Abstract
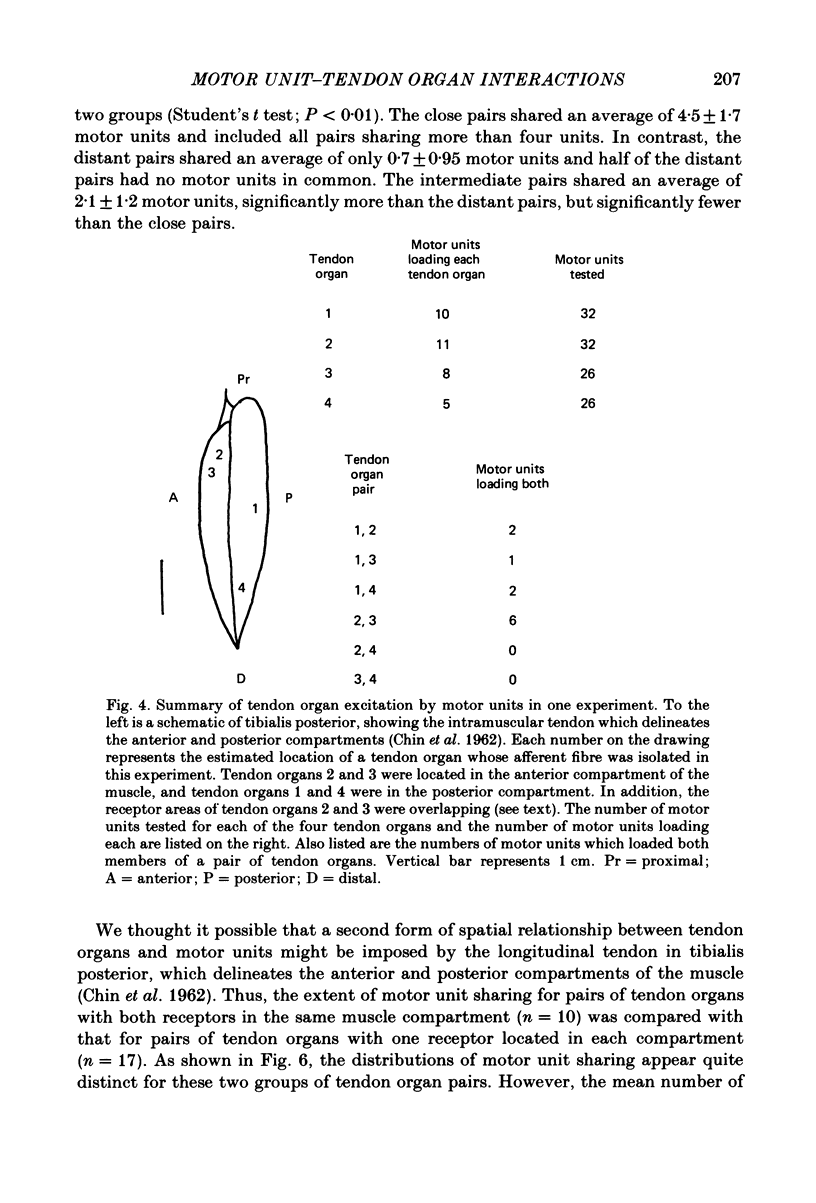
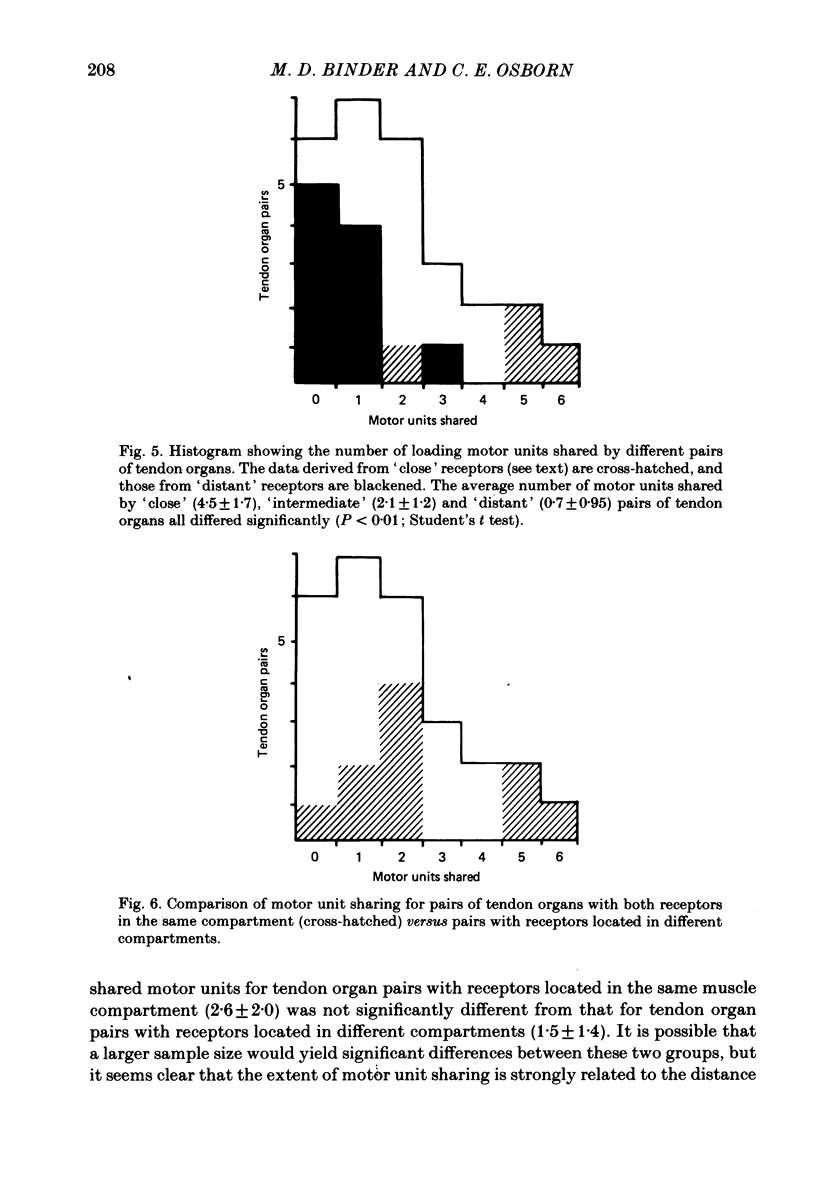
The responses of Golgi tendon organs to single motor unit contractions were studied to determine whether receptors located in the same muscle region respond to a common set of motor units. In each of five experiments we isolated a large fraction (25-65%) of the motor units of the cat tibialis posterior muscle and determined to which of the units each of several tendon organs was responsive. Each tendon organ was excited by from two to fifteen of the isolated motor units, including units which produced very small forces. However, there was a much greater probability for large force units to excite a given receptor than for small force units to do so. The number of motor units which produced either an 'unloading' or an 'off response' exceeded, on average, the number of motor units which excited the same tendon organ. The extent to which single motor units excited both of a pair of tendon organs was examined statistically in relation to the mutual proximity of the receptors within the muscle. It was found, on average, that the closer were two receptors, the greater was the number of motor units that excited both of them. These results suggest that despite the extensive territories of individual motor units, the spike trains of tendon organs may still encode information about localized muscle activity.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder M. D. Further evidence that the Golgi tendon organ monitors the activity of a discrete set of motor units within a muscle. Exp Brain Res. 1981;43(2):186–192. doi: 10.1007/BF00237762. [DOI] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stuart D. G. The response of Golgi tendon organs to single motor unit contractions. J Physiol. 1977 Oct;271(2):337–349. doi: 10.1113/jphysiol.1977.sp012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Stuart D. G. Responses of Ia and spindle group II afferents to single motor-unit contractions. J Neurophysiol. 1980 Mar;43(3):621–629. doi: 10.1152/jn.1980.43.3.621. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman C. F. Comparisons in structure of tendon organs in the rat, cat and man. J Comp Neurol. 1970 Mar;138(3):369–372. doi: 10.1002/cne.901380308. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. "Sensory partitioning" of cat medial gastrocnemius muscle by its muscle spindles and tendon organs. J Neurophysiol. 1981 Jul;46(1):32–47. doi: 10.1152/jn.1981.46.1.32. [DOI] [PubMed] [Google Scholar]
- Crago P. E., Houk J. C., Rymer W. Z. Sampling of total muscle force by tendon organs. J Neurophysiol. 1982 Jun;47(6):1069–1083. doi: 10.1152/jn.1982.47.6.1069. [DOI] [PubMed] [Google Scholar]
- Fukami Y. Responses of isolated Golgi tendon organs of the cat to muscle contraction and electrical stimulation. J Physiol. 1981 Sep;318:429–443. doi: 10.1113/jphysiol.1981.sp013876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami Y., Wilkinson R. S. Responses of isolated Golgi tendon organs of the cat. J Physiol. 1977 Mar;265(3):673–689. doi: 10.1113/jphysiol.1977.sp011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Proske U. The responses of Golgi tendon organs to stimulation of different combinations of motor units. J Physiol. 1979 Oct;295:251–262. doi: 10.1113/jphysiol.1979.sp012966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Jami L., Petit J. Frequency of tendon organ discharges elicited by the contraction of motor units in cat leg muscles. J Physiol. 1976 Oct;261(3):633–645. doi: 10.1113/jphysiol.1976.sp011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Petit J. Heterogeneity of motor units activating single Golgi tendon organs in cat leg muscles. Exp Brain Res. 1976 Mar 15;24(5):485–493. doi: 10.1007/BF00234965. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Binder M. D. Topographic factors in distribution of homonymous group Ia-afferent input to cat medial gastrocnemius motoneurons. J Neurophysiol. 1984 Jan;51(1):50–63. doi: 10.1152/jn.1984.51.1.50. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Cope T. C., Binder M. D. Analysis of individual Ia-afferent EPSPs in a homonymous motoneuron pool with respect to muscle topography. J Neurophysiol. 1984 Jan;51(1):64–74. doi: 10.1152/jn.1984.51.1.64. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. Tetrapartite classification of motor units of cat tibialis posterior. J Neurophysiol. 1980 Oct;44(4):696–712. doi: 10.1152/jn.1980.44.4.696. [DOI] [PubMed] [Google Scholar]
- Meyer-Lohmann J., Riebold W., Robrecht D. Mechanical influence of the extrafusal muscle on the static behaviour of deefferented primary muscle spindle endings in cat. Pflugers Arch. 1974;352(3):267–278. doi: 10.1007/BF00590491. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stephens J. A., Stuart D. G. The tendon organs of cat medial gastrocnemius: significance of motor unit type and size for the activation of Ib afferents. J Physiol. 1975 Sep;250(3):491–512. doi: 10.1113/jphysiol.1975.sp011067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. K., Stephens J. A. The tendon organs of cat soleus: static sensitivity to active force. Exp Brain Res. 1975 Sep 29;23(3):279–291. doi: 10.1007/BF00239740. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Reinking R. M., Stuart D. G. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975 Sep;38(5):1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Goslow G. E., Mosher C. G., Reinking R. M. Stretch responsiveness of Golgi tendon organs. Exp Brain Res. 1970 Jun 25;10(5):463–476. doi: 10.1007/BF00234263. [DOI] [PubMed] [Google Scholar]
- Sturart D. G., Mosher C. G., Gerlach R. I., Reinking R. M. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–292. doi: 10.1007/BF00816163. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Considerations on mechanisms of focussed signal transmission in the multi-channel muscle stretch reflex system. Biol Cybern. 1978 Nov 24;31(2):81–90. doi: 10.1007/BF00344238. [DOI] [PubMed] [Google Scholar]
- Windhorst U., Meyer-Lohmann J. The influence of extrafusal muscle activity on discharge patterns of primary muscle spindle endings. Pflugers Arch. 1977 Dec 12;372(2):131–138. doi: 10.1007/BF00585326. [DOI] [PubMed] [Google Scholar]
- Zelená J., Soukup T. The development of Golgi tendon organs. J Neurocytol. 1977 Apr;6(2):171–194. doi: 10.1007/BF01261504. [DOI] [PubMed] [Google Scholar]



