Abstract
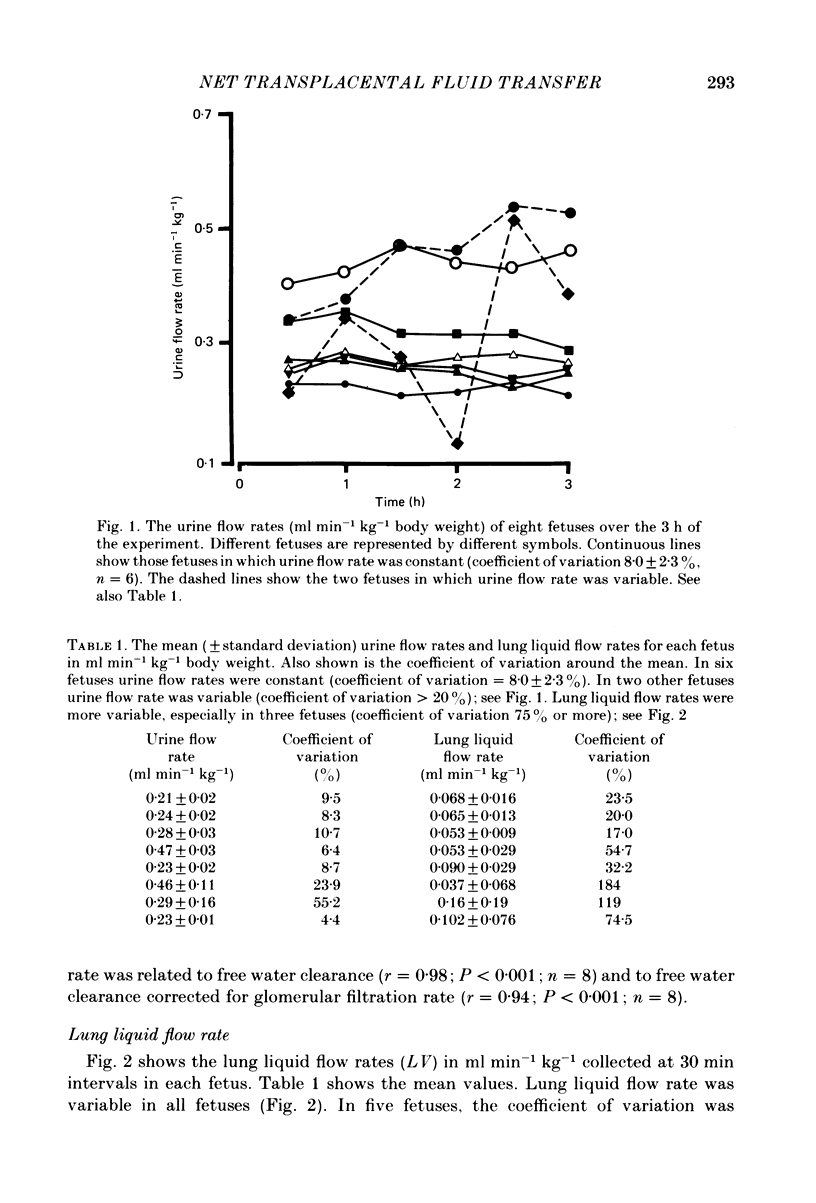
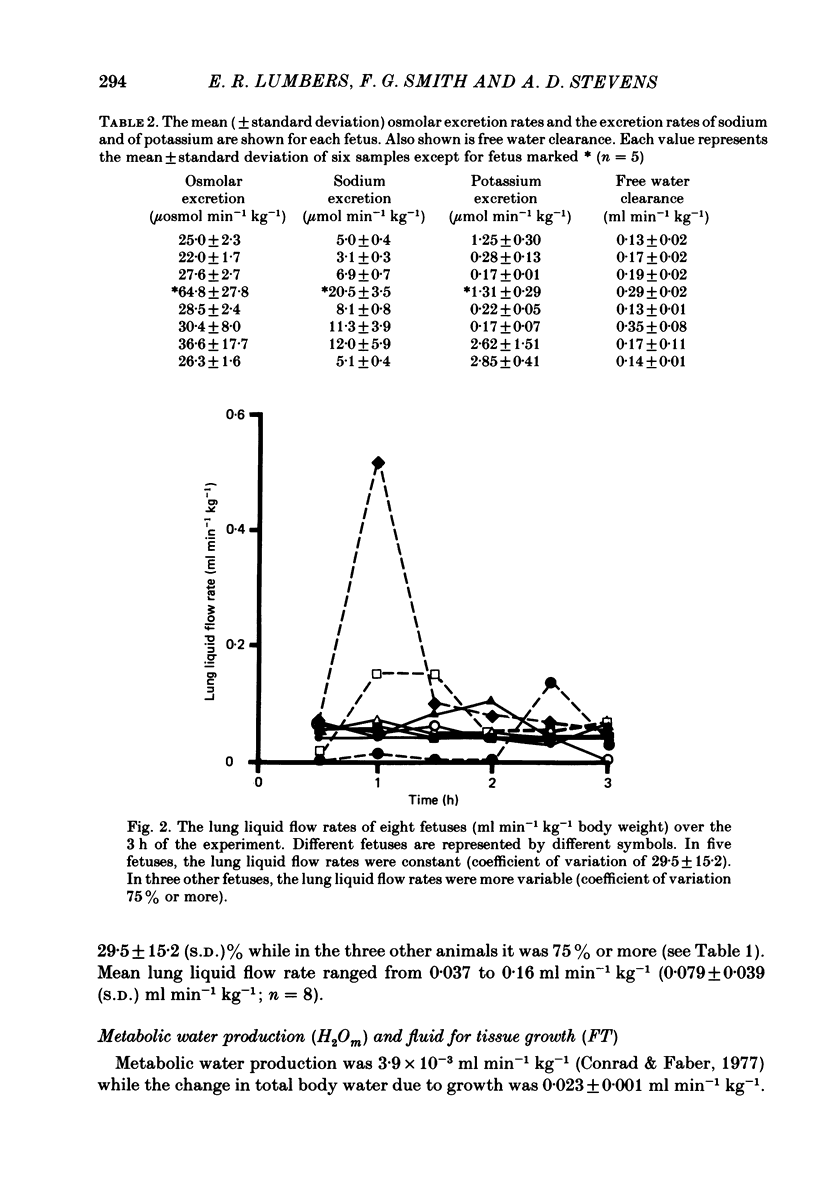
If fetal drinking activity is prevented and it is assumed that in the latter third of gestation the fetus is capable of maintaining itself in fluid balance, then the net amount of fluid gained across the placenta by the fetus is equal to the amount of fluid lost from the fetus, by routes other than the placenta, plus fluid deposited in growing tissues minus the amount of water produced as a result of oxidative metabolism. Net transplacental transfer of fluid to the fetus over a 3 h period was measured in eight chronically catheterized fetal sheep in which drinking activity was prevented by ligating the oesophagus. Urine and lung liquid flow rates were measured. In the latter third of gestation, these are the only significant sources of fluid loss from these fetuses during the 3 h experimental period. Water produced as a result of oxidative metabolism was calculated, as was the amount of fluid deposited in growing tissues during the course of the experiment. The weight of the fetus at the beginning of the experiment and the change in weight that occurred during the experiment was calculated by measuring the weight of the fetus at death (within 30 h) and applying an equation which describes the body weight-gestation age relationship for merino sheep. Net transplacental fluid transfer was 0.40 +/- 0.09 ml min-1 kg-1 (range 0.30-0.54 ml min-1 kg-1). Fetal urine flow rate averaged 0.30 +/- 0.11 ml min-1 kg-1. It was 72.8 +/- 10.0% of the volumes used to calculate net transplacental fluid transfer to the fetus. Lung liquid flow rate was 0.079 +/- 0.039 ml min-1 kg-1. It was 20.2 +/- 9.2% of the volumes used to calculate net fluid intake. The amount of fluid deposited as a result of tissue growth was 0.023 +/- 0.001 ml min-1 kg-1; it was 5.94 +/- 1.1% of the volumes used in the equation, while the production of water as a result of metabolism was 3.9 X 10(-3) ml min-1 kg-1 (Conrad & Faber, 1977) and constituted 1.01 +/- 0.22% of the volumes used in the equation. This method of measuring net transplacental fluid transfer to the fetus can be used to measure fetal fluid intake over relatively short periods of time. It also means that the effects of disturbances in maternal fluid and electrolyte balance on fluid transfer to the fetus can be studied and quantitated.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. Quantitative development of adipose tissue in foetal sheep. Aust J Biol Sci. 1978 Oct;31(5):489–503. doi: 10.1071/bi9780489. [DOI] [PubMed] [Google Scholar]
- Armentrout T., Katz S., Thornburg K. L., Faber J. J. Osmotic flow through the placental barrier of chronically prepared sheep. Am J Physiol. 1977 Oct;233(4):H466–H474. doi: 10.1152/ajpheart.1977.233.4.H466. [DOI] [PubMed] [Google Scholar]
- BRUNS P. D., HELLEGERS A. E., SEEDS A. E., Jr, BEHRMAN R. E., BATTAGLIA F. C. EFFECTS OF OSMOTIC GRADIENTS ACROSS THE PRIMATE PLACENTA UPON FETAL AND PLACENTAL WATER CONTENTS. Pediatrics. 1964 Sep;34:407–411. [PubMed] [Google Scholar]
- BURNS P. D., LINDER R. O., DROSE V. E., BATTAGLIA F. The placental transfer of water from fetus to mother following the intravenous infusion of hypertonic mannitol to the maternal rabbit. Am J Obstet Gynecol. 1963 May 15;86:160–167. doi: 10.1016/0002-9378(63)90425-3. [DOI] [PubMed] [Google Scholar]
- Conrad E. E., Jr, Faber J. J. Water and electrolyte acquisition across the placenta of the sheep. Am J Physiol. 1977 Oct;233(4):H475–H487. doi: 10.1152/ajpheart.1977.233.4.H475. [DOI] [PubMed] [Google Scholar]
- Dreszer M. Fluid and electrolyte requirements in the newborn infant. Pediatr Clin North Am. 1977 Aug;24(3):537–546. doi: 10.1016/s0031-3955(16)33463-0. [DOI] [PubMed] [Google Scholar]
- Egan E. A., Olver R. E., Strang L. B. Changes in non-electrolyte permeability of alveoli and the absorption of lung liquid at the start of breathing in the lamb. J Physiol. 1975 Jan;244(1):161–179. doi: 10.1113/jphysiol.1975.sp010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN E. A., GRAY M. J., HUTCHINSON D. L., PLENTL A. A. The role of the monkey fetus in the exchange of the water and sodium of the amniotic fluid. J Clin Invest. 1959 Jun;38(6):961–970. doi: 10.1172/JCI103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J. J., Green T. J. Foetal placental blood flow in the lamb. J Physiol. 1972 Jun;223(2):375–393. doi: 10.1113/jphysiol.1972.sp009853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Hansen B. Body water metabolism in early infancy. Acta Paediatr Scand Suppl. 1982;296:44–48. doi: 10.1111/j.1651-2227.1982.tb09594.x. [DOI] [PubMed] [Google Scholar]
- Langlands J. P., Sutherland H. A. An estimate of the nutrients utilized for pregnancy by Merino sheep. Br J Nutr. 1968 May;22(2):217–227. doi: 10.1079/bjn19680027. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R. A brief review of fetal renal function. J Dev Physiol. 1984 Feb;6(1):1–10. [PubMed] [Google Scholar]
- Lumbers E. R., Stevens A. D. Changes in fetal renal function in response to infusions of a hyperosmotic solution of mannitol to the ewe. J Physiol. 1983 Oct;343:439–446. doi: 10.1113/jphysiol.1983.sp014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher E. J., Platzker A. C., Ballard P. L., Kitterman J. A., Clements J. A., Tooley W. H. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. J Appl Physiol. 1975 Dec;39(6):1017–1021. doi: 10.1152/jappl.1975.39.6.1017. [DOI] [PubMed] [Google Scholar]
- Rattray P. V., Garrett W. N., East N. E., Hinman N. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J Anim Sci. 1974 Mar;38(3):613–626. doi: 10.2527/jas1974.383613x. [DOI] [PubMed] [Google Scholar]
- Stevens A. D., Lumbers E. R. The relationship between plasma renin activity and renal electrolyte excretion in the fetal sheep. J Dev Physiol. 1981 Apr;3(2):101–110. [PubMed] [Google Scholar]
- Wilbur W. J., Power G. G., Longo L. D. Water exchange in the placenta: a mathematical model. Am J Physiol. 1978 Sep;235(3):R181–R199. doi: 10.1152/ajpregu.1978.235.3.R181. [DOI] [PubMed] [Google Scholar]


