified using the CHO expression system, after which they were subjected to ELISA to assess their binding affinity for the ligand TRAIL. As shown in Fig. 4D, the EC50 for TRAIL and wild-type DR5 was 333.2 ng/mL, whereas the EC50 for TRAIL and the DR5-A62D mutant was 677.3 ng/mL. This finding suggests a greater affinity between TRAIL and wild-type DR5-than between TRAIL and the DR5-A62D mutant, suggesting that the A62D mutation affects the binding of DR5 to its ligand, TRAIL. The induction of apoptosis is initiated by the interaction between DR5 and TRAIL. To determine the effect of the decreased binding of DR5-A62D to TRAIL, we examined apoptosis by measuring caspase-3/7 activity following KSHV infection. As shown in Fig. 4E, caspase-3/7 activity was decreased in the DR5-A62D group. Taken together, our results indicate that the reverse mutation of one amino acid (A62D) affects the binding of DR5 to its ligand, TRAIL, thereby influencing DR5-induced cell apoptosis, consequently impacting the antiviral function of DR5.
KSHV K5 Interacts with DR5 and Promotes DR5 Degradation.
These results indicated that DR5 functions as a host restriction factor to inhibit KSHV lytic replication. If a restriction factor poses a true threat to viral replication, the virus will inevitably evolve equally effective countermeasures against this restriction. Viruses have evolved multiple mechanisms to antagonize the functions of these restriction factors, including inducing protein degradation, causing mislocalization of the restriction factors and thus downregulating functional expression, or mimicking the substrate of the restriction factor (29). To determine whether KSHV has evolved a mechanism to antagonize the antiviral function of DR5, we analyzed proteomics data collected during KSHV reactivation in iSLK cells (30) and observed a decrease in DR5 protein levels. Additionally, we noted a reduction in DR5 protein expression during the early stages of KSHV lytic reactivation (SI Appendix, Fig. S1 B and D–F). These findings suggest that KSHV may antagonize the inhibitory activity of DR5 by degrading DR5.
KSHV encodes three E3 ligases that target and degrade host restriction factors. RTA mainly targets immunomodulatory factors and transcriptional repressors such as IRF7, MyD88, Hey-1, and the SMC5/6 complex (31–34). Moreover, K3 and K5 play pivotal roles in downregulating many cell-surface receptors, including MHC-I (35). We next examined the interaction between DR5 and the KSHV-encoded E3 ligases K3, K5, and RTA and found that only K5 can interact with DR5, unlike RTA and K3 (SI Appendix, Fig. S9 A–D). This result is consistent with the finding that TRAIL-R2 is regulated by K5 (30). Furthermore, we generated a FLAG-tagged K5 construct in SLK cells and used endogenous immunoprecipitation to confirm the interaction between DR5 and K5 (Fig. 5 A and B). In addition to immunoprecipitation, we confirmed the interaction between K5 and DR5 via immunofluorescence. As shown in Fig. 5C, K5 was distributed mainly in the cytoplasm, whereas DR5 was distributed in both the cytoplasm and the nucleus, which is consistent with their previously reported distributions (36, 37). When cells were cotransfected with K5 and DR5, they displayed obvious colocalization in the cytoplasm. To further investigate the interaction between K5 and DR5, K5 truncation mutants and DR5 truncation mutants were generated. Immunoprecipitation assays with the truncated DR5 and K5 mutants revealed that the DR5–K5 interaction site is located within the region of K5 spanning amino acids 67 to 145 (SI Appendix, Fig. S9E). Conversely, immunoprecipitation assays with the truncated K5 and DR5 mutants revealed that the site in DR5 that facilitates the DR5–K5 interaction is within the region of DR5 spanning amino acids 1 to 169 (SI Appendix, Fig. S9F). Taken together, these data demonstrate that K5 interacts with DR5.
Fig. 5.

KSHV K5 interacts with DR5 and degrades DR5. (A–C) KSHV K5 interacts with DR5. (A) A stable SLK cell line expressing the FLAG-K5 gene was established via lentiviral transduction. Cell lysates were immunoprecipitated with FLAG antibody-conjugated agarose beads and analyzed via western blotting with an anti-DR5 antibody. (B) Cell lysates from SLK cells expressing FLAG-K5 were immunoprecipitated with DR5 antibody and protein A/G-conjugated agarose beads. Subsequently, the samples were analyzed by western blotting with an anti-FLAG antibody. (C) HeLa cells were transfected with FLAG-DR5, HA-K5, or FLAG-DR5 and HA-K5 and then stained with anti-HA and anti-FLAG antibodies and Alexa Fluor 488 and Alexa Fluor 555 secondary antibodies. Colocalization was viewed by a DM6000B fluorescence microscope. (D–G) KSHV K5 degrades DR5. (D) SLK cells expressing K5 or transfected with the empty control were stained with anti-DR5-PE or anti-IgG1-PE and analyzed by flow cytometry. (E) HEK293T cells were transfected with HA-K5 or FLAG-DR5 or FLAG-DR5 and HA-K5, and co-IP was performed with FLAG antibody-conjugated agarose beads. Ubiquitination was detected with an anti-Ub antibody. (F and G) DR5 expression in wild-type RGB cells and mutant RGB cells in which K5 was deleted. A recombinant K5 deletion virus was constructed and introduced into iSLK cells, after which the cells were treated with Dox for 8, 24, 36, 48, or 72 h. The expression of DR5 was measured via western blotting.
Since K5 interacts with DR5, we tested whether K5 can downregulate DR5 expression. We analyzed the expression of DR5 in the presence of K5. With increasing K5 expression, DR5 expression decreased in a dose-dependent manner (SI Appendix, Fig. S10A). As DR5 is located primarily on the cell surface, we investigated whether K5 diminishes the expression of DR5 on the cell surface. As shown in Fig. 5D, flow cytometry revealed that the cell-surface expression of DR5 was markedly lower in K5-expressing cells than in control cells. Next, cycloheximide (CHX) assays revealed that the ectopic expression of K5 was associated with a decreased DR5 half-life (SI Appendix, Fig. S10B). Since protein degradation is usually related to ubiquitination, we used the ubiquitin—proteasome system inhibitor MG132 and the autophagy—lysosome pathway inhibitors NH4Cl and 3-methyladenine (3MA) to determine whether the downregulation of DR5 is ubiquitin dependent. In the presence of all the inhibitors, the reduction in DR5 protein levels was almost completely reversed (SI Appendix, Fig. S10C), indicating that K5 can degrade DR5 in two ways. Furthermore, we performed a ubiquitination assay and found that K5 promotes the ubiquitination of DR5 (Fig. 5E). Mutational analysis of a lysine residue in the cytosolic tail of DR5 revealed that Lys245 of DR5 is essential for K5-induced degradation (SI Appendix, Fig. S11). Finally, we examined whether the sequence of K5 is also important for the KSHV-mediated downregulation of DR5. Recombinant viruses lacking the coding sequence (CDS) of the K5 gene were generated from the KSHV red-green-blue bacterial artificial chromosome (BAC) 16 (RGB-BAC16) genome and then introduced into iSLK cells as previously described (38). iSLK cells harboring the wild-type virus or the virus lacking the K5 gene were treated with doxycycline for different durations, and the expression of DR5 was analyzed via qPCR and western blotting. In the absence of K5, the downregulation of DR5 protein expression was abolished (Fig. 5 F and G and SI Appendix, Fig. S10 D and E), which excludes the possibility that K5 affects DR5 gene transcription, thereby reducing DR5 protein expression. These results reaffirm the requirement of K5 for KSHV infection and the downregulation of DR5 expression.
DR5 Has Broad Antiviral Activity Against Various Herpesviruses.
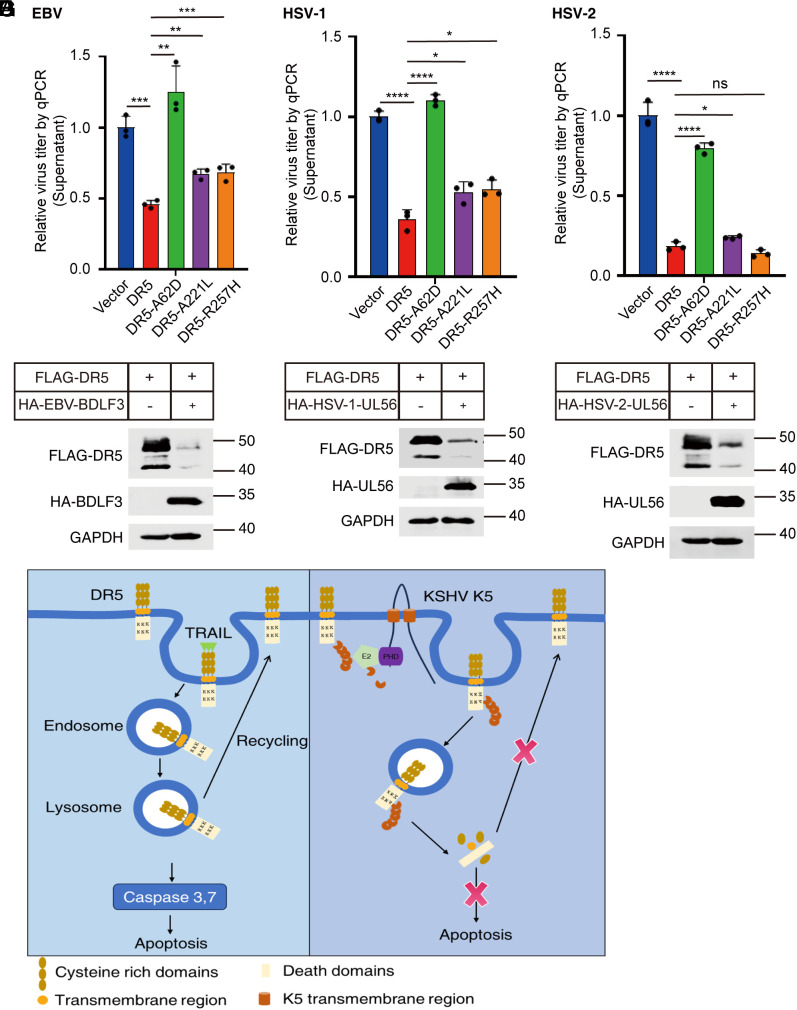
Apoptosis is a widely conserved antiviral defense mechanism that efficiently curtails viral dissemination by orchestrating the self-destruction of infected cells. We found that DR5 can inhibit the lytic replication of KSHV. Therefore, we were interested in exploring whether DR5 also inhibits other distinct herpesviruses. First, we constructed HEK293 cell lines harboring different constructs: an empty vector, wild-type DR5, and three DR5 mutants (DR-A62D, DR-A221L, and DR-R257H). These cell lines were subsequently infected with the B95.8 strain of EBV, another γ-herpesvirus. After 24 h of infection, viral titers in the extracellular fraction were quantified via qPCR. As shown in Fig. 6A, DR5 significantly decreased the DNase-treated extracellular viral loads by approximately 60%. These outcomes strongly imply that DR5 can inhibit EBV infection. Notably, we also found that the DR5-A62D mutation attenuated the antiviral activity of DR5 against EBV, whereas the other two mutants retained their antiviral activities. We next investigated the ability of DR5 to restrict the replication of HSV-1 and HSV-2, both of which are α-herpesviruses. HSV-1 strain 17 and the HSV-2 G strain were used to infect these cell lines at a multiplicity of infection (MOI) of 0.01. At 24 h after infection, the viral titers in the supernatants were significantly lower in the cells expressing DR5 than in the negative control cells (Fig. 6 B and C). However, nearly equivalent levels of viral DNA were detected in the A62D mutant group and the negative control group (Fig. 6 B and C). These results highlight the pivotal role of DR5 as a potent restriction factor against herpesviruses. Importantly, the spectrum of viruses susceptible to DR5-induced inhibition likely extends beyond our current investigation, indicating a broader impact than initially observed.
Fig. 6.
DR5 has broad antiviral activity against EBV, HSV-1, and HSV-2. (A) DR5 inhibits EBV infection. HEK293 cells expressing wild-type DR5 and the DR5-A62D, DR5-A221L, and DR5-R257H mutants and control cells were infected with the EBV B95.8 strain for 24 h. The extracellular viral loads were subsequently quantified via qPCR after DNase treatment. To ensure DNA extraction quality, pGL3-luc plasmid DNA was introduced during viral DNA extraction. Relative DNA copy numbers were determined via qPCR using primers for EBV-Bam-W and pGL3. The control values were normalized to 1. (B) DR5 inhibits HSV-1 infection. Cell lines were infected with HSV-1 for 24 h. The viral loads in the supernatants were subsequently quantified via qPCR via primers for HSV-1 UL30 and pGL3. The control values were normalized to 1. (C) DR5 inhibits HSV-2 infection. Cell lines were infected with HSV-2 for 24 h. The viral loads in the supernatants were quantified via qPCR using primers for HSV-2 gD and pGL3. The control values were normalized to 1. Representative results from three biological replicates are presented. The error bars indicate the SDs. The data were analyzed with Student’s multiple t tests (ns, no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (D) EBV BDLF3 Mediates DR5 Degradation. HEK293T cells were transfected with FLAG-DR5 and HA-Vector, or FLAG-DR5 and HA-BDLF3 for 36 h, followed by cell lysis. The levels of DR5 and BDLF3 were assessed via western blot using anti-HA and anti-FLAG antibodies. (E) HSV-1 Induces DR5 Degradation. HEK293T cells were transfected with FLAG-DR5 and HA-Vector, or FLAG-DR5 and HSV-1 HA-UL56 for 36 h, then underwent cell lysis. The expression of DR5 and HSV-1 UL56 was analyzed by western blot using anti-HA and anti-FLAG antibodies. (F) HSV-2 promotes DR5 degradation. HEK293T cells were transfected with FLAG-DR5 and HA-Vector, or FLAG-DR5 and HSV-2 HA-UL56 for 36 h, then lysed. The levels of DR5 and HSV-2 UL56 were determined via western blot using anti-HA and anti-FLAG antibodies. (G) Schematic depicting the role of DR5 in regulating KSHV lytic replication. The host protein DR5 acts as a restriction factor, suppressing KSHV lytic replication through the induction of lysosomal-dependent apoptosis. To antagonize its inhibitory impact on KSHV lytic replication, the K5 protein interacts with DR5, promoting DR5 degradation.
The K5 protein is unique to KSHV. To explore the mechanisms by which other herpesviruses counteract the antiviral function of DR5, we wanted to determine whether EBV, HSV-1, and HSV-2 utilize their encoded membrane-associated E3 ubiquitin ligases or viral proteins located on the cell membrane that can recruit cellular E3 ligases to regulate the degradation of DR5 during lytic replication. BDLF3 is a late lytic gene of EBV that can induce the downregulation of cell-surface MHC class I and MHC class II molecules (39). However, the mechanism of BDLF3 is different from that of the KSHV K5 protein. Unlike K5, BDLF3 itself has not been known to be an E3 ubiquitin ligase. BDLF3 likely recruits membrane-associated RING-CH (MARCH) proteins to decrease the expression of MHC class I and MHC class II molecules by inducing ubiquitination (39). After cotransfection with DR5, BDLF3 downregulated DR5 protein expression (Fig. 6D). The HSV UL56 gene is conserved among most α-herpesviruses and is classified as a type II membrane protein (40). HSV UL56 has been reported to interact with several members of the neuronal precursor cell-expressed developmentally downregulated 4 (NEDD4) ubiquitin ligase family, including Nedd4 and Itch (41, 42). HSV-1 pUL56 directly interacts with Golgi-associated PDZ and coiled-coil motif-containing protein (GOPC), facilitating the ubiquitination and subsequent proteasomal degradation of GOPC, resulting in decreased expression of cell-surface toll-like receptor 2 (TLR2). Moreover, pUL56 induces degradation of the interleukin-18 (IL-18) receptor (43). Additionally, ORF1, a homolog of HSV UL56 found in equine herpesvirus type 4 (EHC-4), significantly reduces the expression of MHC-I during the early stages of viral infection (44). To investigate the impact of HSV-UL56 on DR5 expression, we cotransfected cells with DR5 and HSV-UL56 and found that the protein levels of DR5 decreased upon the expression of HSV-UL56 (Fig. 6 E and F). Collectively, these results demonstrate that the downregulation of DR5, facilitated by membrane-associated E3 ubiquitin ligases encoded by herpesviruses or viral proteins located on cell membranes that interact with cellular E3 ligases, represents a common mechanism to counteract the inhibitory activity of DR5.
Discussion
Most cells undergo apoptosis after viral infection, which reduces the release of progeny viruses (45). Apoptosis is a pivotal antiviral mechanism that can be initiated through two distinct signaling pathways: the intrinsic and extrinsic apoptotic pathways. The extrinsic apoptotic pathway is initiated in response to extracellular stress, which triggers the activation of death receptors such as FasR, TNFR1, DR3, DR4, and DR5. In our study, we found that the mRNA expression of DR5 was upregulated in KSHV-positive PEL cells during KSHV lytic replication, especially in the late stages of lytic replication. DR5 is a typical death receptor expressed on the cell surface that induces cell apoptosis by binding the ligand TRAIL, thereby executing immune surveillance of virus-infected cells or transformed cells (46). The role of DR5 in viral infection is complex. During porcine epidemic diarrhea virus (PEDV) infection, DR5 promotes PEDV replication by regulating caspase-8-dependent apoptosis and facilitates PEDV entry (47). However, respiratory syncytial virus (RSV) infection strongly upregulates the expression of DR5 and its ligand, TRAIL, which in turn increases the sensitivity of RSV-infected cells to exogenous TRAIL-triggered apoptosis, rendering RSV more easily eliminated by immune cells (including natural killer cells and CD4+ cells expressing membrane-bound TRAIL) through the TRAIL pathway (48). In addition, HIV and hepatitis C virus (HCV) infections also upregulate DR5 expression, and HCV infection can sensitive host cells to TRAIL-induced apoptosis (49, 50). In our study, we that DR5 can suppress KSHV lytic replication and that this inhibitory effect depends on DR5-induced apoptosis. Additionally, we reported that DR5 exhibits broad antiviral activity against herpesviruses, including EBV, HSV-1, and HSV-2. DR5-mediated apoptosis can be triggered through three distinct mechanisms. In type I cells, the interaction of DR5 with TRAIL leads to the activation of caspase-8, which can directly induce the activation of caspase-3 and caspase-7, leading to apoptosis (6). In type II cells, DR5-induced apoptosis involves caspase-8-mediated cleavage of the BH3-only protein BID to form a truncated form (tBID). tBID then triggers MOMP and the release of cytochrome C, initiating effector caspase activation and apoptosis (51). Type I and type II cells can be differentiated by differences in the formation of the DISC. In type I cells, caspase-8 activation at the DISC is very efficient and leads to the direct activation of caspase-3 and subsequent caspase cascades. In contrast, type II cells exhibit little DISC formation, and the cleavage of Bid is required for TRAIL-induced apoptosis (19, 51). Alternatively, in some specific cell types, DR5 can be internalized via endocytosis upon stimulation with TRAIL. DR5 is subsequently transported to lysosomes, leading to lysosomal permeabilization and the release of cathepsin B, further triggering cell death (20, 52). In KSHV-infected cells, DR5 induced apoptosis through an alternative apoptotic pathway, the lysosomal apoptotic pathway, highlighting the necessity of DR5 internalization for the ability of DR5 to induce apoptosis during KSHV lytic replication. When the sites L311 and L312, which are necessary for DR5 internalization, were mutated, the inhibitory effect of DR5 on KSHV lytic replication was lost, and the ability of DR5 to trigger apoptosis was abolished.
Viruses impose a heavy burden on their hosts, forcing them into sustained evolutionary conflict. If a virus is pathogenic to its host, it can exert selective pressure that may increase the frequency of advantageous genetic variations or traits within a population. This process promotes better adaptations and ultimately increases survival and reproduction rates (53). In this study, we compared DR5 sequences from 28 primate species, performed evolutionary analyses, and identified positive selection sites in DR5. Our findings revealed that DR5 has evolved under positive selection in primates and that three sites display strong signatures of diversifying selection. In response to viral infection, positive selection enables individuals with genetic variations that render them immune or tolerant to the pathogen to survive and transmit these advantageous traits to subsequent generations. We scrutinized the evolutionary trajectory of the three positive selection sites in DR5 in primates. Evolutionary analysis and functional analysis of DR5 can reveal the evolutionary dynamics of this arms race, in terms of the development of DR5 and its more recent adaptations. We subsequently conducted the reverse mutation of DR5 to examine the effects of these mutations on herpesvirus replication and found that DR5 restricts diverse herpesviruses, including KSHV, EBV, HSV-1, and HSV-2. Importantly, the A62D mutation of DR5 can alleviate the inhibitory effect of DR5 on infection with these herpesviruses, suggesting that DR5 possesses a significant evolutionary advantage in combating viral infection and that the presence of site 62 in DR5 contributes to enhancing the diversity of antiviral defenses. However, the DR5-A221L and DR5-R257H mutants did not affect antiviral activity, suggesting that sites 221 and 257 may function in other ways. DR5 is a death-inducing receptor that is characterized by an extracellular ligand-binding region containing three cysteine-rich domains, a transmembrane region, and a cytoplasmic DD (54). The A62D mutation of DR5 is located within a crucial cysteine-rich domain essential for the binding of DR5 to its ligand, TRAIL. This mutation may impact the binding affinity between DR5 and its ligand, TRAIL, consequently affecting cell apoptosis triggered by the DR5–TRAIL interaction. Our study confirmed that the A62D mutation notably weakens the ability of DR5 to bind TRAIL, alleviating apoptosis triggered by the DR5–TRAIL interaction. This mutation not only decreases the binding activity of DR5 to its ligand but also reduces the degree to which apoptosis is triggered by herpesvirus infection, suggesting that these sites under positive selection have important effects on DR5 function.
KSHV has evolved with the human immune system for millions of years and has developed diverse mechanisms to antagonize it (55). By analyzing transcriptomic and proteomic data in epithelial cells with KSHV reactivation (30, 56), we found that the DR5 mRNA level was increased, whereas the DR5 protein level was decreased in iSLK cells. This discrepancy implies that DR5 may be an ISG and may undergo posttranslational modifications by KSHV-encoded viral proteins. Thus, we analyzed the sequence upstream of the TSS of DR5 and detected multiple ISREs. Induction with interferons α/β/γ increased both the mRNA and protein expression of DR5, indicating that DR5 is an ISG. The observations that DR5 expression increased upon interferon stimulation in THP-1 cells, whereas DR5 protein levels decreased in iSLK cells during KSHV reactivation, may be contradictory. Upon detecting DR5 expression in various KSHV-negative and KSHV-positive cells, we observed significantly lower levels of DR5 in KSHV-positive BCBL1 cells than in iSLK cells (SI Appendix, Fig. S8B). This may explain why the expression of DR5 was significantly increased in PEL cells but not in iSLK cells during KSHV lytic replication. Furthermore, in KSHV-positive PEL cells, we observed an initial decrease in DR5 protein expression during the early stages of KSHV lytic replication, followed by an increase in the later stages of lytic replication. As viral protein expression increased, DR5 protein levels correspondingly decreased. This dynamic aptly reflects the ongoing arms race between the host and the virus. Reduced DR5 protein expression might represent an antagonistic mechanism that the virus has attained via evolution. We found that only K5 could interact with DR5 and induce DR5 degradation. K5 is a KSHV-specific protein that plays a crucial role in evading host immune surveillance and facilitating infection by degrading cell-surface receptors. Thus, it is imperative to investigate whether EBV or other viruses have developed comparable antagonistic strategies. It has been suggested that HBV can evade antiviral immunity by modulating TRAIL receptor signaling. During HBV infection, the HBV X protein (HBx) induces the macroautophagy/autophagy-mediated degradation of DR5, permitting the survival of virus-infected cells (57).
In summary, our study reports that DR5 serves as a key host restriction factor for herpesviruses, including KSHV. DR5 inhibits KSHV lytic replication by inducing apoptosis, whereas the virus utilizes the K5 protein to mediate the degradation of DR5 (Fig. 6G). Understanding the mechanism of virus-induced death receptor-mediated apoptosis can help us better understand the life cycle of KSHV and thus increase our knowledge of virus–host interactions. However, several questions still remain. In KSHV-infected endothelial cells, DR5 induced apoptosis through an alternative pathway, but we do not know whether DR5 induces apoptosis through this pathway in other types of cells infected with KSHV. It is also unknown whether this alternative pathway is activated in other viruses, including EBV, HSV-1, and HSV-2. Furthermore, other herpesviruses utilize encoded membrane-localized viral proteins to recruit cellular E3 ligases and downregulate the expression of the DR5 protein. However, the specific mechanisms underlying the downregulation of DR5 protein expression by other herpesviruses still need to be explored. Finally, DR5-A221 and DR5-R257 have also undergone positive selection, but the DR5-A221L and DR5-R257H mutants did not alter the antiviral function of DR5. What is the means of positive selection for these two sites? Further exploration is warranted to answer these questions.
Materials and Methods
Details of the materials and methods used in this study, including Antibodies and reagents; cell lines and cell culture; plasmid constructs and qRT-PCR; RNAi interference; Caspase-3/7 activity assay; apoptosis assay; interferons induction; and flow cytometric analysis of cell surface expression of DR5, are provided in SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (TXT)
Dataset S02 (XSLX)
Acknowledgments
This work was supported by the grants from the National Key R&D Program (2023YFC2306600), the Natural Science Foundation of China (32188101), the Natural Science Foundation of Wuhan (2024040701010031) and the Fundamental Research Funds for the Central Universities (2042024kf1025) to K.L. and the grant from the China Postdoctoral Science Foundation (2024M752464) to C.H.
Author contributions
C.H. and K.L. designed research; C.H., C.G., B.S., N.L., and H.Y. performed research; C.H. and C.G. analyzed data; and C.H. and K.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Chang Y., et al. , Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266, 1865–1869 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E., Chang Y., Moore P. S., Said J. W., Knowles D. M., Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Soulier J., et al. , Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86, 1276–1280 (1995). [PubMed] [Google Scholar]
- 4.Kedes D. H., Lagunoff M., Renne R., Ganem D., Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J. Clin. Invest. 100, 2606–2610 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Tang Q., Maul G. G., Yuan Y., Kaposi’s sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: Dual role of replication and transcription activator. J. Virol. 80, 12171–12186 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait S. W., Green D. R., Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Wilson N. S., Dixit V., Ashkenazi A., Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 10, 348–355 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Fischer U., Jänicke R. U., Schulze-Osthoff K., Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 10, 76–100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P., et al. , Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Friborg J. Jr., Kong W., Hottiger M. O., Nabel G. J., p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402, 889–894 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Bélanger C., et al. , Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 4, 62–73 (2001). [PubMed] [Google Scholar]
- 12.Coleman C. B., et al. , A gammaherpesvirus Bcl-2 ortholog blocks B cell receptor-mediated apoptosis and promotes the survival of developing B cells in vivo. PLoS Pathog. 10, e1003916 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo T., Park J., Lee D., Hwang S. G., Choe J., Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 75, 6193–6198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., et al. , The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64, 2774–2781 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Wang H. W., Sharp T. V., Koumi A., Koentges G., Boshoff C., Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 21, 2602–2615 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai T., et al. , Genomic organization and mutation analyses of the DR5/TRAIL receptor 2 gene in colorectal carcinomas. Cancer Lett. 133, 197–204 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Wang T. T., Jeng J., Coordinated regulation of two TRAIL-R2/KILLER/DR5 mRNA isoforms by DNA damaging agents, serum and 17beta-estradiol in human breast cancer cells. Breast Cancer Res. Treat. 61, 87–96 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Bertsch U., Röder C., Kalthoff H., Trauzold A., Compartmentalization of TNF-related apoptosis-inducing ligand (TRAIL) death receptor functions: Emerging role of nuclear TRAIL-R2. Cell Death Dis. 5, e1390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi C., et al. , Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akazawa Y., et al. , Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines. Gastroenterology 136, 2365–2376.e1-7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris R. S., Hultquist J. F., Evans D. T., The restriction factors of human immunodeficiency virus. J. Biol. Chem. 287, 40875–40883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigeno M., et al. , Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene 22, 1653–1662 (2003). [DOI] [PubMed] [Google Scholar]
- 23.McBee R. M., Rozmiarek S. A., Meyerson N. R., Rowley P. A., Sawyer S. L., The effect of species representation on the detection of positive selection in primate gene data sets. Mol. Biol. Evol. 32, 1091–1096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perelman P., et al. , A molecular phylogeny of living primates. PLoS Genet. 7, e1001342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosakovsky Pond S. L., Frost S. D., Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 22, 1208–1222 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Wang S., El-Deiry W. S., TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22, 8628–8633 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Walczak H., Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb. Perspect. Biol. 5, a008698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan F. K., Three is better than one: Pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine 37, 101–107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duggal N. K., Emerman M., Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 12, 687–695 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabaev I., Williamson J. C., Crozier T. W. M., Schulz T. F., Lehner P. J., Quantitative proteomics analysis of lytic KSHV infection in human endothelial cells reveals targets of viral immune modulation. Cell Rep. 33, 108249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y., Wang S. E., Hayward G. S., The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity 22, 59–71 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Zhao Q., et al. , Kaposi’s sarcoma-associated herpesvirus-encoded replication and transcription activator impairs innate immunity via ubiquitin-mediated degradation of myeloid differentiation factor 88. J. Virol. 89, 415–427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould F., Harrison S. M., Hewitt E. W., Whitehouse A., Kaposi’s sarcoma-associated herpesvirus RTA promotes degradation of the Hey1 repressor protein through the ubiquitin proteasome pathway. J. Virol. 83, 6727–6738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han C., et al. , KSHV RTA antagonizes SMC5/6 complex-induced viral chromatin compaction by hijacking the ubiquitin-proteasome system. PLoS Pathog. 18, e1010744 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haque M., et al. , Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 82, 1175–1180 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Leithner K., et al. , Nuclear and cytoplasmic death receptor 5 as prognostic factors in patients with non-small cell lung cancer treated with chemotherapy. Lung Cancer 65, 98–104 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Haque M., et al. , Identification and analysis of the K5 gene of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 74, 2867–2875 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brulois K. F., et al. , Construction and manipulation of a new Kaposi’s sarcoma-associated herpesvirus bacterial artificial chromosome clone. J. Virol. 86, 9708–9720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn L. L., et al. , The missing link in Epstein–Barr virus immune evasion: The BDLF3 gene induces ubiquitination and downregulation of major histocompatibility complex class I (MHC-I) and MHC-II. J. Virol. 90, 356–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koshizuka T., et al. , Identification and characterization of the UL56 gene product of herpes simplex virus type 2. J. Virol. 76, 6718–6728 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ushijima Y., Koshizuka T., Goshima F., Kimura H., Nishiyama Y., Herpes simplex virus type 2 UL56 interacts with the ubiquitin ligase Nedd4 and increases its ubiquitination. J. Virol. 82, 5220–5233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ushijima Y., et al. , Herpes simplex virus UL56 interacts with and regulates the Nedd4-family ubiquitin ligase Itch. Virol. J. 7, 179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soh T. K., et al. , Temporal proteomic analysis of herpes simplex virus 1 infection reveals cell-surface remodeling via pUL56-mediated GOPC degradation. Cell Rep. 33, 108235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Said A., Azab W., Damiani A., Osterrieder N., Equine herpesvirus type 4 UL56 and UL49.5 proteins downregulate cell surface major histocompatibility complex class I expression independently of each other. J. Virol. 86, 8059–8071 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everett H., McFadden G., Apoptosis: An innate immune response to virus infection. Trends Microbiol. 7, 160–165 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Walczak H., et al. , TRAIL-R2: A novel apoptosis-mediating receptor for TRAIL. EMBO J. 16, 5386–5397 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X. Z., Tian W. J., Wang J., You J. L., Wang X. J., Death receptor DR5 as a proviral factor for viral entry and replication of coronavirus PEDV. Viruses 14, 2724 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotelkin A., Prikhod’ko E. A., Cohen J. I., Collins P. L., Bukreyev A., Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 77, 9156–9172 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbeuval J. P., et al. , CD4+ T-cell death induced by infectious and noninfectious HIV-1: Role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 106, 3524–3531 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Z., et al. , Hepatitis C virus sensitizes host cells to TRAIL-induced apoptosis by up-regulating DR4 and DR5 via a MEK1-dependent pathway. PLoS ONE 7, e37700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kantari C., Walczak H., Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 1813, 558–563 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Werneburg N. W., et al. , Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2). J. Biol. Chem. 287, 24427–24437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pritchard J. K., Pickrell J. K., Coop G., The genetics of human adaptation: Hard sweeps, soft sweeps, and polygenic adaptation. Curr. Biol. 20, R208–R215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cha S. S., et al. , Crystal structure of TRAIL-DR5 complex identifies a critical role of the unique frame insertion in conferring recognition specificity. J. Biol. Chem. 275, 31171–31177 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Broussard G., Damania B., KSHV: Immune modulation and immunotherapy. Front. Immunol. 10, 3084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li D. J., Verma D., Mosbruger T., Swaminathan S., CTCF and Rad21 act as host cell restriction factors for Kaposi’s sarcoma-associated herpesvirus (KSHV) lytic replication by modulating viral gene transcription. PLoS Pathog. 10, e1003880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin G. C., Kang H. S., Lee A. R., Kim K. H., Hepatitis B virus-triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy 12, 2451–2466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (TXT)
Dataset S02 (XSLX)
Data Availability Statement
All study data are included in the article and/or supporting information.