Abstract
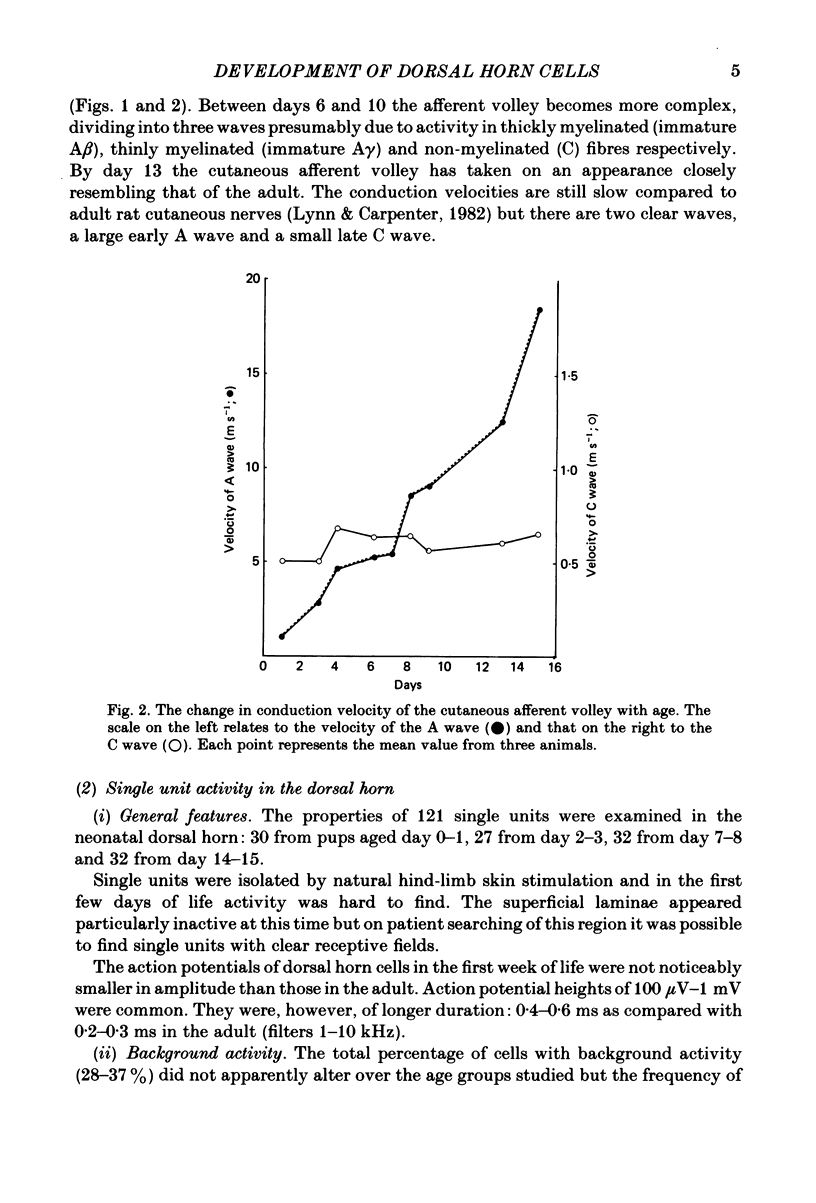
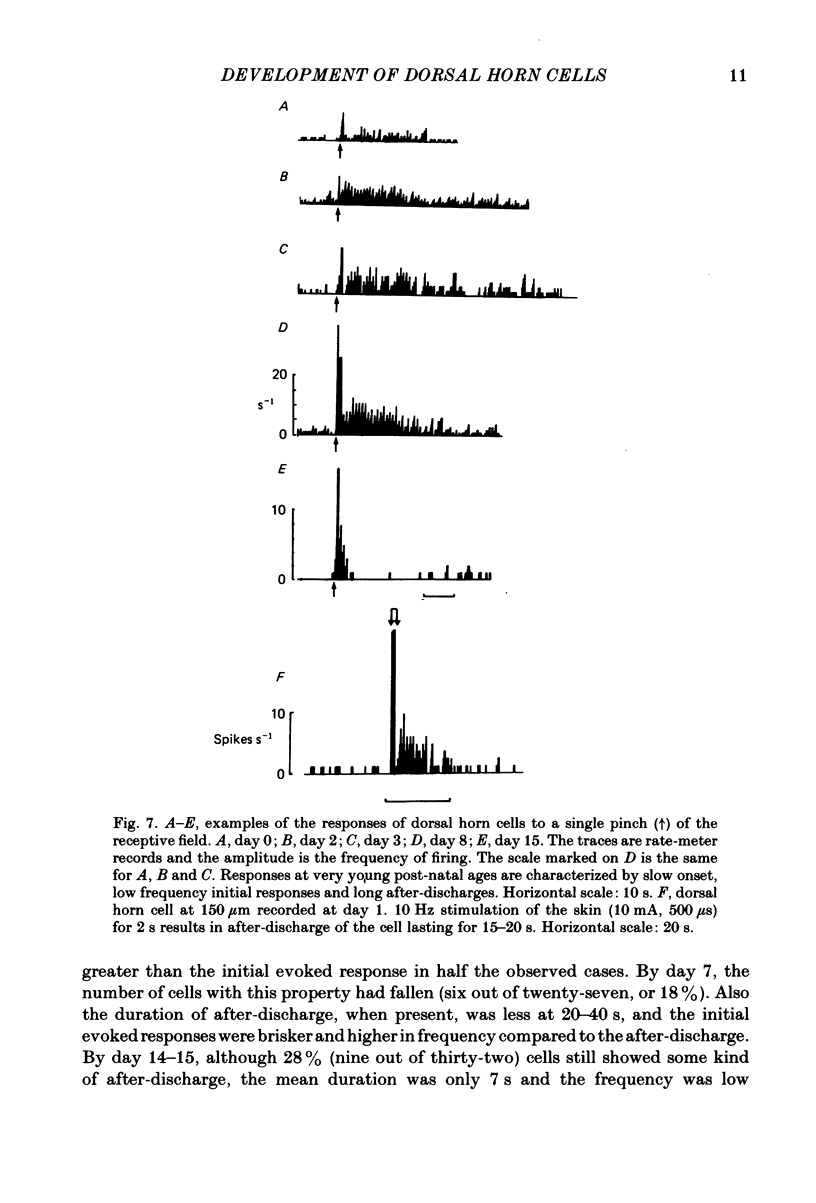
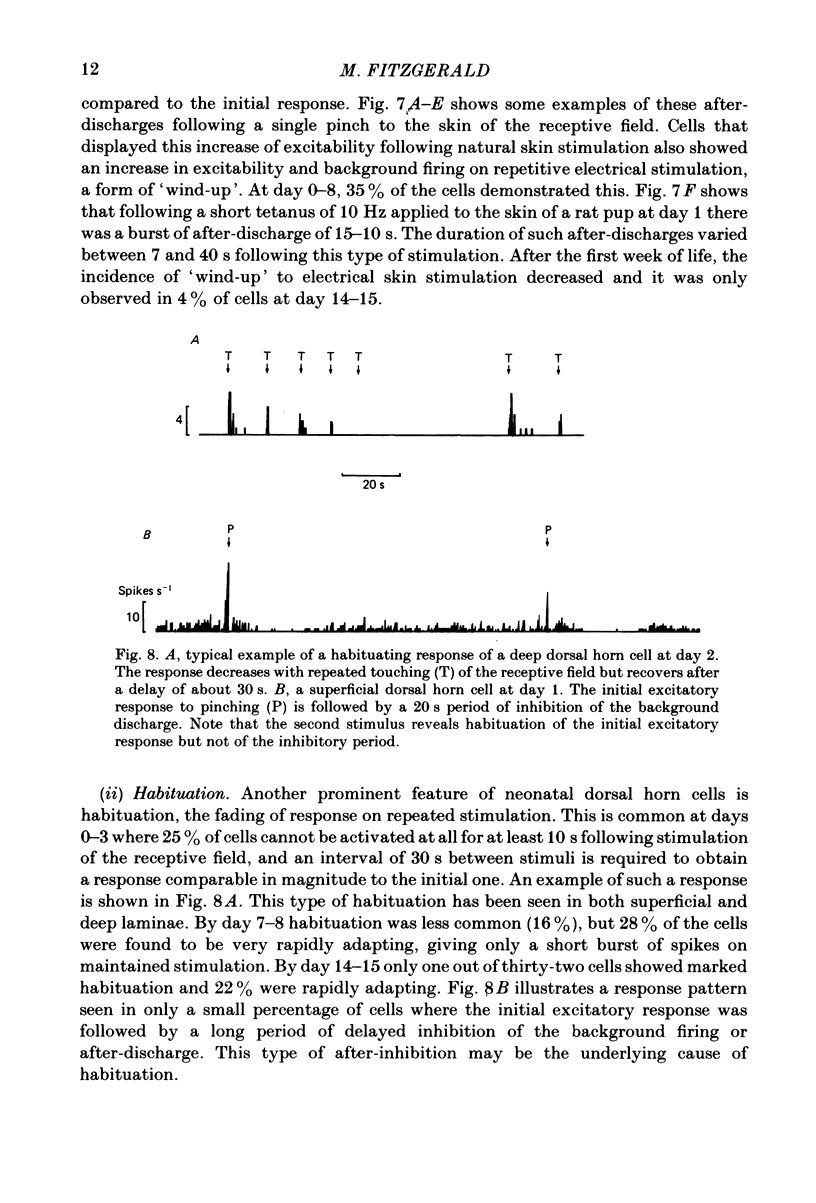
The responses evoked in lumbar dorsal horn cells by both natural and electrical hind-limb skin stimulation were recorded in the spinal cord of rat pups aged 0-15 days under urethane anaesthesia. The input volley was recorded on the L4 dorsal root and consisted of two separate waves from birth. Latency and threshold measurements were consistent with these two waves being immature A (myelinated fibre) waves and C (non-myelinated fibre) waves. On the first 3 days of life background activity of cells in the dorsal horn was low and evoked discharges were sluggish. On electrical stimulation of the skin, neonatal dorsal horn cells frequently responded with only 1 or 2 impulses per input volley with long central delays of up to 20 ms. Synaptic linkage appeared weak and many cells failed to follow stimulation rates of 5 Hz. Natural skin stimulation showed that the majority of cells at days 0-3 responded to pinching the skin only. The development of responses evoked by C fibres in the dorsal horn was delayed compared to that of responses evoked by A fibres. Short and long latency responses corresponding to the early A and late C afferent input volleys could be recorded in the superficial laminae (I, II and III) of the dorsal horn from day 0, but in the deeper laminae only early short latency A responses were evoked until the age of day 7-8. After this time, a long latency C response also appeared and increased in strength with age. Convergence of low and high threshold inputs onto dorsal horn cells was rare at birth but increased gradually over the following two weeks. Receptive field areas, mapped by natural mechanical stimulation of skin, were large at birth and decreased in size with age. At birth the mean receptive field area was 14.2% of the total hind-limb area whereas at day 15 it was 3.6%. This fall in size was particularly marked in cells of the deep dorsal horn. Pinching or brushing the receptive field of many neonatal dorsal horn cells resulted in long-lasting after-discharges (30-90 s) which on days 0-3 could be more pronounced than the initial evoked response. The duration and amplitude of these responses decreased with age. Repetitive electrical skin stimulation of the receptive fields of these cells produced 'wind-up' and prolonged after-discharge. Ipsilateral, contralateral and distant inhibitory components to receptive fields were observed from day 0.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975 Nov;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M. The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex S1. J Physiol. 1975 Apr;246(3):501–538. doi: 10.1113/jphysiol.1975.sp010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell H. R., Jr, Beal J. A. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol. 1984 Jul 10;226(4):508–522. doi: 10.1002/cne.902260406. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce I. C., Tatton W. G. Descending projections to the cervical spinal cord in the developing kitten. Neurosci Lett. 1981 Sep 25;25(3):227–231. doi: 10.1016/0304-3940(81)90396-7. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. J Neurosci. 1981 Jul;1(7):679–684. doi: 10.1523/JNEUROSCI.01-07-00679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm J. Postnatal changes in cutaneous reflexes and in the discharge pattern of cutaneous and articular sense organs. A morphological and physiological study in the cat. Acta Physiol Scand Suppl. 1967;297:1–130. [PubMed] [Google Scholar]
- Fitzgerald M. Alterations in the ipsi- and contralateral afferent inputs of dorsal horn cells produced by capsaicin treatment of one sciatic nerve in the rat. Brain Res. 1982 Sep 23;248(1):97–107. doi: 10.1016/0006-8993(82)91151-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984 Nov;13(3):933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Swett J. The termination pattern of sciatic nerve afferents in the substantia gelatinosa of neonatal rats. Neurosci Lett. 1983 Dec 30;43(2-3):149–154. doi: 10.1016/0304-3940(83)90179-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The contralateral input to the dorsal horn of the spinal cord in the decerebrate spinal rat. Brain Res. 1982 Mar 25;236(2):275–287. doi: 10.1016/0006-8993(82)90714-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Wall P. D. The laminar organization of dorsal horn cells responding to peripheral C fibre stimulation. Exp Brain Res. 1980;41(1):36–44. doi: 10.1007/BF00236677. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968 Oct;27(4):546–570. [PubMed] [Google Scholar]
- Fulton B. P., Miledi R., Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc R Soc Lond B Biol Sci. 1980 Jun 23;208(1170):115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Gilbert M., Stelzner D. J. The development of descending and dorsal root connections in the lumbosacral spinal cord of the postnatal rat. J Comp Neurol. 1979 Apr 15;184(4):821–838. doi: 10.1002/cne.901840413. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hillman P., Wall P. D. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9(4):284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Honig M. G. The development of sensory projection patterns in embryonic chick hind limb. J Physiol. 1982 Sep;330:175–202. doi: 10.1113/jphysiol.1982.sp014336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. I., Hamilton T. C., Hsung J. C., Ulindki P. Gracile nucleus absent in adult opossums after leg removal in infancy. Brain Res. 1972 Mar 24;38(2):421–424. doi: 10.1016/0006-8993(72)90724-x. [DOI] [PubMed] [Google Scholar]
- Kellerth J. O., Mellström A., Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scand. 1971 Sep;83(1):31–41. doi: 10.1111/j.1748-1716.1971.tb05048.x. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Belford G. R. The formation of afferent patterns in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1979 Jan 15;183(2):285–303. doi: 10.1002/cne.901830206. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979 Jun;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Loizou L. A. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res. 1972 May 26;40(2):395–418. doi: 10.1016/0006-8993(72)90142-4. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Mair R. G., Gesteland R. C. Response properties of mitral cells in the olfactory bulb of the neonatal rat. Neuroscience. 1982;7(12):3117–3125. doi: 10.1016/0306-4522(82)90234-2. [DOI] [PubMed] [Google Scholar]
- Mattio T. G., Rosenquist T. H., Kirby M. L. Appearance of acid phosphatase in neonatal rat substantia gelatinosa. Exp Brain Res. 1981;41(3-4):411–413. doi: 10.1007/BF00238899. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. II. INTERACTION AMONG PERIPHERAL INPUTS AND RECURRENT INHIBITION. J Gen Physiol. 1964 May;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan C. H., Fox M. W., Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus). Behaviour. 1971;40(1):100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Preston P. R., Wallis D. I. Dorsal root potentials recorded in the isolated spinal cord of the neonate rat [proceedings]. J Physiol. 1979 Apr;289:84P–84P. [PubMed] [Google Scholar]
- Purpura D. P., Shofer R. J., Scarff T. Properties of synaptic activities and spike potentials of neurons in immature neocortex. J Neurophysiol. 1965 Sep;28(5):925–942. doi: 10.1152/jn.1965.28.5.925. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. J Physiol. 1979 Sep;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Schreyer D. J., Jones E. G. Growth and target finding by axons of the corticospinal tract in prenatal and postnatal rats. Neuroscience. 1982;7(8):1837–1853. doi: 10.1016/0306-4522(82)90001-x. [DOI] [PubMed] [Google Scholar]
- Scott S. A. The development of the segmental pattern of skin sensory innervation in embryonic chick hind limb. J Physiol. 1982 Sep;330:203–220. doi: 10.1113/jphysiol.1982.sp014337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba E., Shiosaka S., Hara Y., Inagaki S., Sakanaka M., Takatsuki K., Kawai Y., Tohyama M. Ontogeny of the peptidergic system in the rat spinal cord: immunohistochemical analysis. J Comp Neurol. 1982 Jun 10;208(1):54–66. doi: 10.1002/cne.902080105. [DOI] [PubMed] [Google Scholar]
- Sima A. Studies on fibre size in developing sciatic nerve and spinal roots in normal, undernourished, and rehabilitated rats. Acta Physiol Scand Suppl. 1974;406:1–55. [PubMed] [Google Scholar]
- Smith C. L. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- Stelzner D. J. The normal postnatal development of synaptic end-feet in the lumbosacral spinal cord and of responses in the hind limbs of the albino rat. Exp Neurol. 1971 Jun;31(3):337–357. doi: 10.1016/0014-4886(71)90237-8. [DOI] [PubMed] [Google Scholar]
- Van der Loos H. Structural changes in the cerebral cortex upon modification of the periphery: barrels in somatosensory cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):373–376. doi: 10.1098/rstb.1977.0049. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Grieshaber J. A. A morphological investigation of an early reflex pathway in developing rat spinal cord. J Comp Neurol. 1973 Mar 15;148(2):177–209. doi: 10.1002/cne.901480205. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Fitzgerald M., Nussbaumer J. C., Van der Loos H., Devor M. Somatotopic maps are disorganized in adult rodents treated neonatally with capsaicin. Nature. 1982 Feb 25;295(5851):691–693. doi: 10.1038/295691a0. [DOI] [PubMed] [Google Scholar]
- Weber E. D., Stelzner D. J. Behavioral effects of spinal cord transection in the developing rat. Brain Res. 1977 Apr 15;125(2):241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983 Dec 10;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]


