Abstract
Cancer stem cells (CSCs), are a critical subpopulation within tumours, and are defined by their capacity for self-renewal, differentiation, and tumour initiation. These unique traits contribute to tumour progression, metastasis, and resistance to conventional treatments like chemotherapy and radiotherapy, often resulting in cancer recurrence and poor patient outcomes. As such, CSCs have become focal points in developing advanced cancer therapies. This review highlights progress in CSC-targeted treatments, including chimeric antigen receptor T-cell (CAR-T) therapy, immunotherapy, molecular targeting, and nanoparticle-based drug delivery systems. Plant-derived compounds and gene-editing technologies, such as clustered regularly interspaced short palindromic repeats (CRISPR), are explored for their potential to enhance precision and minimize side effects. Metabolic pathways integral to CSC survival, such as mitochondrial dynamics, mitophagy (regulated by dynamin-related protein 1 [DRP1] and the PINK1/Parkin pathway), one-carbon metabolism, amino acid metabolism (involving enzymes like glutaminase (GLS) and glutamate dehydrogenase (GDH]), lipid metabolism, and hypoxia-induced metabolic reprogramming mediated by hypoxia-inducible factors (HIF-1α and HIF-2α), are examined as therapeutic targets. The adaptability of CSCs through autophagy, metabolic flexibility, and epigenetic regulation by metabolites like α-ketoglutarate, succinate, and fumarate is discussed. Additionally, extracellular vesicles and nicotinamide adenine dinucleotide (NAD⁺) metabolism are identified as pivotal in redox balance, DNA repair, and epigenetic modifications. Addressing challenges such as tumour heterogeneity, immune evasion, and treatment durability requires interdisciplinary collaboration. Advancing CSC-targeted therapies is essential for overcoming drug resistance and preventing cancer relapse, paving the way for transformative cancer treatments. This review underscores the importance of leveraging innovative technologies and fostering collaboration to revolutionize cancer treatment.
Graphical Abstract
Keywords: Cancer stem cells (CSCs), Tumour initiation and progression, Chemotherapeutic drug resistance, CAR T-cell therapy, Nanoparticular drug delivery, Gene editing methods
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, representing a significant public health challenge and a critical area of biomedical research. The complexity of cancer lies not only in its genetic and molecular heterogeneity but also in the dynamic interactions between tumour cells and their surrounding microenvironment [1–3]. This complexity underpins disease progression, therapy resistance, and ultimately, patient outcomes. Among the various factors driving cancer’s multifaceted nature, cancer stem cells (CSCs) have emerged as pivotal players, reshaping our understanding of tumorigenesis and therapeutic resistance [4]. CSCs represent a subpopulation of tumour cells characterized by their unique ability to self-renew, differentiate into diverse cell types, and resist conventional cancer treatments such as chemotherapy and radiotherapy [5–7]. These cells exhibit properties reminiscent of normal stem cells but are distinguished by their malignant potential. Their self-renewal capability allows them to sustain tumour growth, while their differentiation potential enables them to generate the heterogeneous cell populations that constitute the bulk of a tumour. Notably, CSCs are implicated in metastasis, the primary cause of cancer-related deaths, and are thought to drive tumour recurrence even after aggressive treatment regimens [7, 8]. These attributes make CSCs a critical target for therapeutic intervention, as their eradication could significantly improve treatment outcomes and reduce the likelihood of relapse.
The identification of CSCs has revolutionized cancer biology. Traditionally, cancer was viewed as a homogeneous disease, and therapies were designed to target the rapidly proliferating bulk of tumour cells [9]. However, this approach often proved inadequate, as tumours would frequently recur, sometimes more aggressively, following treatment. This recurrence is now understood to result from the persistence of CSCs, which are often resistant to standard therapies [10]. Unlike bulk tumour cells, CSCs can survive chemotherapy and radiotherapy due to their ability to enter a quiescent state, activate robust DNA repair mechanisms, and exploit protective niches within the tumour microenvironment (TME) [11–13]. Consequently, the classical model of cancer treatment has shifted to recognize the need for therapies that specifically target CSCs alongside bulk tumour cells.
Despite the promise of CSC-targeted therapies, significant challenges remain. One major obstacle is the plasticity of CSCs, which allows them to dynamically transition between stem-like and non-stem-like states in response to environmental cues and therapeutic pressures [14, 15]. This plasticity complicates efforts to define and target CSC populations consistently. Moreover, CSCs are adept at evading immune responses through mechanisms such as the upregulation of immune checkpoint molecules and the secretion of immunosuppressive factors. They also exhibit remarkable metabolic and epigenetic adaptability, enabling them to survive under diverse and often hostile conditions within the TME [15].
The TME plays a crucial role in maintaining CSC stemness and promoting their survival [16]. This dynamic and complex milieu comprises various stromal cells, immune cells, extracellular matrix components, and soluble factors that collectively create a protective niche for CSCs. For instance, fibroblasts and immune cells within the TME secrete cytokines and growth factors that reinforce CSC properties and support tumour progression [17]. Hypoxia, a hallmark of the TME, further enhances CSC stemness and therapy resistance by activating pathways such as epithelial-mesenchymal transition (EMT) and hypoxia-inducible factors (HIFs) [18, 19]. These insights into the role of the TME have spurred efforts to develop therapies that disrupt the interactions between CSCs and their microenvironment, thereby weakening CSCs’ defenses.
One promising avenue of research involves targeting the signaling pathways critical for CSC maintenance and function. Key pathways, including Wnt/β-catenin, Notch, Hedgehog, and PI3K/AKT/mTOR, are often dysregulated in CSCs and contribute to their self-renewal, survival, and therapy resistance [20]. Drugs and natural compounds that inhibit these pathways are under investigation, with some showing encouraging preclinical and clinical results. For example, inhibitors of the Hedgehog pathway, such as vismodegib, have demonstrated efficacy in targeting CSCs in basal cell carcinoma and medulloblastoma. Similarly, Wnt pathway inhibitors are being explored for their potential to disrupt CSC-mediated tumour growth and metastasis [21–23].
Recent innovations in CSC-targeted therapies include advanced molecular approaches such as gene editing, immunotherapy, and nanotechnology-based drug delivery systems [24–26]. The advent of gene-editing tools like CRISPR/Cas9 has enabled precise modifications of genes critical for CSC survival and therapy resistance. By selectively knocking out genes involved in CSC maintenance, researchers can potentially eliminate these cells while sparing normal tissues [27, 28]. Immunotherapy has also shown promise in targeting CSCs. For instance, chimeric antigen receptor (CAR) T-cell therapy, originally developed for hematologic malignancies, is being adapted to target CSC-specific surface antigens [29, 30]. Additionally, immune checkpoint inhibitors, which have revolutionized cancer therapy, are being evaluated for their ability to enhance immune responses against CSCs [31–33]. Nanotechnology offers another innovative approach to CSC-targeted therapy. Nanoparticles can be engineered to deliver drugs specifically to CSCs, minimizing off-target effects and reducing systemic toxicity. For example, nanoparticles loaded with chemotherapeutic agents or siRNA molecules can be functionalized with ligands that bind to CSC-specific markers, ensuring precise delivery [34]. Furthermore, nanoparticles can be designed to release their payload in response to stimuli such as pH changes or enzymatic activity within the TME, enhancing their therapeutic efficacy [35]. In addition to these molecular and technological approaches, natural compounds and phytochemicals are being explored for their potential to modulate CSC pathways [36, 37]. Compounds such as curcumin, resveratrol, and sulforaphane have demonstrated the ability to inhibit CSC proliferation and induce differentiation, making them attractive candidates for combination therapies. These natural agents often exhibit lower toxicity compared to synthetic drugs, making them suitable for long-term use in cancer prevention and management.
Despite these advances, the development of effective CSC-targeted therapies faces several challenges. Tumour heterogeneity remains a significant hurdle, as CSCs within a single tumour can exhibit diverse phenotypes and molecular profiles [38, 39]. This heterogeneity complicates the identification of universal CSC markers and necessitates the development of personalized therapeutic strategies. Moreover, the ability of CSCs to adapt and evolve under therapeutic pressure underscores the need for combination therapies that target multiple pathways and mechanisms simultaneously.
The integration of CSC-targeted therapies with conventional treatments holds significant promise. By addressing both bulk tumour cells and CSCs, combination therapies could achieve more durable responses and reduce the likelihood of relapse. For instance, combining chemotherapy with CSC-targeting agents could eliminate both the rapidly dividing tumour cells and the therapy-resistant CSCs. Similarly, integrating CSC-targeted immunotherapies with immune checkpoint inhibitors could enhance anti-tumour immune responses and overcome immune evasion mechanisms [40]. To advance the field of CSC research and therapy development, a comprehensive understanding of CSC biology and the TME is essential. This includes elucidating the molecular mechanisms underlying CSC plasticity, identifying novel CSC-specific biomarkers, and exploring the interplay between CSCs and immune cells. Additionally, robust preclinical models that accurately recapitulate human tumours are needed to evaluate the efficacy and safety of CSC-targeted therapies before clinical translation.
This review aims to provide a detailed analysis of emerging approaches to CSC-targeted therapy. It explores innovative strategies that address the TME, key signaling pathways, and CSC-specific surface biomarkers. Furthermore, it examines novel stem cell treatments and advanced drug delivery systems that hold promise for improving therapeutic outcomes. While the challenges of targeting CSCs are substantial, the potential rewards—in terms of reducing cancer recurrence and improving patient survival—make this an area of immense scientific and clinical importance. By synthesizing current knowledge and identifying gaps, this review seeks to contribute to the ongoing efforts to develop effective and personalized cancer treatments.
Search method
This review employs databases such as PubMed, Google Scholar, Scopus, Web of Science, ClinicalTrials.gov, and Embase to retrieve biomedical literature, research papers, and resources. The search terms encompass cancer stem cells, Cancer Stem cells (CSC) treatment, cancer stem cell markers, immunotherapy, resistance mechanisms, and clinical trials. Advanced search phrases encompass "cancer stem cells" and "drug resistance," or "tumour recurrence" and "therapeutic innovation," or "novel therapy.
Current innovations in cancer stem cell (CSC) therapy
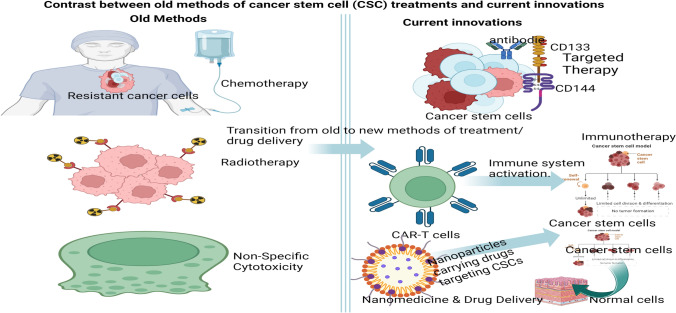
Cancer stem cells (CSCs) have emerged as pivotal players in the development, progression, and recurrence of various cancers [5, 41, 42]. CSCs represent a unique and critical population within tumours, making them prime targets for novel therapies designed to eliminate cancer at its root. These cells have a remarkable capacity for self-renewal and are the primary drivers of tumour growth and metastasis, which highlights their pivotal role in cancer progression and recurrence. A significant challenge in cancer treatment is that CSCs often exhibit resistance to conventional therapies, contributing to relapse and metastasis [42]. This has led to an increasing focus on developing therapies that specifically target CSCs. Current advancements in CSC therapy involve several strategies, including gene editing technologies, targeted drug delivery, immunotherapy, small molecule inhibitors, natural agents, and metabolism-based approaches [41]. These methods are being explored to eradicate CSCs and improve clinical outcomes for cancer patients. Key developments include the creation of targeted nano-therapeutic platforms that interact with CSC micro-niches, promoting CSC differentiation and enhancing antitumour efficacy. Additionally, the combination of immunotherapy with epigenetic compounds shows promise in selectively targeting CSCs and overcoming therapeutic resistance, paving the way for precision medicine in cancer treatment [43]. These advancements highlight the necessity of a multi-faceted approach to effectively combat CSCs and revolutionize cancer therapy. These innovations are represented in Fig. 1, indicating a contrast between old and current innovations in cancer stem cells treatment.
Fig. 1.
The contrast between old methods of cancer stem cell (CSC) treatments and current innovations (Created in BioRender.com)
Cancer stem cells (CSCs) often exhibit resistance to conventional therapies due to their unique biological properties, including enhanced DNA repair mechanisms, overexpression of drug efflux pumps, and a quiescent cell cycle state [44, 45]. Unlike the bulk of tumour cells, CSCs can remain in a slow-dividing or dormant state, rendering them less susceptible to treatments like chemotherapy and radiotherapy, which typically target rapidly dividing cells [46, 47]. Additionally, CSCs have been shown to overexpress ATP-binding cassette (ABC) transporters, such as ABCG2, which actively pump chemotherapeutic agents out of the cells, reducing intracellular drug concentrations and diminishing therapeutic efficacy [48].
Another critical factor contributing to CSC resistance is their robust ability to activate key signaling pathways involved in survival and self-renewal, such as Wnt/β-catenin, Notch, and Hedgehog pathways [49]. These pathways not only promote the maintenance of the CSC phenotype but also enhance resistance to apoptosis and oxidative stress induced by treatments. Furthermore, the tumour microenvironment, including interactions with stromal cells, hypoxia, and immune evasion strategies, plays a pivotal role in protecting CSCs from therapy-induced damage. Understanding these mechanisms is crucial for developing targeted approaches that can effectively eliminate CSCs and improve therapeutic outcomes.
Signalling pathways involved in cancer stem cells maintenance
Numerous signalling pathways that are crucial for the survival, growth, self-renewal, and differentiation of normal stem cells become dysregulated during tumorigenesis and in cancer stem cells (CSCs). These pathways can be either excessively activated or suppressed. A variety of endogenous and exogenous genes, along with microRNAs, play a significant role in regulating these intricate pathways. Furthermore, these signalling mechanisms can lead to the expression of downstream genes, including those related to cytokines, growth factors, apoptosis, anti-apoptotic functions, proliferation, and metastasis in CSCs [50, 51]. These signalling pathways do not operate independently; rather, they form complex networks of signalling mediators that collectively influence CSC growth. Therefore, this section will explore how these signalling pathways are interconnected in contributing to the regulation of CSC growth.
The Wnt signaling pathway plays a pivotal role in CSCs through the interaction of Wnt ligands with their receptors. The Wnt family consists of nineteen glycoproteins that function as ligands [52]. These ligands bind to one of the ten Frizzled (Fz) receptors, which are classified as seven-transmembrane G-protein coupled receptors (GPCRs). There are two primary Wnt pathways: Canonical Pathway: This pathway relies on the low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptor and the Non-Canonical Pathway: This β-catenin-independent pathway involves co-receptors such as Receptor Tyrosine Kinase-Like Orphan Receptor 1 (ROR1) Receptor Tyrosine Kinase-Like Orphan Receptor 2 (ROR2), and Related to Tyrosine Kinase (RYK) [52, 53]. Both pathways are crucial for regulating various cellular functions in CSCs, including self-renewal and differentiation. Dysregulation of these pathways can lead to enhanced tumorigenesis and metastasis, highlighting their importance in cancer biology. Wnt signalling pathway can be divided into two distinct branches: The Planar Cell Polarity (Wnt/PCP) pathway and the Wnt/Ca2 + pathway [54, 55]. A key feature of the canonical Wnt pathway is the involvement of β-catenin, which, upon entering the nucleus, influences gene expression by forming complexes with transcription factors such as T-cell/lymphoid enhancer factor (TCF/Lef) through the recruitment of cAMP Response Element-Binding Protein Binding Protein (CBP) [54]. In scenarios where Wnt ligands do not activate their receptors, β-catenin is directed towards a degradation complex that includes the tumour suppressor Adenomatous Polyposis Coli (APC), Axis Inhibitor 1/2 (AXIN1/2), and kinases CK1α/GSK-3β [56].Tankyrase (TNKS) is an enzyme that facilitates the ubiquitination and subsequent degradation of AXIN1/2; notably, inhibitors of tankyrase can enhance the function of the β-catenin degradation complex, making it a potential target for therapeutic interventions [57, 58]. Under typical physiological conditions, the Wnt pathway is crucial for embryonic development and cell cycle regulation, where β-catenin supports the differentiation and maturation of specific T-cells, dendritic cells, and various tissue systems [54, 58]. Cancer stem cells (CSCs) often exploit the Wnt signaling pathway, leading to unregulated growth of cancer cells. Over time, mutations in tumour suppressor genes and oncogenes in specific cancer subtypes result in persistent activation of Wnt signaling. The numerous complexes formed between Wnt ligands and receptors initiate various intricate cascades that significantly influence multiple cancer types. For instance, activation of the Wnt pathway is associated with decreased survival rates in more than 50% of breast cancer patients, affects gastrointestinal cancers, and the interplay between canonical and non-canonical Wnt signalling has been linked to the progression of melanoma [59]. Despite the potential of targeting these pathways, many current therapeutic strategies focused on Wnt signaling are stalled at preclinical or early clinical trial phases [51, 60]. Nevertheless, continued research into combined therapeutic approaches that target Wnt signaling alongside other treatments could yield critical insights into the complexities of CSCs.
In the tumour microenvironment (TME), Wnt proteins, along with inhibitors such as bone morphogenetic protein (BMP) and Delta, are produced, which activate the self-renewal capabilities of cancer stem cells (CSCs) [61]. Proto-oncogenes like c-Myc, in conjunction with the subsequent upregulation of Cyclin D1, have been shown to facilitate the transition of dormant CSCs into active CSCs through the activation of the Wnt pathway in colorectal cancer. The Wnt pathway is particularly associated with CD44 + /CD133 + isolated colorectal CSCs [50, 62]. Beyond colorectal cancer, canonical Wnt signalling also plays a crucial role in sustaining various types of CSCs.
The Notch signalling pathway is essential not only for embryonic development but also for the regulation of CSC proliferation, maintenance, and differentiation, with a significant role in angiogenesis [63, 64]. Traditionally, Notch functions as both a tumour suppressor and an oncogene, influencing tumorigenesis based on the tissue type or specific genetic mutations. The expression of Notch can vary depending on the microenvironment; it is often downregulated in cancers such as prostate, skin, lung, liver, and certain breast cancers, while it tends to be upregulated in gastric, colon, pancreatic, and some breast cancers [63, 65]. Under hypoxic conditions, the Notch pathway can undergo epithelial-mesenchymal transition (EMT), leading to an upregulation of Notch signalling in CSCs. This suggests that CSCs typically enhance the Notch pathway to support their survival and proliferation.
The Notch signalling pathway is crucial for enhancing the stemness properties of CSCs. This pathway is activated when Delta/Serrate/Lag2 (DSL) transmembrane ligands, such as Delta-like 1, 3, and 4 or Jagged 1 and 2, bind to Notch receptors (Notch1–4) in a juxtacrine manner [66]. This interaction triggers proteolytic cleavage by the ADAM-10 protease, followed by further cleavage by gamma-secretase (γ-secretase), which releases the intracellular domain of Notch from the plasma membrane. The resulting Notch intracellular domain (NICD) then translocate to the nucleus, where it binds to the CBF1/Suppressor of Hairless/Lag-1 (CSL) transcription factor, activating target genes [67].Given that activation of the Notch signalling pathway in CSCs can influence their replication, survival, and differentiation, targeting this pathway may provide a means to regulate CSC renewal and mitigate CSC-driven tumour formation and recurrence.
The Hedgehog (Hh) signalling pathway consists of three homologs: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh), with Shh being the most extensively studied [68]. Under typical circumstances, this pathway is vital for embryonic development and cellular differentiation. However, abnormal activation of the Hh pathway has been associated with various cancer types, and cancer stem cells (CSCs) exhibit elevated levels of Hh activation [52]. When extracellular Hh ligands bind to the twelve-pass transmembrane receptor Patched (PTCH), this interaction initiates a signalling cascade that primarily activates downstream GLI transcription factors, including Gli1, Gli2, and Gli3. The aberrant activation of the Hh pathway has been implicated in the proliferation and maintenance of CSCs across multiple cancers, highlighting its importance in tumour biology [69, 70]. In the absence of ligand-receptor activation, patched (PTCH) functions as a negative regulator, inhibiting the seven-pass transmembrane G-protein coupled receptor Smoothened (SMO) and thereby preventing signal transduction. Likewise, in the absence of a ligand, the suppressor of fused (SUFU) protein binds to GLI transcription factors, anchoring them in the cytoplasm and blocking their ability to activate GLI target genes, which effectively inhibits Hedgehog (Hh) signalling [60, 68, 71]. Targeted inhibition of Hh signalling has emerged as an effective strategy for impeding cancer progression and curbing the metastasis of cancer stem cells (CSCs).
Several other signaling pathways, such as NF-κB, JAK/STAT, PI3K/AKT, and PPAR, play pivotal roles in regulating CSC metabolism, influencing their survival and function. NF-κB is a key regulator of inflammation, immune response, and cell survival, and its dysregulation is frequently observed in various cancers [72]. It enhances metabolic plasticity in CSCs, enabling a switch between glycolysis and oxidative phosphorylation (OXPHOS) based on environmental cues. NF-κB drives the expression of glycolytic enzymes like hexokinase-2 (HK2) and pyruvate kinase M2 (PKM2), promoting a Warburg-like phenotype in CSCs [72].
JAK/STAT is crucial in controlling cell proliferation, differentiation, and immune evasion in cancer cells, including CSCs [8]. It upregulates glucose transporter 1 (GLUT1) and key glycolytic enzymes, supporting enhanced glycolytic flux in CSCs [73]. It also contributes to mitochondrial biogenesis and OXPHOS regulation by increasing the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α). PI3K/AKT is a central regulator of growth and survival in cancer cells, including CSCs, and plays a critical role in metabolic reprogramming. It boosts glycolytic flux by increasing the expression of GLUTs and glycolytic enzymes, promotes lipid synthesis, and regulates fatty acid oxidation (FAO) to meet CSC energy demands. PPARs are nuclear receptors that regulate lipid metabolism, glucose homeostasis, and inflammation [73]. They are implicated in CSC metabolic adaptation, providing an energy source for CSCs in nutrient-scarce environments, promoting glucose uptake and lipid storage, maintaining stemness, and modulating the interaction between CSCs and their niche. In sum, targeting glycolysis, inhibitors of key glycolytic enzymes (e.g., HK2 inhibitors) may selectively target CSCs reliant on glycolysis. For the inhibition of lipid metabolism, drugs targeting SREBPs or CPT1 can disrupt lipid metabolism, starving CSCs of essential resources. While in modulating Pathway Activity, Specific inhibitors of NF-κB (e.g., IκB kinase inhibitors), JAK/STAT (e.g., ruxolitinib), PI3K/AKT (e.g., LY294002), and PPAR pathways (e.g., PPARγ antagonists) have shown promise in preclinical models.
Emerging pathways in cancer stem cells metabolism
The emerging pathways in Cancer Stem Cell (CSC) metabolism include mitochondrial dynamics and mitophagy, one-carbon metabolism, amino acid metabolism, lipid metabolism, hypoxia-induced metabolic reprogramming, autophagy and metabolic flexibility, metabolism signaling and epigenetic regulation, extracellular vesicles and metabolic crosstalk, and NAD + metabolism [74–77].
Mitochondrial dynamics and mitophagy are key regulators of CSCs, with key regulators being Dynamin-Related Protein (DRP1) and the PINK1/Parkin pathway. Inhibitors of these pathways can selectively impair CSC survival. One-carbon metabolism supports nucleotide biosynthesis, methylation reactions, and redox balance, all of which are critical for CSC proliferation and survival. Glutamine metabolism is essential for energy production, membrane biosynthesis, and signaling, with key enzymes such as GLS and GDH playing a role. Inhibitors of GLS and targeting cystine uptake show potential. Lipid metabolism is crucial for energy production, membrane biosynthesis, and signaling, with pathways like Fatty Acid Oxidation (FAO) providing ATP and maintaining redox balance [78].
Hypoxia-induced metabolic reprogramming involves hypoxic tumour microenvironments causing Hypoxia-Inducible Factor-1 Alpha (HIF-1α) and Hypoxia-Inducible Factor-2 Alpha (HIF-2α) to reprogram CSC metabolism towards glycolysis and alter lipid and amino acid metabolism [79, 80]. Autophagy and metabolic flexibility can be reduced by autophagy inhibitors. Metabolites like α-ketoglutarate, succinate, and fumarate act as signaling molecules and epigenetic modulators, with key enzymes like isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) mutations leading to the production of 2-hydroxyglutarate. Extracellular vesicles and metabolic crosstalk involve CSCs releasing exosomes containing metabolites, enzymes, and lipids that reprogram the tumour microenvironment. Inhibitors of exosome production can disrupt CSC communication with the tumour microenvironment [81–83]. NAD + metabolism is crucial for redox reactions, DNA repair, and epigenetic modifications, with pathways like Nicotinamide Phosphoribosyltransferase (NAMPT) synthesis mediating this process [84–86]. These emerging pathways highlight the metabolic versatility of CSCs and their ability to adapt to environmental challenges. Targeting these pathways, either alone or in combination with conventional therapies, offers new opportunities to eliminate CSCs and improve cancer treatment outcomes. Further research into the metabolic dependencies of CSCs will be crucial for developing innovative therapies.
Tumour micro-environment and cancer stem cells dynamics
Cancer stem cells (CSCs) are integral to tumour proliferation, recurrence, and metastasis, with their destiny predominantly influenced by the tumour microenvironment (TME). The TME serves as a specialized home for cancer stem cells CSCs, facilitating their self-renewal, dormancy, and reactivation, while also offering protection against immune responses and therapeutic interventions [50, 65]. CSCs aggressively construct and change the TME, enlisting and converting normal cells to establish an immunosuppressive habitat that facilitates tumour development. The CSC niche encompasses several facets of cancer progression, including initial tumour proliferation, metastasis establishment, and the epithelial-mesenchymal transition [61, 87]. Considering the essential function of the TME in sustaining CSCs and promoting tumour advancement, targeting this niche has surfaced as a viable therapeutic approach to address cancer and surmount drug resistance.
The TME refers to the cellular surroundings in which tumour cells exist, encompassing both cellular and non-cellular elements. This environment includes various stromal cells such as fibroblasts, lymphocytes, macrophages, and endothelial cells, along with immune cells like T and B lymphocytes. Additionally, it contains extracellular components such as cytokines, growth factors, hormones, and the extracellular matrix, all of which are supported by blood vessels that nourish the tumour [51, 65, 70]. The TME creates a conducive environment for cancer cells to proliferate, evade host immune responses, and develop resistance to anticancer therapies. As research progresses in understanding the TME, it is becoming increasingly clear that these interactions play a critical role in tumour initiation, progression, and metastasis. The TME not only supports cancer cell survival but also actively participates in modulating the behaviour of these cells through complex signalling networks [88].
The microenvironment surrounding cancer stem cells (CSCs) plays a crucial role in their metabolic plasticity. Factors such as cancer-associated fibroblasts (CAFs), endothelial cells, inflammatory agents, and the presence of immunomodulatory cells, along with conditions like inflammation and hypoxia, significantly influence the metabolic shifts in CSCs [61, 63]. With the ongoing advancements in understanding CSCs and the TME, the specific microenvironment that supports CSCs, known as the CSC niche, has become a focal point for researchers. This niche is crucial for the survival of CSCs, as it regulates their characteristics through both direct cell-to-cell interactions and the secretion of various factors [61]. Therefore, a thorough understanding of the interplay between CSCs and the TME needed to be elucidated.
Role of TME as a key player in metabolism of tumour micro-cells
A high concentration of ketone bodies and lactate, indicative of glycolysis and ketogenesis in the tumour microenvironment (TME), alongside factors like TGF-β and HIF-1α, may contribute to the induction or maintenance of stemness in cancer stem cells (CSCs) [89, 90]. Rapid proliferation coupled with insufficient blood vessel formation leads to hypoxic conditions in most solid tumors, which in turn induces HIF. The hypoxic environment can drive alterations in the TME, such as epithelial-mesenchymal transition (EMT), apoptosis suppression, metabolic changes, tumour invasion, infiltration of regulatory cells, and neovascularization. [91] Additionally, hypoxia supports stemness and maintain undifferentiated characteristics in CSCs. The ability to enhance glucose transporters on the cell surface and shift towards glycolysis in response to nutrient deprivation and hypoxia showcases the remarkable adaptability of cancer stem cells (CSCs) [42, 89].
Epigenetic regulation: influence of epignetic modifaiction on CSC behaviour
Epigenetics include any processes that control gene expression, without altering the DNA sequence, through the alteration of structures of chromosomes [92, 93]. This involves for example the events commonly known as methylation and demethylation of CpG regions. The chromatin's structure can be altered through at least three interconnected methods: Covalent histone modifications that occur after the incorporation of core histones into nucleosomes, ATP-dependent chromatin remodeling, and histone exchange. Further, non-coding RNAs can regulate the genes on the basis of their connection with transcription factors. In addition to two-dimensional changes, epigenetic regulation encompasses complex architectural changes, for example promoter–enhancer interactions and regulatory DNA loops that are necessary for the cell’s identity [94, 95].
It was previously reported that DNA methylation regulates the plasticity of CSCs through gene expression by targeting CpG dinucleotide sites known as CpG islands that can be located at a promoter region [96, 97]. This process is facilitated by DNA methyltransferases also known as DNMTs, where extra methyl groups are added to the cytosines making it 5-methylcytosine or abbreviated as 5mC. 5mC can lead to gene silencing either directly by attracting proteins that are themselves repressive or indirectly by preventing the binding of transcription factor. To counter that silencing, Ten-Eleven Translocation (TET) proteins can convert 5mC to 5-hydroxymethylcytosine (5hmC), which can be demethylated either passively or actively [98]. That passive demethylation means that 5mC marks are not maintained during cell division, while active demethylation is demethylation of 5hmC to 5fC and 5caC and its substitution by T via Base Excision Repair (BER.) In cancer, methylation abnormalities are presented; localized hypermethylation of tumour suppressor gene promoters cause their inactivation, referred to as the “CpG island methylator phenotype” (CIMP). At the same time, the global DNA hypomethylation in intergenic space can cause the activation of oncogenes and to genomic instability. It is suggested that these alterations in the CSC subset are important for tumour initiation and growth because certain methylation changes are needed for generating CSCs and for enabling their malignant properties [99].
Epigenetic regulation of cancer stem cells’ (CSCs) plasticity involves the chemical modifications that occur in histone tails including acetylation, methylation, and ubiquitylation [93, 100]. These modifications happen mostly at the promoter and enhancer sites to define particular chromatin conformation favorable to or unfavorable to gene activity. The state invoked here is euchromatin, which allows transcription, and on the other side of the spectrum, there is heterochromatin, which does not allow transcription. The proteins of the Trithorax group (TrxG) are involved in euchromatin formation, whereas proteins of the Polycomb group (PcG) specify heterochromatin formation [101].
The most investigated histone changes belong to histone H3, which relates to active enhancers (H3K9ac, H3K27ac), promoters (H3K4me3) and within genes that are being actively transcribed (H3K36me3) [102, 103]. Examples of repressive marks are H3K27me3 and H3K9me3. Interestingly, about 30% of the baseline genome is characterized by variations in chromatin organization between differentiated and pluripotent stem cells; in the latter, particularly in euchromatin, transcriptional permission is significantly higher than in somatic cells [103]. This is the case of higher levels of acetylation and reduced repressive marks of chromatin. Histone-modifying enzymes are involved in the setting of cell fate; for example, the Polycomb repressive complexes PRC1 and PRC2 are required for the definition of pluripotency. Nevertheless, reports shows that the overall level of histone modifications was similar between PSCs and CSCs; however, tumor-specific features are present. EZH2, is a component of PRC2, isogenic for H3K27me3 and involved in CSC development in some types of cancer [104, 104]. Down-regulation of Enhancer of Zeste Homolog 2 (EZH2) has been reported to promote tumorigenesis in some tumour types whereas its oncogenic roles have been described in breast cancer and pancreatic ductal adenocarcinoma [105]. Additionally, loss-of-function mutations in demethylases including Ubiquitously Transcribed Tetratricopeptide Repeat on X Chromosome (UTX3) and Jumonji Domain-Containing Protein 3 (JMJD3) may have biphasic effects on tumour initiation in T-cell acute lymphoblastic leukemia (T-ALL) suggesting multifarious roles of histone modification in determining CSC behaviors [106, 107]
Targeted drug delivery for cancer stem cells
Nanoparticles and liposomes
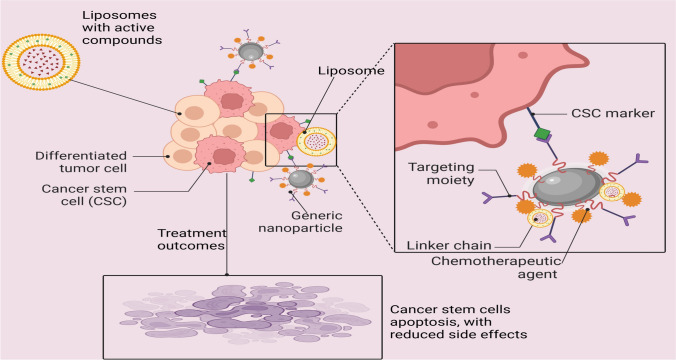
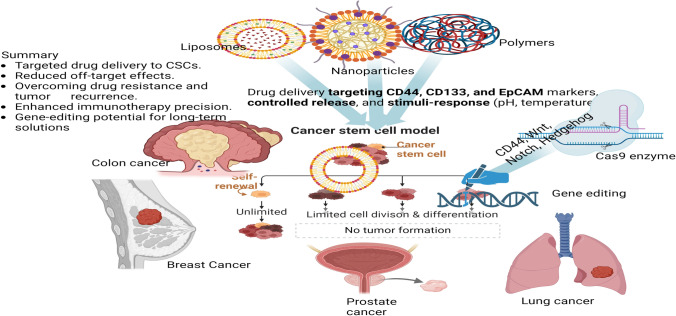
Targeted drug delivery systems utilizing nanoparticles, particularly liposomes, have shown great promise in addressing the challenges posed by cancer stem cells (CSCs) in various cancer types, including breast and colorectal cancer [35, 35]. These systems offer advantages in delivering therapeutic agents specifically to targeted locations, such as the mitochondria in tumour cells. By actively targeting CSCs, these nanocarriers can enhance drug efficacy and reduce off-target effects, potentially improving treatment outcomes [108, 109]. The use of functionalized liposomes has emerged as a leading strategy to overcome biological barriers and improve drug delivery for cancer therapeutics, offering a potential solution to the limitations of conventional treatments in effectively targeting CSCs and reducing side effects in cancer therapy [109, 110]. By concentrating on CSCs in particular, targeted drug delivery methods like liposomes and nanoparticles aim to increase the effectiveness and decrease the negative effects of anticancer medications. To guarantee that the therapeutic chemicals are delivered to the CSCs, these delivery systems can be designed to identify and bind to markers unique to CSCs [111, 112] (Fig. 2) as summarized.
Fig. 2.
Targeted Drug Delivery for Cancer Stem Cells Using Nano Particles, and Liposomes as Delivery agents (Created in BioRender.com)
Nanoparticles: Nanoparticles are an innovative and versatile tool in modern drug delivery and cancer therapy, offering the potential for highly targeted treatments [4]. The fabrication of nanoparticles involves the use of various materials such as metals (e.g., gold, silver), lipids (e.g., lipid-based nanoparticles), and polymers (e.g., poly(lactic-co-glycolic acid) or PLGA) that each contribute unique properties suited to specific therapeutic needs [113]. These nanoparticles can be engineered to be functionalized with ligands that specifically bind to cancer stem cell (CSC) markers such as CD44, CD133, and EpCAM, which are present on the surface of CSCs [111, 114]. This selective targeting allows for precision in delivering therapeutic agents directly to the CSCs, which are often responsible for tumour initiation, metastasis, and recurrence, thus improving treatment efficacy.
An exemplary application of this approach is the use of gold nanoparticles conjugated with anti-CD44 antibodies, which can selectively bind to CD44 receptors on CSCs, enabling targeted killing of these cells both in vitro and in vivo [115]. The ability to specifically target and eliminate CSCs holds promise for reducing tumour relapse and enhancing the effectiveness of cancer therapies. The use of nanoparticles in drug delivery offers several advantages over traditional methods. These include their ability to penetrate deep into tissues, which is particularly beneficial for targeting solid tumours, as well as their capacity for controlled release. Nanoparticles can be designed to release their payload in a controlled manner over time, ensuring that therapeutic agents are delivered at a consistent rate. This controlled release minimizes the need for frequent dosing and reduces side effects. Furthermore, nanoparticles exhibit a high drug-loading capacity, allowing for the incorporation of significant amounts of therapeutic agents, which can improve the therapeutic outcome [116, 117].
In addition, nanoparticles can be engineered to respond to specific environmental stimuli, such as temperature, pH, or enzyme activity, ensuring that the release of the encapsulated drug occurs at the targeted site, where such stimuli are present. For instance, nanoparticles can be designed to release their contents in response to the acidic microenvironment of tumours or changes in temperature at the site of inflammation. This feature allows for even more precise control over when and where the drug is released, maximizing therapeutic efficiency while minimizing off-target effects. [110, 117]. Overall, nanoparticles represent a promising avenue for the development of more effective and personalized therapies, particularly in the treatment of cancers, where targeting CSCs and ensuring localized drug delivery could lead to significant advancements in clinical outcomes.
Liposomes: Made of phospholipid bilayers, liposomes are spherical vesicles that can hold both hydrophilic and hydrophobic medications. They can deliver medications to CSCs specifically by adding targeting moieties, like peptides or antibodies, to the liposome surface [109]. For example, glioma CSCs have been targeted and eliminated using liposomes modified with an anti-CD133 antibody. Pharmaceuticals can be better absorbed and distributed by liposomes, which also increase drug solubility and prevent pharmaceuticals from degradation. Their versatility in targeted medication administration stems from their biocompatibility and capacity to encapsulate a broad spectrum of medicinal substances [110, 112].
Immunotherapy approaches
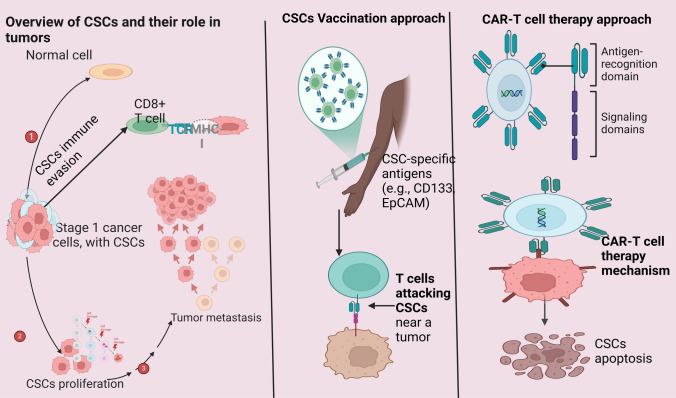
The ultimate goal of immunotherapy is to harness the body's immune system to recognize and eliminate cancer cells, including the elusive and resilient cancer stem cells (CSCs). CSCs are a subpopulation of tumour cells known for their ability to drive tumour initiation, progression, recurrence, and resistance to conventional therapies. Therefore, targeting CSCs has become a critical focus in the development of effective and durable cancer treatments. [62]. Recent advancements in CSC-targeted immunotherapy include the development of vaccines and adoptive cell therapies, such as chimeric antigen receptor (CAR)-T cell therapy. Vaccines designed to target CSC-specific antigens aim to stimulate a robust and sustained immune response against CSCs. For example, dendritic cell (DC) vaccines loaded with CSC-associated antigens have shown significant promise in preclinical studies. In models of glioblastoma and melanoma, these vaccines have successfully elicited immune responses specifically targeting CSCs, demonstrating their potential as a novel therapeutic strategy for combating aggressive and recurrent cancers. [118]. CAR-T cell therapy represents another groundbreaking approach in CSC-directed immunotherapy. This technique involves the genetic engineering of T cells to express chimeric antigen receptors that can precisely recognize and bind to antigens uniquely expressed by CSCs. Preclinical studies have demonstrated the efficacy of CAR-T cells targeting CSC-specific markers such as CD133 and EpCAM. These engineered T cells have shown the ability to selectively eradicate CSCs, significantly reducing tumour recurrence and progression [62, 118]. One of the most significant advantages of CAR-T cell therapy is its highly targeted nature, which minimizes off-target effects while maximizing therapeutic efficacy. Moreover, given the complexity and heterogeneity of tumors, designing T cells capable of recognizing multiple CSC markers could enhance the therapy’s effectiveness by addressing tumour diversity and overcoming resistance mechanisms. This multipronged strategy could represent a crucial step forward in improving the outcomes of immunotherapy for cancer patients. (Fig. 3).
Fig. 3.
Cancer Stem Cells-Targeted Immunotherapy Approaches (Created in BioRender.com)
Gene editing technology
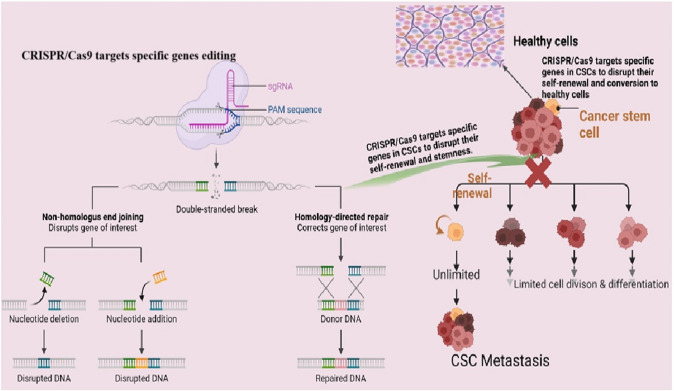
The ground-breaking gene-editing technique known as CRISPR/Cas9-clustered regularly interspaced short palindromic repeats, or CRISPR—and CRISPR-associated protein 9 (Cas9) enable accurate and effective modification of DNA sequences (Fig. 4). To drive the Cas9 nuclease to a particular genomic location and cause a double-strand break, the mechanism makes use of a guide RNA (gRNA). Targeted gene disruption, insertion, or correction can then result from the repair of this break via a variety of methods [119, 120].
Fig. 4.
CRISPR/Cas9 and Its Role in Targeting Cancer Stem Cells (CSCs)(Created in BioRender.com)
Genes are essential to the survival and maintenance of CSCs can be deleted using CRISPR/Cas9. Targeting genes related to stemness, self-renewal, and resistance mechanisms, for instance, can lower the number of CSCs and make tumours more susceptible to traditional treatments [121]. For instance, it has been demonstrated that deleting CD44, a cell surface marker linked to CSCs, using CRISPR/Cas9 can decrease the stemness and carcinogenic potential of breast cancer cells [119, 122]. Moreso, CSC survival and upkeep frequently depend on particular signalling pathways, including Wnt, Notch, and Hedgehog. Crucial elements of these pathways can be altered using CRISPR/Cas9, impairing CSC activity [119]. If two gene mutations combine to cause cell death, this is known as synthetic lethality; if one of these genes is mutated alone, this is not the case [123]. Artificially created fatal interactions unique to CSCs can be found and exploited using CRISPR/Cas9. An example of this would be the potential to selectively destroy BRCA1-deficient CSCs while preserving normal cells by using CRISPR/Cas9 to alter DNA damage response genes [120, 124]. Base editing and prime editing are two more advanced CRISPR-based techniques that enable more exact genetic alterations without breaking DNA strands. While prime editing can add or remove short DNA sequences, base editing can only modify a single DNA base. The possibility of using these technologies to fix the mutations causing the characteristics of CSCs is being investigated. Therefore, enhancing CRISPR/Cas9 component distribution to CSCs is essential for clinical applications. Utilising exosomes, viral vectors, and nanoparticles to improve the effectiveness and selectivity of gene editing in CSCs is one recent development [122, 124].
Other gene therapy tools
TALENs (Transcription Activator-Like Effector Nucleases): Engineered proteins known as TALENs can be made to target particular DNA sequences and cause double-strand breaks. They utilize distinct recognition methods, yet they operate similarly to CRISPR/Cas9. TALENs have been applied to CSCs to target genes related to drug resistance and CSC maintenance [125].
ZNFs (Zinc Finger Nucleases): These are synthetic DNA-binding proteins that can be tailored to identify particular DNA sequences and induce double-strand breaks. They were among the first instruments for altering DNA to be created. ZNFs have been applied to CSCs to modify genes linked to specific CSC traits [126].
RNA Interference (RNAi): In RNAi, targeted mRNA molecules are neutralised by RNA molecules, thereby inhibiting the production of genes. RNA interference (RNAi) can be used to silence genes relevant to cancer stem cells, however, it is not a technique for gene editing per se. RNA interference (RNAi) has been applied to silence genes related to CSC signalling cascades [127, 128]. A summary of the drug delivery process is presented in Fig. 5, illustrating the interplay between different targeted drug delivery systems (nanoparticles, liposomes), immunotherapy, and gene-editing technologies for combating cancer stem cells (CSCs).
Fig. 5.
Illustration of the interplay between different targeted drug delivery systems (nanoparticles, liposomes), immunotherapy, and gene-editing technologies for combating cancer stem cells (CSCs) in different organs (Created in BioRender.com)
Natural compounds and phytochemicals
Natural substances and phytochemicals obtained from plants and other natural sources have demonstrated the ability to target and suppress the characteristics of CSCs (Table 1). These substances can be utilized as adjunctive therapy and frequently have lesser toxicity than traditional chemotherapeutics [112, 129].
Table 1.
Current Innovations in Cancer Stem Cell Therapy: A Review of Plant-Derived Compounds and Their Therapeutic Potential
| Serial number | Compound | Plant source | Current research | Current level of application | Success/Failures | References |
|---|---|---|---|---|---|---|
| 1 | Curcumin | Turmeric | Anti-CSC effects by targeting Wnt, Notch, and Hedgehog pathways in cancer models. | Adjunctive therapy in preclinical and some clinical studies. | Shown to reduce CSC populations and enhance chemotherapy effectiveness in various cancer models, but limited clinical translation. | [130, 131] |
| 2 | Resveratrol | Grapes, Berries | Targets CSCs in prostate, colon, and breast cancers via NF-κB and PI3K/Akt pathways. | Preclinical studies with ongoing clinical trials. | Demonstrated inhibition of CSC features, but variable bioavailability in vivo. | [132, 133] |
| 3 | Epigallocatechin Gallate (EGCG) | Green Tea | Targets Notch and Hedgehog pathways; effective in glioblastoma and pancreatic cancer CSCs. | Preclinical studies with limited clinical application. | Anti-CSC effects observed in models, but needs more clinical trials for efficacy confirmation. | [134, 135] |
| 4 | Quercetin | Onions, Apples | Inhibits CSCs via modulation of NF-κB and Wnt/β-catenin signaling in colon and breast cancers | Preclinical studies. | Shown to reduce CSC properties in several cancer types, but issues with solubility and delivery limit its therapeutic potential. | [136, 137] |
| 5 | Genistein | Soybeans | Downregulates CSC characteristics in breast and prostate cancers by targeting the Notch and Hedgehog pathways. | Preclinical research with some clinical trials. | Successfully inhibits CSC markers, but bioavailability and metabolism hinder its clinical use. | [138, 139] |
| 6 | Silibinin | Milk Thistle | Modulates CSC characteristics through inhibition of STAT3 signaling in lung and breast cancers. | Preclinical and early-phase clinical trials. | Shown to reduce CSCs, with potential for clinical development, but limited large-scale human trials. | [140, 141] |
| 7 | Piperine | Black Pepper | Targets CSCs in colorectal cancer by modulating the Wnt/β-catenin pathway. | Preclinical studies. | Demonstrates anti-CSC effects, but needs further study to assess long-term effects and toxicity. | [142, 143] |
| 8 | Berberine | Goldenseal | Inhibits CSC proliferation via modulation of AMPK and Wnt pathways in liver and colon cancers. | Preclinical studies, ongoing trials | Demonstrates potent anti-CSC properties but low bioavailability in-vivo is a challenge. | [144, 145] |
| 9 | Sulforaphane | Broccoli | Suppresses CSCs in breast and pancreatic cancer by targeting Wnt and Hedgehog pathways. | Clinical trials in combination with chemotherapy | Shown to enhance chemotherapy effectiveness, with good clinical potential, but optimal dosing needs further investigation. | [146, 147] |
| 10 | Thymoquinone | Black Cumin Seed | Inhibits CSCs in colorectal and breast cancers via modulation of PI3K/Akt and Wnt pathways. | Preclinical research. | Exhibits anti-CSC activity, but limited clinical data. | [148, 149] |
| 11 | Lycopene | Tomatoes | Targets CSCs in prostate cancer by modulating NF-κB and PI3K/Akt pathways. | Preclinical studies. | Demonstrated anti-CSC effects, but limited bioavailability and stability in therapeutic applications. | [150–152] |
| 12 | Apigenin | Parsley, Celery | Suppresses CSC characteristics in prostate and breast cancers by targeting Wnt and Notch pathways. | Preclinical studies | Promising anti-CSC effects, but issues with poor solubility and bioavailability. | [153–155] |
| 13 | Fisetin | Strawberries | Inhibits CSCs in lung and prostate cancers by modulating PI3K/Akt and Hedgehog pathways. | Preclinical studies | Potent anti-CSC effects observed, but limited clinical trials to confirm efficacy. | [156–158] |
| 14 | Baicalein | Scutellaria baicalensis | Targets CSCs in liver and lung cancers by inhibiting Notch and Wnt signaling pathways. | Preclinical research. | Promising anti-CSC effects in preclinical models, but further studies required for clinical application. | [159, 160] |
| 15 | Emodin | Rhubarb | Targets CSCs in liver and lung cancers by modulating the PI3K/Akt and Wnt pathways. | Preclinical studies with limited clinical translation. | Demonstrates anti-CSC effects in various cancer models, but challenges with stability and delivery in vivo. | [161, 162] |
| 16 | Betulinic Acid | Birch Tree Bark | Inhibits CSC features in melanoma and glioblastoma by modulating the NF-κB pathway. | Preclinical studies. | Promising anti-CSC activity, but limited by poor solubility and bioavailability in therapeutic contexts. | [163, 164] |
| 17 | Ginsenoside Rh2 | Ginseng | Targets CSCs in gastric and colorectal cancers by inhibiting the Wnt/β-catenin pathway. | Preclinical and clinical studies. | Potent anti-CSC effects with promising clinical potential, though large-scale human studies are limited. | [165, 166] |
| 18 | Honokiol | Magnolia Bark | Inhibits CSC proliferation in lung and breast cancers by modulating the NF-κB and PI3K/Akt pathways. | Preclinical studies. | Exhibits anti-CSC effects, but limited clinical applications due to solubility and bioavailability issues. | [167–169] |
| 19 | Isothiocyanates | Cruciferous Vegetables | Suppresses CSCs in lung and breast cancers by modulating PI3K/Akt and Wnt/β-catenin pathways. | Preclinical studies. | Potent anti-CSC effects demonstrated, though limited by stability and bioavailability. | [170–172] |
| 20 | Diosgenin | Wild Yam | Inhibits CSC features in colon and liver cancers by modulating Hedgehog and PI3K/Akt pathways. | Preclinical studies. | Promising anti-CSC effects in preclinical models, with further studies needed for clinical translation. | [173–175] |
Combination therapies in cancer stem cells
Combination therapy have arisen as a potential strategy to target cancer stem cells (CSCs), which are accountable for tumour recurrence and medication resistance [4, 65, 176]. CSCs have distinctive characteristics, including self-renewal, multipotential differentiation, and improved survival mechanisms, rendering them resistant to conventional monotherapies. Diverse combination tactics have been investigated, encompassing the targeting of particular signaling pathways, stem cell-based therapeutics, and photodynamic therapy [42, 69, 110, 177]. Moreover, novel strategies such as differentiation treatment, anti-angiogenic agents, immunotherapy, and epigenetic enzyme inhibition have demonstrated promise in specifically targeting cancer stem cells [42, 178, 179]. Combination treatments in breast cancer treatment, which include molecular-targeted therapy, hormone therapy, immunotherapy, and chemotherapy, have shown enhanced effectiveness and less toxicity relative to monotherapies. Notwithstanding obstacles, the amalgamation of diverse technologies such as nanotechnology and computer technology may enhance combination therapy for cancer stem cells [42, 60, 91].
Combination therapy aimed at cancer stem cells (CSCs) provide a viable approach for enhancing cancer treatment efficacy. These medicines can address both cancer stem cells and the predominant tumour mass, providing the possibility of sustained remission and decreased recurrence rates. Nonetheless, issues including as toxicity, tumour heterogeneity, and drug resistance must be resolved to fully exploit the potential of combination therapy in oncology. Conventional chemotherapy predominantly targets quickly proliferating cells while frequently neglecting the dormant cancer stem cell population. Integrating chemotherapy with medicines that precisely target cancer stem cells can enhance treatment results.
Challenges in cancer stem cells research
Cancer stem cells (CSCs) provide considerable obstacles in cancer therapy owing to their capacity for self-renewal, differentiation, and resistance to standard treatments. Targeting cancer stem cells (CSCs) is a potential approach for successful cancer eradication; yet, their variability poses a significant challenge. Current research emphasizes the development of innovative therapeutic methods that integrate traditional therapies with techniques specifically targeting cancer stem cells (CSCs). This encompasses the targeting of surface biomarkers, signaling pathways, drug-efflux pumps, and microenvironmental signals. CAR-T treatment has promise in targeting cancer stem cells; nonetheless, issues related to toxicity, durability, and tumour heterogeneity must be resolved [43, 62, 178]. The inhibition of CSC-regulating mechanisms, has shown increased effectiveness of conventional treatments in preclinical investigations [42, 70]. Numerous CSC-targeting medicines are presently being assessment in preclinical and clinical studies, aiming to enhance cancer therapy efficacy and avert recurrence [109, 110].
Research on cancer stem cells (CSCs) encounters numerous challenges, such as the heterogeneity of CSCs within tumors, the absence of universal markers, dynamic cell populations, inter- and intra-tumour variability, resistance to conventional therapies, the intricacy of the tumour microenvironment, and the deficiency of effective models. Cancer stem cells exhibit greater resistance to standard cancer therapies, frequently resulting in recurrence and metastasis. Comprehending the molecular pathways that contribute to this resistance is difficult. They can also enter a latent state, rendering them less vulnerable to therapy aimed at quickly proliferating cells. The interplay between cancer stem cells and their microenvironment, comprising immune cells, stromal cells, and extracellular matrix components, is crucial for their survival and proliferation [50, 61, 90].
The intricacy of the tumour microenvironment (TME) presents a further hurdle, since it significantly influences tumour survival and growth. Numerous cancer stem cells inhabit hypoxic microenvironments, complicating their targeting without harming adjacent healthy tissues. Moreover, there exists a deficiency of useful models, including insufficient in-vitro and animal models [41, 61]. The processes by which metastasis and cancer stem cells contribute to metastasis remain ambiguous and intricate. Targeting CSC-specific pathways presents challenges because to the negative effects associated with route targeting and the emergence of resistance mechanisms. Clinical translation is a significant hurdle, with the creation of biomarkers being an unmet necessity. Creating pharmaceuticals that specifically target cancer stem cells while preserving normal stem cells is a significant barrier, and only a limited number of CSC-specific treatments have advanced to clinical trials [70, 109]. Nonetheless, technological advancements, like single-cell sequencing, enhanced in-vitro models, and targeted therapeutics, are facilitating the resolution of some challenges in cancer stem cell research [110].
Future perspectives
Looking ahead, the continued exploration of novel cancer gene therapy methods holds great promise for the future of CSC-targeted therapies. One potential avenue is the development of gene-editing technologies beyond CRISPR, such as base editors or prime editing, which offer enhanced precision and fewer off-target effects. These advanced tools could enable the targeted correction of genetic mutations in CSCs, reducing the risk of recurrence and metastasis. Additionally, the integration of advanced artificial intelligence (AI) and machine learning (ML) in cancer research could facilitate the discovery of novel CSC markers and therapeutic targets, as well as optimize patient-specific treatment regimens. AI-driven systems could also assist in designing personalized approaches by integrating genomic, proteomic, and metabolic data to predict CSC behavior and therapy response.
Another promising frontier involves the use of synthetic biology to engineer novel therapeutic strategies, such as engineered oncolytic viruses or bacteria, designed to specifically target CSCs and their unique metabolic profiles. These engineered entities could selectively deliver therapeutic agents or activate immune responses against CSCs while sparing healthy tissue. Moreover, the exploitation of the tumour microenvironment (TME) as a therapeutic target offers potential to disrupt CSC survival and metastasis. By understanding the role of stromal cells, extracellular matrix components, and immune cell interactions in CSC regulation, new strategies could emerge to manipulate the TME and reprogram it to suppress CSCs.
Lastly, there is a need for further research into combining CSC-targeted therapies with emerging technologies, such as nanomedicine and advanced drug delivery systems, to enhance the effectiveness of treatments while minimizing off-target toxicity. This integrated approach could overcome current challenges in CSC eradication and contribute to the development of highly specific, personalized cancer therapies. These future research directions are crucial for overcoming the complexities associated with CSC biology, addressing treatment resistance, and ultimately achieving lasting therapeutic breakthroughs in cancer care.
Summary/conclusion
The review discusses the role of Cancer Stem Cells (CSCs) in tumour initiation, progression, and therapy resistance. It highlights the potential of innovative therapies like CRISPR/Cas9 gene editing, CAR-T cell immunotherapy, and nanoparticle-based drug delivery systems in targeting CSCs, offering precision, reduced side effects, and enhanced efficacy. The study also explores metabolic and epigenetic pathways in CSC survival, highlighting the role of natural compounds like curcumin and resveratrol in CSC-targeting abilities. The tumour microenvironment (TME) is examined as a key target for innovative interventions, presenting it as a key target for innovative interventions. The revie suggest that combining conventional and CSC-targeted therapies can reduce relapse and overcome resistance. The review concludes that CSC-focused strategies in cancer therapy have transformative potential, improving treatment durability and reducing recurrence rates. However, challenges such as tumour heterogeneity, immune evasion, and effective clinical translation remain.
Author contributions
Equally contributing authors.
Funding
None.
Data availability
All data used are available within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All Authors read and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Statement of animal rights
Not applicable.
Statement of informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communicat Signal. 2020;18:59. 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bareham B, Dibble M, Parsons M. Defining and modeling dynamic spatial heterogeneity within tumor microenvironments. Curr Opin Cell Biol. 2024;90: 102422. 10.1016/j.ceb.2024.102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat GR, Sethi I, Sadida HQ, Rah B, Mir R, Algehainy N, Albalawi IA, Masoodi T, Subbaraj GK, Jamal F, Singh M, Kumar R, Macha MA, Uddin S, Akil ASA-S, Haris M, Bhat AA. Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024;43:197–228. 10.1007/s10555-024-10172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johari B, Ebrahimi-Rad M, Maghsood F, Lotfinia M, Saltanatpouri Z, Teimoori-Toolabi L, Sharifzadeh Z, Karimipoor M, Kadivar M. Myc Decoy oligodeoxynucleotide inhibits growth and modulates differentiation of mouse embryonic stem cells as a model of cancer stem cells. Anticancer Agents Med Chem. 2017;17:1786–95. 10.2174/1871521409666170412142507. [DOI] [PubMed] [Google Scholar]
- 5.Chu X, Tian W, Ning J, Xiao G, Zhou Y, Wang Z, Zhai Z, Tanzhu G, Yang J, Zhou R. Cancer stem cells: advances in knowledge and implications for cancer therapy. Sig Transduct Target Ther. 2024;9:1–63. 10.1038/s41392-024-01851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y-R, Fang Y, Lyu Z, Zhu Y, Yang L. Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: implications for novel therapeutic strategies. J Transl Med. 2023;21:686. 10.1186/s12967-023-04575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Z, Fu M, Hu Y, Wei Y, Wei X, Luo M. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer. 2023;22:172. 10.1186/s12943-023-01877-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmati M, Johari B, Kadivar M, Rismani E, Mortazavi Y. Suppressing the metastatic properties of the breast cancer cells using STAT3 decoy oligodeoxynucleotides: a promising approach for eradication of cancer cells by differentiation therapy. J Cell Physiol. 2020;235:5429–44. 10.1002/jcp.29431. [DOI] [PubMed] [Google Scholar]
- 9.Maleki EH, Bahrami AR, Matin MM. Cancer cell cycle heterogeneity as a critical determinant of therapeutic resistance. Genes Diseases. 2024;11:189–204. 10.1016/j.gendis.2022.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, He G, Yu Y, Li X, Peng X, Yang L. Exosome crosstalk between cancer stem cells and tumor microenvironment: cancer progression and therapeutic strategies. Stem Cell Res Ther. 2024;15:449. 10.1186/s13287-024-04061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie MS, Ward CM, Davies CC. DNA repair and therapeutic strategies in cancer stem cells. Cancers. 2023;15:1897. 10.3390/cancers15061897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivares-Urbano MA, Griñán-Lisón C, Marchal JA, Núñez MI. CSC radioresistance: a therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells. 2020;9:1651. 10.3390/cells9071651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Fiore R, Suleiman S, Drago-Ferrante R, Subbannayya Y, Pentimalli F, Giordano A, Calleja-Agius J. Cancer stem cells and their possible implications in cervical cancer: a short review. Int J Mol Sci. 2022;23:5167. 10.3390/ijms23095167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul R, Dorsey JF, Fan Y. Cell plasticity, senescence, and quiescence in cancer stem cells: biological and therapeutic implications. Pharmacol Ther. 2022;231: 107985. 10.1016/j.pharmthera.2021.107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thankamony AP, Saxena K, Murali R, Jolly MK, Nair R. Cancer stem cell plasticity–a deadly deal. Front Mol Biosci. 2020. 10.3389/fmolb.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nallasamy P, Nimmakayala RK, Parte S, Are AC, Batra SK, Ponnusamy MP. Tumor microenvironment enriches the stemness features: the architectural event of therapy resistance and metastasis. Mol Cancer. 2022;21:225. 10.1186/s12943-022-01682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen P, Hsu W-H, Han J, Xia Y, DePinho RA. Cancer stemness meets immunity: from mechanism to therapy. Cell Rep. 2021;34: 108597. 10.1016/j.celrep.2020.108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian M, Ahmadlou M, Salehi R, Sadeghi B, Manian M. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21:62. 10.1186/s12935-020-01719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo CD, Kang N, Choi SY, Kim BN, Park CK, Kim JW, Kim YK, Kim SJ. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: a possible link to epigenetic regulation. Korean J Intern Med. 2017;32:589–99. 10.3904/kjim.2016.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talukdar J, Srivastava TP, Sahoo OS, Karmakar A, Rai AK, Sarma A, Gogoi G, Alqahtani MS, Abbas M, Dhar R, Karmakar S. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm Oncol. 2023;2:e62. 10.1002/mog2.62. [Google Scholar]
- 21.Habashy S, Jafri A, Osman HO, Thomas NE, Udekwe S, Heindl SE. Hedgehog pathway inhibitors: clinical implications and resistance in the treatment of basal cell carcinoma. Cureus. 2021;13:13859. 10.7759/cureus.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lospinoso Severini L, Ghirga F, Bufalieri F, Quaglio D, Infante P, Di Marcotullio L. The SHH/GLI signaling pathway: a therapeutic target for medulloblastoma. Expert Opin Ther Targets. 2020;24:1159–81. 10.1080/14728222.2020.1823967. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen NM, Cho J. Hedgehog pathway inhibitors as targeted cancer therapy and strategies to overcome drug resistance. Int J Mol Sci. 2022;23:1733. 10.3390/ijms23031733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan H, Liu Y, Gao Z, Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. 2021;11:55–70. 10.1016/j.apsb.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajendran A, Rajan RA, Balasubramaniyam S, Elumalai K. Nano delivery systems in stem cell therapy: transforming regenerative medicine and overcoming clinical challenges. Nano TransMed. 2025;4: 100069. 10.1016/j.ntm.2024.100069. [Google Scholar]
- 26.Tiwari H, Rai N, Singh S, Gupta P, Verma A, Singh AK, Kajal SP, Singh SK, Gautam V. Recent advances in nanomaterials-based targeted drug delivery for preclinical cancer diagnosis and therapeutics. Bioengineering. 2023;10:760. 10.3390/bioengineering10070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansori, A. M., Antonius, Y., Susilo, R. K., Hayaza, S., Kharisma, V. D., Parikesit, A. A., Zainul, R., Jakhmola, V., Saklani, T., Rebezov, M., Ullah, M. E., Maksimiuk, N., Derkho, M., Burkov, P.: Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J. 3, 184 (2023). 10.52225/narra.v3i2.184 [DOI] [PMC free article] [PubMed]
- 28.Chehelgerdi M, Chehelgerdi M, Khorramian-Ghahfarokhi M, Shafieizadeh M, Mahmoudi E, Eskandari F, Rashidi M, Arshi A, Mokhtari-Farsani A. Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy. Mol Cancer. 2024;23:9. 10.1186/s12943-023-01925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masoumi J, Jafarzadeh A, Abdolalizadeh J, Khan H, Philippe J, Mirzaei H, Mirzaei HR. Cancer stem cell-targeted chimeric antigen receptor (CAR)-T cell therapy: challenges and prospects. Acta Pharm Sin B. 2021;11:1721–39. 10.1016/j.apsb.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Accardo C, Porcelli G, Mangiapane LR, Modica C, Pantina VD, Roozafzay N, Di Franco S, Gaggianesi M, Veschi V, Lo Iacono M, Todaro M, Turdo A, Stassi G. Cancer cell targeting by CAR-T cells: a matter of stemness. Front Mol Med. 2022;2:1055028. 10.3389/fmmed.2022.1055028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O’Byrne K, Kulasinghe A. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–60. 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roarty K. Unlocking the secrets of cancer stem cells: Immune checkpoint inhibitors face their formidable foes. Cell Stem Cell. 2023;30:743–4. 10.1016/j.stem.2023.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Zhao J, Zhang Y, Jiang H, Liu D. Revisiting immune checkpoint inhibitors: new strategies to enhance efficacy and reduce toxicity. Front Immunol. 2024;15:1490129. 10.3389/fimmu.2024.1490129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes Q, Therachiyil L, Khan AQ, Bedhiafi T, Korashy HM, Bhat AA, Uddin S. Shrinking the battlefield in cancer therapy: nanotechnology against cancer stem cells. Eur J Pharm Sci. 2023;191: 106586. 10.1016/j.ejps.2023.106586. [DOI] [PubMed] [Google Scholar]
- 35.Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M, Viktor P, Lakshmaiya N, Saadh MJ, Amajd A, Abo-Zaid MA, Castillo-Acobo RY, Ismail AH, Amin AH, Akhavan-Sigari R. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22:169. 10.1186/s12943-023-01865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan MM, Chen R, Fong D. Targeting cancer stem cells with dietary phytochemical–repositioned drug combinations. Cancer Lett. 2018;433:53–64. 10.1016/j.canlet.2018.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alum EU, Tufail T, Uti DE, Aja PM, Offor CE, Ibiam UA, Ukaidi CUA, Alum BN. Utilizing Indigenous flora in East Africa for breast cancer treatment: an overview. Anti-Cancer Agents in Med Chem. 2025;25:99–113. 10.2174/0118715206338557240909081833. [DOI] [PubMed] [Google Scholar]
- 38.Aramini B, Masciale V, Grisendi G, Bertolini F, Maur M, Guaitoli G, Chrystel I, Morandi U, Stella F, Dominici M, Haider KH. Dissecting tumor growth: the role of cancer stem cells in drug resistance and recurrence. Cancers. 2022;14:976. 10.3390/cancers14040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzobo K, Senthebane DA, Ganz C, Thomford NE, Wonkam A, Dandara C. Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells. 2020;9:1896. 10.3390/cells9081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang B, Miao L, Liu J, Zhang J, Li Y. A promising antitumor method: targeting CSC with immune cells modified with CAR. Front Immunol. 2022;13: 937327. 10.3389/fimmu.2022.937327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mai Y, Su J, Yang C, Xia C, Fu L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol Cancer. 2023;22:1–24. 10.1186/s12943-023-01867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayani H, Chávez-González A, Vázquez-Santillan K, Contreras J, Guzman ML. Cancer stem cells: biology and therapeutic implications. Arch Med Res. 2022;53:770–84. 10.1016/J.ARCMED.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Fiori ME, Villanova L, De Maria R. Cancer stem cells: at the forefront of personalized medicine and immunotherapy. Curr Opin Pharmacol. 2017;35:1. [DOI] [PubMed] [Google Scholar]
- 44.Phi LTH, Sari IN, Yang Y-G, Lee S-H, Jun N, Kim KS, Lee YK, Kwon HY. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:5416923. 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mengistu BA, Tsegaw T, Demessie Y, Getnet K, Bitew AB, Kinde MZ, Beirhun AM, Mebratu AS, Mekasha YT, Feleke MG, Fenta MD. Comprehensive review of drug resistance in mammalian cancer stem cells: implications for cancer therapy. Cancer Cell Int. 2024;24:406. 10.1186/s12935-024-03558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doustmihan A, Fathi M, Mazloomi M, Salemi A, Hamblin MR, Jahanban-Esfahlan R. Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: a narrative review. J Control Release. 2023;363:57–83. 10.1016/j.jconrel.2023.09.029. [DOI] [PubMed] [Google Scholar]
- 47.Santos-de-Frutos K, Djouder N. When dormancy fuels tumour relapse. Commun Biol. 2021;4:1–12. 10.1038/s42003-021-02257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng Y, Ma L, Sun Q. Clinically-relevant ABC transporter for anti-cancer drug resistance. Front Pharmacol. 2021. 10.3389/fphar.2021.648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020;13:60. 10.1186/s13045-020-00901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng Z, Fu M, Hu Y, Wei Y, Wei X, Luo M. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer. 2023;22:1–31. 10.1186/S12943-023-01877-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manni W, Min W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. MedComm. 2022. 10.1002/MCO2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. Springer. 2020. 10.1186/s13045-020-00901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Jiang X, Duan L, Xiong Q, Yuan Y, Liu P, Jiang L, Shen Q, Zhao S, Yang C, Chen Y. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol Cancer. 2021. 10.1186/S12943-021-01469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, Tao Q, Xu H. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Springer. 2021;21:144. 10.1186/s12943-022-01616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhen H, Deng H, Song Q, Zheng M, Yuan Z, Cao Z, Pang Q, Zhao B. The Wnt/Ca2+ signaling pathway is essential for the regeneration of GABAergic neurons in planarian Dugesia japonica. Wiley Online Library. 2020;34:16567–80. 10.1096/fj.201903040RR. [DOI] [PubMed] [Google Scholar]
- 56.Borlongan MC, Wang H. Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics. Front Cell Develop Bio. 2023. 10.3389/FCELL.2023.1125174/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khatun B, Kamath V, Sathyanarayana MB, Pai A, Gupta R, Malviya R. Emerging role of Wnt/beta-catenin signalling pathways in cancer progression and role of small molecule tankyrase inhibitors in combating multistage cancers. Curr Cancer Therapy Rev. 2021;17:304–11. 10.2174/1573394717666210628122306. [Google Scholar]
- 58.Gambini A, Stein P, Savy V, Grow EJ, Papas BN, Zhang Y, Kenan AC, Padilla-Banks E, Cairns BR, Williams CJ. Developmentally programmed tankyrase activity upregulates β-catenin and licenses progression of embryonic genome activation. Dev Cell. 2020;53:545-560.e7. 10.1016/j.devcel.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gajos-Michniewicz A, Czyz M. WNT signaling in melanoma. Int J Mol Sci. 2020;21:4852. 10.3390/IJMS21144852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borlongan MC, Wang H. Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics. Front Cell Developmen Bio. 2023;11:1125174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diaz MBN, Martin MJ, Gentili C. Tumor microenvironment involvement in colorectal cancer progression via Wnt/β-catenin pathway: Providing understanding of the complex mechanisms of chemoresistance. World J Gastroenterol. 2022;28:3027. 10.3748/WJG.V28.I26.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izadpanah A, Mohammadkhani N, Masoudnia M, Ghasemzad M, Saeedian A, Mehdizadeh H, Poorebrahim M, Ebrahimi M. Update on immune-based therapy strategies targeting cancer stem cells. Cancer Med. 2023;12:18960. 10.1002/CAM4.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akil A, Gutiérrez-García AK, Guenter R, Rose JB, Beck AW, Chen H, Ren B. Notch signaling in vascular endothelial cells, angiogenesis, and tumor progression: an update and prospective. Front Cell Develop Bio. 2021. 10.3389/FCELL.2021.642352/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reichrath J, Reichrath S. The impact of notch signaling for carcinogenesis and progression of nonmelanoma skin cancer: lessons learned from cancer stem cells, tumor angiogenesis, and beyond. Adv Exp Med Biol. 2021;1287:123–54. 10.1007/978-3-030-55031-8_9. [DOI] [PubMed] [Google Scholar]
- 65.Shrestha S, Banstola A, Jeong JH, Seo JH, Yook S. Targeting cancer stem cells: therapeutic and diagnostic strategies by the virtue of nanoparticles. J Controll Release. 2022;348:518–36. 10.1016/J.JCONREL.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Sen P, Ghosh SS. The intricate notch signaling dynamics in therapeutic realms of cancer. ACS Pharmacol Translat Sci. 2023;6:651–70. 10.1021/ACSPTSCI.2C00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sachan N, Sharma V, Mutsuddi M, Mukherjee A. Notch signalling: multifaceted role in development and disease. FEBS J. 2024;291:3030–59. 10.1111/FEBS.16815. [DOI] [PubMed] [Google Scholar]
- 68.Kumar V, Vashishta M, Kong L, Wu X, Lu JJ, Guha C, Dwarakanath BS. The role of Notch, Hedgehog, and Wnt signaling pathways in the resistance of tumors to anticancer therapies. Front Cell Develop Bio. 2021. 10.3389/FCELL.2021.650772/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bisht S, Nigam M, Kunjwal SS, Sergey P, Mishra AP, Sharifi-Rad J. Cancer stem cells: from an insight into the basics to recent advances and therapeutic targeting. Stem Cells Int. 2022. 10.1155/2022/9653244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aramini B, Masciale V, Grisendi G, Bertolini F, Mauer M, Guaitoli G, Chrystel I, Morandi U, Stella F, Dominici M, Haider KH. Dissecting tumor growth: the role of cancer stem cells in drug resistance and recurrence. Cancers. 2022. 10.3390/CANCERS14040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou H, Xiong Y, Peng L, Wang R, Zhang H, Fu Z. LncRNA-cCSC1 modulates cancer stem cell properties in colorectal cancer via activation of the Hedgehog signaling pathway. J Cell Biochem. 2020;121:2510–24. 10.1002/JCB.29473. [DOI] [PubMed] [Google Scholar]
- 72.Ageeva T, Rizvanov A, Mukhamedshina Y. NF-κB and JAK/STAT signaling pathways as crucial regulators of neuroinflammation and astrocyte modulation in spinal cord injury. Cells. 2024;13:581. 10.3390/cells13070581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sahoo OS, Pethusamy K, Srivastava TP, Talukdar J, Alqahtani MS, Abbas M, Dhar R, Karmakar S. The metabolic addiction of cancer stem cells. Front Oncol. 2022. 10.3389/fonc.2022.955892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Sahli S, Wang L. Cancer stem cell-associated pathways in the metabolic reprogramming of breast cancer. Int J Mol Sci. 2020;21:9125. 10.3390/ijms21239125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du J, Qin H. Lipid metabolism dynamics in cancer stem cells: potential targets for cancers. Front Pharmacol. 2024;15:1367981. 10.3389/fphar.2024.1367981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kovale L, Singh MK, Kim J, Ha J. Role of autophagy and AMPK in cancer stem cells: therapeutic opportunities and obstacles in cancer. Int J Mol Sci. 2024;25:8647. 10.3390/ijms25168647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Hout M, Cosialls E, Mehrpour M, Hamaï A. Crosstalk between autophagy and metabolic regulation of cancer stem cells. Mol Cancer. 2020;19:27. 10.1186/s12943-019-1126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Liu H-H, Cao Y-T, Zhang L-L, Huang F, Yi C. The role of mitochondrial dynamics and mitophagy in carcinogenesis, metastasis and therapy. Front Cell Development Bio. 2020;8:413. 10.3389/fcell.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belisario DC, Kopecka J, Pasino M, Akman M, De Smaele E, Donadelli M, Riganti C. Hypoxia dictates metabolic rewiring of tumors: implications for chemoresistance. Cells. 2020;9:2598. 10.3390/cells9122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int J Mol Sci. 2021;22:5703. 10.3390/ijms22115703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao H, Li Y. Cancer metabolism and intervention therapy. Mol Biomed. 2021;2:5. 10.1186/s43556-020-00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge T, Gu X, Jia R, Ge S, Chai P, Zhuang A, Fan X. Crosstalk between metabolic reprogramming and epigenetics in cancer: updates on mechanisms and therapeutic opportunities. Cancer Commun. 2022;42:1049–82. 10.1002/cac2.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao Y, Xia Z, Xia W, Jiang P. Metabolic reprogramming, sensing, and cancer therapy. Cell Rep. 2024;43: 115064. 10.1016/j.celrep.2024.115064. [DOI] [PubMed] [Google Scholar]
- 84.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–41. 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navas LE, Carnero A. Nicotinamide Adenine dinucleotide (NAD) metabolism as a relevant target in cancer. Cells. 2022;11:2627. 10.3390/cells11172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myong S, Nguyen AQ, Challa S. Biological functions and therapeutic potential of NAD+ metabolism in gynecological cancers. Cancers. 2024;16:3085. 10.3390/cancers16173085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng Z, Fu M, Hu Y, Wei Y, Wei X, Cancer ML-M. 2023, undefined: Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Springer. 2023;22:172. 10.1186/s12943-023-01877-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richard V, Kumar TRS, Pillai RM. Transitional dynamics of cancer stem cells in invasion and metastasis. Translat Oncol. 2021;14: 100909. 10.1016/J.TRANON.2020.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu W, Weng J, Xu M, Zhou Q, Liu S, Hu Z, Ren N, Zhou C, Shen Y. Functions of key enzymes of glycolytic metabolism in tumor microenvironment. Cell Reprogram. 2023;25:91–8. 10.1089/CELL.2023.0010/ASSET/IMAGES/CELL.2023.0010_FIGURE2.JPG. [DOI] [PubMed] [Google Scholar]
- 90.Zhang P, Cheng S, Sheng X, Dai H, He K, Du Y. The role of autophagy in regulating metabolism in the tumor microenvironment. Genes Diseas. 2023;10:447–56. 10.1016/J.GENDIS.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo Q, Zhou Y, Xie T, Yuan Y, Li H, Shi W, Zheng L, Li X, Zhang W. Tumor microenvironment of cancer stem cells: perspectives on cancer stem cell targeting. Genes Diseas. 2024;11: 101043. 10.1016/J.GENDIS.2023.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Breiling A, Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenet Chromat. 2015;8:24. 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conteduca V, Hess J, Yamada Y, Ku SY, Beltran H. Epigenetics in prostate cancer: clinical implications. Transl Androl Urol. 2021;10:3104–16. 10.21037/tau-20-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar S, Gonzalez EA, Rameshwar P, Etchegaray J-P. Non-coding RNAs as mediators of epigenetic changes in malignancies. Cancers. 2020;12:3657. 10.3390/cancers12123657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferrer J, Dimitrova N. Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat Rev Mol Cell Biol. 2024;25:396. 10.1038/s41580-023-00694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.French R, Pauklin S. Epigenetic regulation of cancer stem cell formation and maintenance. Int J Cancer. 2020;148:2884. 10.1002/ijc.33398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar VE, Nambiar R, Souza CD, Nguyen A, Chien J, Lam KS. Targeting epigenetic modifiers of tumor plasticity and cancer stem cell behavior. Cells. 2022;11:1403. 10.3390/cells11091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joshi K, Liu S, Sj PB, Zhang J. Mechanisms that regulate the activities of TET proteins. Cell Mol Life Sci. 2022;79:363. 10.1007/s00018-022-04396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo M, Peng Y, Gao A, Du C, Herman JG. Epigenetic heterogeneity in cancer. Biomarker Research. 2019;7:23. 10.1186/s40364-019-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sibuh B, Quazi S, Panday H, Parashar R, Jha N, Mathur R, Jha S, Taneja P, Jha A. The emerging role of epigenetics in metabolism and endocrinology. Biology. 2023;12:256. 10.3390/biology12020256. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Morrison O, Thakur J. Molecular complexes at euchromatin, heterochromatin and centromeric chromatin. Int J Mol Sci. 2021;22:6922. 10.3390/ijms22136922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Igolkina AA, Zinkevich A, Karandasheva KO, Popov AA, Selifanova MV, Nikolaeva D, Tkachev V, Penzar D, Nikitin DM, Buzdin A. H3K4me3, H3K9ac, H3K27ac, H3K27me3 and H3K9me3 histone tags suggest distinct regulatory evolution of open and condensed chromatin landmarks. Cells. 2019;8:1034. 10.3390/cells8091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herz H-M, Mohan M, Garruss AS, Liang K, Takahashi Y, Mickey K, Voets O, Verrijzer CP, Shilatifard A. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26:2604. 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Angrand P-O. Structure and function of the polycomb repressive complexes PRC1 and PRC2. Int J Mol Sci. 2022;23:5971. 10.3390/ijms23115971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun S, Yu F, Xu D, Zheng H, Li M. EZH2, a prominent orchestrator of genetic and epigenetic regulation of solid tumor microenvironment and immunotherapy. Biochimica et Biophysica Acta Rev Cancer. 2022;1877:188700. 10.1016/j.bbcan.2022.188700. [DOI] [PubMed] [Google Scholar]
- 106.Zhang P, Zhang M. Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma. Clin Epigenetics. 2020;12:169. 10.1186/s13148-020-00962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Chen J, Liu H, Mi R, Huang R, Li X, Fan F, Xie X, Ding J. The role of histone methylase and demethylase in antitumor immunity: a new direction for immunotherapy. Front Immunol. 2023. 10.3389/fimmu.2022.1099892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sher EK, Kalić A, Džidić-Krivić A, Zećo MB, Pinjić E, Sher F. Cellular therapeutic potential of genetically engineered stem cells in cancer treatment. Biotechnol Genet Eng Rev. 2023;00:1–36. 10.1080/02648725.2023.2204720. [DOI] [PubMed] [Google Scholar]
- 109.Abballe L, Spinello Z, Antonacci C, Coppola L, Miele E, Catanzaro G, Miele E. Nanoparticles for drug and gene delivery in pediatric brain tumors’ cancer stem cells: current knowledge and future. Perspectives. 2023;15:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doustmihan A, Fathi M, Mazloomi MA, Salemi A, Hamblin MR, Jahanban-Esfahlan R. Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: a narrative review. J Controll Release. 2023;363:57–83. [DOI] [PubMed] [Google Scholar]
- 111.Grover R, Drall S, Poonia N, Kumar Jain G, Aggarwal G, Lather V, Kesharwani P, Pandita D, Goyal RK. CD44 and CD133 aptamer directed nanocarriers for cancer stem cells targeting. Eur Polym J. 2023;183:111770. [Google Scholar]
- 112.Hatami M, Kouchak M, Kheirollah A, Khorsandi L, Rashidi M. Effective inhibition of breast cancer stem cell properties by quercetin-loaded solid lipid nanoparticles via reduction of Smad2/Smad3 phosphorylation and β-catenin signaling pathway in triple-negative breast cancer. Biochem Biophys Res Commun. 2023;664:69–76. 10.1016/j.bbrc.2023.03.077. [DOI] [PubMed] [Google Scholar]
- 113.Puri A, Mohite P, Maitra S, Subramaniyan V, Kumarasamy V, Uti DE, Sayed AA, El-Demerdash FM, Algahtani M, El-Kott AF, Shati AA, Albaik M, Abdel-Daim MM, Atangwho IJ. From nature to nanotechnology: The interplay of traditional medicine, green chemistry, and biogenic metallic phytonanoparticles in modern healthcare innovation and sustainability. Biomed Pharmacother. 2024;170: 116083. 10.1016/j.biopha.2023.116083. [DOI] [PubMed] [Google Scholar]
- 114.Devi A, Chaitanya NSN. Designing of peptide aptamer targeting the receptor-binding domain of spike protein of SARS-CoV-2: an in silico study. Mol Diversity. 2022;26:157–69. 10.1007/s11030-020-10171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalyane D, Polaka S, Vasdev N, Tekade RK. CD44-Receptor targeted gold-doxorubicin nanocomposite for pulsatile chemo-photothermal therapy of triple-negative breast cancer cells. Pharmaceutics. 2022;14:2734. 10.3390/pharmaceutics14122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li B, Li Q, Mo J, Dai H. Drug-loaded polymeric nanoparticles for cancer stem cell targeting. Front Pharmacol. 2017;8:51. 10.3389/fphar.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ertas YN, Abedi Dorcheh K, Akbari A, Jabbari E. Nanoparticles for targeted drug delivery to cancer stem cells: a review of recent advances. Nanomaterials. 2021;11:1755. 10.3390/nano11071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Köseer AS, Di Gaetano S, Arndt C, Bachmann M, Dubrovska A. Immunotargeting of cancer stem cells. Cancers. 2023. 10.3390/CANCERS15051608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dashtaki ME, Ghasemi S. CRISPR/Cas9-based gene therapies for fighting drug resistance mediated by cancer stem cells. Curr Gene Ther. 2022;23:41–50. 10.2174/1566523222666220831161225. [DOI] [PubMed] [Google Scholar]
- 120.He C, Jaffar Ali D, Qi Y, Li Y, Sun B, Liu R, Sun B, Xiao Z. Engineered extracellular vesicles mediated CRISPR-induced deficiency of IQGAP1/FOXM1 reverses sorafenib resistance in HCC by suppressing cancer stem cells. J Nanobiotechnol. 2023. 10.1186/s12951-023-01902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang S, Yang R, Ouyang Y, Shen Y, Hu L, Xu C. Cancer stem cells: a target for overcoming therapeutic resistance and relapse. Cancer Bio Med. 2024;20:985. 10.20892/j.issn.2095-3941.2023.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D’Antonio L, Fieni C, Ciummo SL, Vespa S, Lotti L, Sorrentino C, Di Carlo E. Inactivation of interleukin-30 in colon cancer stem cells via CRISPR/Cas9 genome editing inhibits their oncogenicity and improves host survival. J Immuno Therapy Cancer. 2023. 10.1136/jitc-2022-006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du Y, Luo L, Xu X, Yang X, Yang X, Xiong S, Yu J, Liang T, Guo L. Unleashing the power of synthetic lethality: augmenting treatment efficacy through synergistic integration with chemotherapy drugs. Pharmaceutics. 2023;15:2433. 10.3390/pharmaceutics15102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Canak-Ipek T, Avci-Adali M, EkentokAtici C, Şalva E, Özbas S. Chitosan-based delivery of CRISPR-Cas9 plasmid in breast cancer stem cells. J Res Pharm. 2023;27:86–96. 10.29228/jrp.292. [Google Scholar]
- 125.Yu AQ, Ding Y, Li CL, Yang Y, Yan SR, Li DS. TALEN-induced disruption of Nanog expression results in reduced proliferation, invasiveness and migration, increased chemosensitivity and reversal of EMT in HepG2 cells. Oncol Rep. 2016;35:1657–63. 10.3892/OR.2015.4483. [DOI] [PubMed] [Google Scholar]
- 126.Saraswat P, Chaturvedi A, Ranjan R. Zinc finger nuclease (ZFNs) and Transcription activator-like effector nucleases (TALENs) based genome editing in enhancement of anticancer activity of plants. In: plant-derived anticancer drugs in the omics era: biosynthesis, functions, and applications. pp. 281–293. Apple Academic Press (2023)
- 127.Allahyari E, Velaei K, Sanaat Z, Jalilzadeh N, Mehdizadeh A, Rahmati M. RNA interference: promising approach for breast cancer diagnosis and treatment. Cell Biol Int. 2023;47:833–47. 10.1002/CBIN.11979. [DOI] [PubMed] [Google Scholar]
- 128.Wong B, Birtch R, Rezaei R, Jamieson T, Crupi MJF, Diallo JS, Ilkow CS. Optimal delivery of RNA interference by viral vectors for cancer therapy. Mol Ther. 2023;31:3127–45. 10.1016/J.YMTHE.2023.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alnasser SM. Advances and challenges in cancer stem cells for onco-therapeutics. Stem Cells Int. 2023. 10.1155/2023/8722803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346:197–205. 10.1016/j.canlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 131.Shen X, Gao C, Li H, Liu C, Wang L, Li Y, Liu R, Sun C, Zhuang J. Natural compounds: Wnt pathway inhibitors with therapeutic potential in lung cancer. Front Pharmacol. 2023. 10.3389/fphar.2023.1250893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cotino-Nájera S, Herrera LA, Domínguez-Gómez G, Díaz-Chávez J. Molecular mechanisms of resveratrol as chemo and radiosensitizer in cancer. Front Pharmacol. 2023;14:1287505. 10.3389/fphar.2023.1287505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Torquato HF, Goettert MI, Justo GZ, Paredes-Gamero EJ. Anti-cancer phytometabolites targeting cancer stem cells. Curr Genomics. 2017;18:156. 10.2174/1389202917666160803162309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talib WH, Awajan D, Alqudah A, Alsawwaf R, Althunibat R, Abu AlRoos M, Al Safadi A, Abu Asab S, Hadi RW, Al Kury LT. Targeting cancer hallmarks with epigallocatechin gallate (EGCG): mechanistic basis and therapeutic targets. Molecules. 2024;29:1373. 10.3390/molecules29061373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Farooqi AA, Pinheiro M, Granja A, Farabegoli F, Reis S, Attar R, Sabitaliyevich UY, Xu B, Ahmad A. EGCG mediated targeting of deregulated signaling pathways and non-coding RNAs in DIFFERENT CANCERS: FOCUS On JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers. 2020;12:951. 10.3390/cancers12040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kamal R, Paul P, Thakur S, Singh SK, Awasthi A. Quercetin in oncology: a phytochemical with immense therapeutic potential. CDT. 2024;25:740–51. 10.2174/0113894501292466240627050638. [DOI] [PubMed] [Google Scholar]
- 137.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66:177–93. 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 138.Li E, Zhang T, Sun X, Li Y, Geng H, Yu D, Zhong C. Sonic hedgehog pathway mediates genistein inhibition of renal cancer stem cells. Oncol Lett. 2019. 10.3892/ol.2019.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naujokat C, McKee DL. The “big five” phytochemicals targeting cancer stem cells: curcumin, egcg, sulforaphane. Resveratrol Genistein CMC. 2021;28:4321–42. 10.2174/0929867327666200228110738. [DOI] [PubMed] [Google Scholar]
- 140.Ray PP, Islam MA, Islam MS, Han A, Geng P, Aziz MdA, Mamun AA. A comprehensive evaluation of the therapeutic potential of silibinin: a ray of hope in cancer treatment. Front Pharmacol. 2024;15:1349745. 10.3389/fphar.2024.1349745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu Y, Dong Z, Liu K. Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery. J Exp Clin Cancer Res. 2024;43:23. 10.1186/s13046-024-02949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia J, Guo P, Yang J, Zhang T, Pan K, Wei H. Piperine induces autophagy of colon cancer cells: dual modulation of AKT/mTOR signaling pathway and ROS production. Biochem Biophys Res Commun. 2024;728: 150340. 10.1016/j.bbrc.2024.150340. [DOI] [PubMed] [Google Scholar]
- 143.He K, Gan W-J. Wnt/β-catenin signaling pathway in the development and progression of colorectal cancer. CMAR. 2023;15:435–48. 10.2147/CMAR.S411168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tarawneh N, Hamadneh L, Abu-Irmaileh B, Shraideh Z, Bustanji Y, Abdalla S. Berberine inhibited growth and migration of human colon cancer cell lines by increasing phosphatase and tensin and inhibiting aquaporins 1, 3 and 5 expressions. Molecules. 2023;28:3823. 10.3390/molecules28093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun Q, Tao Q, Ming T, Tang S, Zhao H, Liu M, Yang H, Ren S, Lei J, Liang Y, Peng Y, Wang M, Xu H. Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine. 2023;114: 154792. 10.1016/j.phymed.2023.154792. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, Zhang T, Korkaya H, Liu S, Lee H-F, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sailo BL, Liu L, Chauhan S, Girisa S, Hegde M, Liang L, Alqahtani MS, Abbas M, Sethi G, Kunnumakkara AB. Harnessing sulforaphane potential as a chemosensitizing agent: a comprehensive review. Cancers. 2024;16:244. 10.3390/cancers16020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kurowska N, Madej M, Strzalka-Mrozik B. Thymoquinone: a promising therapeutic agent for the treatment of colorectal cancer. CIMB. 2023;46:121–39. 10.3390/cimb46010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shabani H, Karami MH, Kolour J, Sayyahi Z, Parvin MA, Soghala S, Baghini SS, Mardasi M, Chopani A, Moulavi P, Farkhondeh T, Darroudi M, Kabiri M, Samarghandian S. Anticancer activity of thymoquinone against breast cancer cells: Mechanisms of action and delivery approaches. Biomed Pharmacother. 2023;165: 114972. 10.1016/j.biopha.2023.114972. [DOI] [PubMed] [Google Scholar]
- 150.Khalaf RA, Awad M. Lycopene as a potential bioactive compound: chemistry, extraction, andanticancer prospective. CCDT. 2023;23:634–42. 10.2174/1568009623666230131124236. [DOI] [PubMed] [Google Scholar]
- 151.Koukourakis IM, Platoni K, Kouloulias V, Arelaki S, Zygogianni A. Prostate cancer stem cells: biology and treatment implications. IJMS. 2023;24:14890. 10.3390/ijms241914890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Verma P, Shukla N, Kumari S, Ansari MS, Gautam NK, Patel GK. Cancer stem cell in prostate cancer progression, metastasis and therapy resistance. Biochimica et Biophysica Acta Rev Cancer. 2023;1878:188887. 10.1016/j.bbcan.2023.188887. [DOI] [PubMed] [Google Scholar]
- 153.Naponelli V, Rocchetti MT, Mangieri D. Apigenin: molecular mechanisms and therapeutic potential against cancer spreading. IJMS. 2024;25:5569. 10.3390/ijms25105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghanbari-Movahed M, Shafiee S, Burcher JT, Lagoa R, Farzaei MH, Bishayee A. Anticancer potential of apigenin and isovitexin with focus on oncogenic metabolism in cancer stem cells. Metabolites. 2023;13:404. 10.3390/metabo13030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pandey P, Khan F, Upadhyay TK. Deciphering the modulatory role of apigenin targeting oncogenic pathways in human cancers. Chem Biol Drug Des. 2023;101:1446–58. 10.1111/cbdd.14206. [DOI] [PubMed] [Google Scholar]
- 156.Qaed E, Al-Hamyari B, Al-Maamari A, Qaid A, Alademy H, Almoiliqy M, Munyemana JC, Al-Nusaif M, Alafifi J, Alyafeai E, Safi M, Geng Z, Tang Z, Ma X. Fisetin’s promising antitumor effects: uncovering mechanisms and targeting for future therapies. Glob Med Genet. 2023;10:205–20. 10.1055/s-0043-1772219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mazurakova A, Koklesova L, Csizmár SH, Samec M, Brockmueller A, Šudomová M, Biringer K, Kudela E, Pec M, Samuel SM, Kassayova M, Hassan STS, Smejkal K, Shakibaei M, Büsselberg D, Saso L, Kubatka P, Golubnitschaja O. Significance of flavonoids targeting PI3K/Akt/HIF-1α signaling pathway in therapy-resistant cancer cells–a potential contribution to the predictive, preventive, and personalized medicine. J Adv Res. 2024;55:103–18. 10.1016/j.jare.2023.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar RM, Kumar H, Bhatt T, Jain R, Panchal K, Chaurasiya A, Jain V. Fisetin in cancer: attributes, developmental aspects, and nanotherapeutics. Pharmaceuticals. 2023;16:196. 10.3390/ph16020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J, Zhang D, Wang S, Yu P, Sun J, Zhang Y, Meng X, Li J, Xiang L. Baicalein induces apoptosis by inhibiting the glutamine-mTOR metabolic pathway in lung cancer. J Adv Res. 2024. 10.1016/j.jare.2024.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu P, Li J, Luo Y, Sun J, Hu Y, Lin B, Meng X, Xiang L. Mechanistic Role of Scutellaria baicalensis Georgi in Breast Cancer Therapy. Am J Chin Med. 2023;51:279–308. 10.1142/S0192415X23500155. [DOI] [PubMed] [Google Scholar]
- 161.Liu W, Qaed E, Zhu Y, Tian W, Wang Y, Kang L, Ma X, Tang Z. Research progress and new perspectives of anticancer effects of emodin. Am J Chin Med. 2023;51:1751–93. 10.1142/S0192415X23500787. [DOI] [PubMed] [Google Scholar]
- 162.Gao Y, Li Y, Zhu Y, Luo Q, Lu Y, Wen K, Du B, Xi X, Li G. Emodin is a potential drug targeting CD44-positive hepatocellularcancer. CCDT. 2024;24:510–8. 10.2174/0115680096256913231101103719. [DOI] [PubMed] [Google Scholar]
- 163.Fernandes S, Vieira M, Prudêncio C, Ferraz R. Betulinic acid for glioblastoma treatment: reality challenges and perspectives. IJMS. 2024;25:2108. 10.3390/ijms25042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adepoju FO, Duru KC, Li E, Kovaleva EG, Tsurkan MV. Pharmacological potential of betulin as a multitarget compound. Biomolecules. 2023;13:1105. 10.3390/biom13071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu L, Li J, Hou N, Han F, Sun X, Li Q. 20(S)-Ginsenoside Rh2 inhibits hepatocellular carcinoma by suppressing angiogenesis and the GPC3-mediated Wnt/β-catenin signaling pathway. ABBS. 2024. 10.3724/abbs.2024038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li S, Han W, He Q, Wang Y, Jin G, Zhang Y. Ginsenoside Rh2 suppresses colon cancer growth by targeting the miR-150-3p/SRCIN1/Wnt axis. ABBS. 2023;55:633–48. 10.3724/abbs.2023032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang J, Mu H-J, Sun Y-L, Yuan B, Wang Y. Use of honokiol in lung cancer therapy: a mini review of its pharmacological mechanism. J Asian Nat Prod Res. 2023;25:1029–37. 10.1080/10286020.2023.2193695. [DOI] [PubMed] [Google Scholar]
- 168.Prasher P, Fatima R, Sharma M, Tynybekov B, Alshahrani AM, Ateşşahin DA, Sharifi-Rad J, Calina D. Honokiol and its analogues as anticancer compounds: current mechanistic insights and structure-activity relationship. Chem Biol Interact. 2023;386: 110747. 10.1016/j.cbi.2023.110747. [DOI] [PubMed] [Google Scholar]
- 169.Mikhaevich EI, Sorokin DV, Scherbakov AM. Honokiol inhibits the growth of hormone-resistant breast cancer cells: its promising effect in combination with metformin. Res Pharm Sci. 2023;18:580–91. 10.4103/1735-5362.383712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ezzat MS, Merghany MR, AbdelBaki MP, AliAbdelrahim N, Osman MS, Salem A, Peña-Corona S, Cortés H, Kiyekbayeva L, Leyva-Gómez G, Sharifi-Rad J. Nutritional sources and anticancer potential of phenethyl isothiocyanate: molecular mechanisms and therapeutic insights. Mol Nutrit Food Res. 2024;68:2400063. 10.1002/mnfr.202400063. [DOI] [PubMed] [Google Scholar]
- 171.Yuan Y, Long H, Zhou Z, Fu Y, Jiang B. PI3K–AKT-targeting breast cancer treatments: natural products and synthetic compounds. Biomolecules. 2023;13:93. 10.3390/biom13010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rizwan D, Masoodi FA. Brassica -derived isothiocyanates as anticancer therapeutic agents and their nanodelivery. Phytother Res. 2024;38:331–48. 10.1002/ptr.8042. [DOI] [PubMed] [Google Scholar]
- 173.Ren Q, Wang Q, Zhang X, Wang M, Hu H, Tang J, Yang X, Ran Y, Liu H, Song Z, Liu J, Li X. Anticancer activity of diosgenin and its molecular mechanism. Chin J Integr Med. 2023;29:738–49. 10.1007/s11655-023-3693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mohseni-Moghaddam P, Khanmohammadi M, Roghani M. Literature review on hepatoprotective effects of diosgenin: possible mechanisms of action. Front Pharmacol. 2023;14:1226548. 10.3389/fphar.2023.1226548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ebrahimi N, Afshinpour M, Fakhr SS, Kalkhoran PG, Shadman-Manesh V, Adelian S, Beiranvand S, Rezaei-Tazangi F, Khorram R, Hamblin MR, Aref AR. Cancer stem cells in colorectal cancer: Signaling pathways involved in stemness and therapy resistance. Crit Rev Oncol Hematol. 2023;182: 103920. 10.1016/j.critrevonc.2023.103920. [DOI] [PubMed] [Google Scholar]
- 176.Ghorbani R, Gharbavi M, Sharafi A, Rismani E, Rezaeejam H, Mortazavi Y, Johari B. Targeted anti-tumor synergistic effects of Myc decoy oligodeoxynucleotides-loaded selenium nanostructure combined with chemoradiotherapy on LNCaP prostate cancer cells. Oncol Res. 2023;32:101–25. 10.32604/or.2023.044741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johari B, Tavangar-Roosta S, Gharbavi M, Sharafi A, Kaboli S, Rezaeejam H. Suppress the cell growth of cancer stem-like cells (NTERA-2) using Sox2-Oct4 decoy oligodeoxynucleotide−encapsulated niosomes-zinc hybrid nanocarriers under X-irradiation. Heliyon. 2024;10: e34096. 10.1016/j.heliyon.2024.e34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Labrie M, Brugge JS, Mills GB, Zervantonakis IK. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat Rev Cancer. 2022;22:323–39. 10.1038/S41568-022-00454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shams F, Pourjabbar B, Hashemi N, Farahmandian N, Golchin A, Nuoroozi G, Rahimpour A. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: from mechanisms to therapy. Biomed Pharmacother. 2023;167: 115505. 10.1016/J.BIOPHA.2023.115505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used are available within the manuscript.