Abstract
This research employs the density functional theory method to study the coordination and capture of the “five toxic” heavy metal ions (Pb, Hg, Cr, Cd, and arsenic) by quinoline—derivative molecular probes. The study has determined the microscopic molecular configurations, structural parameters, and natural charge distributions of the coordinating atoms in the optimal structures of the complexes formed by the probes and each heavy metal ion. Additionally, the coordination bond orders of all the complexes have been calculated and analyzed. Furthermore, by calculating the infrared vibration frequencies of the optimal structures, the stability of the structures has been verified. Moreover, the binding energies, frontier molecular orbital energy levels, and energy gap values of the complexes formed by the probes and each heavy metal ion through capture have been further obtained, and the changes in the ultraviolet—visible absorption spectra and molecular fluorescence spectra of the probes have been analyzed. The study also investigated the probe’s ability to capture and detect the “five toxic” heavy metal ions.
Keywords: “five—toxic” heavy metal ions, Quinoline derivatives, Fluorescent probes, Heavy metal detection and treatment
Subject terms: Environmental sciences, Environmental chemistry
Introduction
Heavy metal pollution is an extremely serious major environmental problem in today’s society, posing far—reaching and severe potential hazards to human health and the ecological environment1,2. In the fields of environmental science and public health, the so—called "five—toxic heavy metals", namely mercury, lead, cadmium, chromium, and arsenic, are frequently highlighted3. These ions can cause severe acute toxic reactions in organisms even at relatively low doses4,5. For example, in LD50 experiments, half of the experimental rats may die after ingesting a very small amount of mercury compounds orally6. Heavy metal ions can also cause rapid damage to organs and systems7–10. For instance, when a certain amount of lead ions enter the body, they can quickly affect the nervous and blood systems10–12. In cases of acute lead poisoning, it may also rapidly lead to nervous system dysfunction. Heavy metal ions can also exhibit chronic toxicity13,14. For example, cadmium ions can gradually disrupt the normal functions of kidney cells and may even lead to chronic renal failure15. Arsenic ions and chromium ions, especially hexavalent chromium, accumulate in the human body and are highly carcinogenic, potentially leading to cellular canceration16,17. Mercury exists in various forms in the environment, including inorganic mercury and organic mercury. Organic mercury (such as methylmercury) is highly resistant to decomposition in the environment, and it can accumulate in organisms and be transferred through the food chain, demonstrating strong environmental persistence18. When plankton ingest trace amounts of methylmercury, its concentration can increase significantly as it moves up the food chain, potentially reaching levels many times higher at the top of the chain19.
There are numerous methods for detecting heavy metal ions. Among them, the molecular probe detection method is typically economical, fast, and simple. The probe is a type of ligand that can specifically recognize and bind to heavy metal ions20. It is a molecule capable of indicating the presence, concentration, and other information of heavy metal ions through the detectable signal changes after binding to heavy metal ions, and it is an important means for qualitative and quantitative detection. Structurally, molecular probes generally consist of a recognition group and a multidentate ligand structure21. The former is mainly used to recognize and bind to heavy metal ions, while the latter enhances recognition specificity and binding affinity, making it an efficient approach for detection in the presence of multiple coexisting ions22. Additionally, a signal—reporting unit is also required. When the probe is not bound to heavy metal ions, intramolecular charge transfer, structural rearrangement, and other processes can alter the intensity and position of the absorption peak in the ultraviolet–visible absorption spectrum, leading to changes in the fluorescence emission wavelength, intensity, etc.22–24. Among them, fluorescence signal changes exhibit higher sensitivity in probe applications25.
Molecular fluorescent probes are a class of compounds with specific structures, that can recognize and monitor specific molecules or ions through changes in fluorescent signals26. These probes typically consist of one or more fluorophores, a recognition unit, and a sensing unit. The fluorophore itself has the ability to generate fluorescent signals27. The recognition unit is responsible for specifically binding to the target molecule or ion, while the sensing unit facilitates binding and induces changes in the fluorescent signals. Molecular fluorescent probes are widely used in heavy metal ion detection and have remarkable characteristics27. Firstly, they are highly sensitive. Fluorescent probes can detect extremely low concentrations of heavy metal ions, which is particularly crucial for environmental monitoring and biomedical research, as applications in these fields often require the detection of pollutants at extremely low concentrations. Secondly, they are highly selective. The recognition units used to construct fluorescent probes are typically designed with precision to specifically recognize a particular heavy metal ion and generate a significant fluorescent signal. Simultaneously, these units are engineered to minimize interference from other ions, either by not reacting with them or by producing signals that are significantly weaker or different. This design enhances the accuracy and reliability of detection26. Fluorescent probes can also be used for real—time monitoring of the dynamics of heavy metal ions in organisms or the environment, providing a powerful tool for studying the behavior and effects of these ions. Fluorescent probes are generally easy to detect, as the detection of fluorescent signals typically only requires the use of devices such as fluorescence spectrometers. These devices are user—friendly, and the fluorescent signals are intuitive, facilitating observation and analysis28. The applications of fluorescent probes are extensive. They can be used not only in laboratory research but also in multiple fields such as on-site monitoring, food safety, and environmental pollution control. The recognition response of fluorescent probes in heavy metal ion detection is rapid, which aids in the timely discovery and treatment of heavy metal pollution problems. With the development of molecular design and synthesis technologies, new types of molecular fluorescent probes are continuously being developed. These probes exhibit improved performance in terms of accuracy and efficiency in heavy metal ion detection, which is of great significance for environmental protection and public health.
In terms of microscopic basic structures, molecular fluorescent probes primarily consist of the conjugated structure of molecules, electron arrangement, as well as the rigid and planar structures of molecules29–31. Among these, the conjugated structure enhances electron delocalization within molecules, enabling electrons to transition among multiple atoms, thereby improving the fluorescence efficiency of the molecules29. Common conjugated structures include aromatic rings, heterocycles, and conjugated double bonds. The electron arrangement in molecules is also critical for fluorescence emission. A favorable electron arrangement promotes the stabilization of the excited state, thereby increasing the probability of fluorescence emission30. For example, the π—π* transition (from the ground state to the excited state of the π-electron cloud) is the primary mechanism for fluorescence emission in many aromatic compounds32.
When a molecule contains both electron donors and electron acceptors, a charge-transfer complex can be formed. This complex is typically more stable in the excited state than a single molecule, thereby promoting fluorescence emission. Rigid and planar molecular structures help reduce vibrational energy in the excited state, thus improving the efficiency of fluorescence emission. Quinoline and its derivatives are excellent metal-ion chelators33. Additionally, quinoline itself possesses a rigid structure, a large conjugated system, and good water solubility34. As a result, it readily forms complexes with metal ions and is well-suited as a fluorescence—enhanced molecular probe for the detection of metal ions. (((pyridine—2,6—diylbis(methylene))bis(oxy))bis(quinoline—8,5—diyl))dimethanol (hereinafter referred to as “Probe” for short) is a typical quinoline derivative, which has the most basic structural characteristics and requirements of a fluorescent probe. It will be collectively referred to as “Probe” hereafter, and its molecular structure is illustrated in Fig. 1.
Fig. 1.

The molecular structure diagram of Probe.
This molecule not only exhibits good water solubility but also contains two fluorophores (quinoline groups). Upon capturing suitable metal ions, if the resulting metal complex adopts a rigid planar structure, the fluorescence intensity can be significantly enhanced, thereby achieving the desired effect of a fluorescent molecular probe. Furthermore, due to the presence of excellent ligand-binding sites in the fluorescent probe, it can also be applied to the capture and treatment of heavy metal ions in water. If Probe is utilized for research on the microscopic-level identification and detection of typical "five-toxic heavy metal" ions such as Pb, Hg, Cr, Cd, and As, the research findings will provide significant support for the application of quinoline-type probe molecules in the monitoring and treatment of heavy metal ions in aquatic environments, demonstrating substantial research value. With advancements in computer technology, as observed in previous studies, the results of computer-simulated predictions using density functional theory can exhibit a high degree of consistency with experimental research results35–39. In our preliminary studies, we have also confirmed that the structural data of the complexes obtained using the B3LYP method within density functional theory (DFT) exhibit a high degree of consistency with the single-crystal structural data obtained experimentally36. By employing DFT techniques, this research investigates the detection and capture of heavy metal toxic ions using probes. If significant probe detection signals and the ability to capture heavy metal ions are observed, it will contribute to the development of innovative solutions for real-time monitoring and remediation of heavy metal pollution in aquatic environments. This holds immense potential for advancing environmental protection and public health initiatives. Furthermore, this study establishes a foundation for sustainable strategies aimed at addressing heavy metal pollution, thereby promoting long-term ecological and societal benefits.
Search methods, contents and processes
The research employs Gaussian 16 quantum computational chemistry software and selects the B3LYP computational method in DFT40,41. For elements such as C, O, N, Cl and H, the 6—311 g* atomic basis set is adopted; for atoms such as Pb, Hg, Cr, Cd and As, the LanL2MB basis set is used42–45. Using water as the solvent under the SMD model, the structural optimization of the probe molecule is performed before and after capturing Pb, Hg, Cr, Cd and As; and under the optimal structures of the probe and the complexes formed by the probe and various heavy—metal ions (Pb, Hg, Cr, Cd and As), infrared vibration frequency analysis is carried out, and the stability of the optimal structures of the probe and each complex is confirmed by the absence of imaginary frequencies in the frequencies. For the probe under the optimal structure and the complexes formed by the probe and various metals, analyses are carried out in terms of structural parameters, Wiberg Bond Indices (WBIs), frontier molecular orbital energy levels, natural charges, etc. Moreover, ultraviolet—visible (UV–Vis) absorption calculations are carried out under the optimal stable structure, and on the basis of the UV–Vis absorption state structure, fluorescence spectral analysis and exploration are carried out on the emission light from the easily excited state back to the original ground state.
Research results and analysis
Structural parameter
Figure 2 shows the structural optimization diagrams of the complexes formed after the Probe captures heavy—metal ions such as Pb, Hg, Cr, Cd, and As, specifically including Probe—Pb, Probe—Hg, Probe—Cr, Probe—Cd, and Probe—As. From the figure, it can be clearly observed that the structure of the Probe—Pb complex exhibits perfect planarity, and the Pb atom is located in the central position of the complex, showing good symmetry. Based on the basic conditions for fluorescence generation46, it can be speculated that Probe—Pb may have a relatively strong fluorescence intensity.
Fig. 2.
The structural optimization diagrams of the probe and the complexes formed by the probe and various heavy—metal ions.
In contrast, the aromatic rings on both sides of the Probe—Cd complex show a certain angular misalignment, and the Cd atom is located between the two misaligned planes. The Probe—Hg complex is rather unique. The aromatic ring at the lower part of its structure is almost in an upright state, while the left—and—right—side aromatic ring systems and the Hg atom are well maintained in the same plane. However, the structures of Probe—Cr and Probe—As are significantly disrupted. Table 1 lists the data of bond lengths, bond angles, and main dihedral angles formed between the metal center and the coordinating atoms. Notably, when the bond lengths formed between the metal ions and the N and O atoms in the probe are within the range of 0.25—0.26 nm, the metal atom can be exactly located at the center of the cavity of the probe molecule, and the central plane of the probe will not be disrupted. In Probe—Pb, the bond lengths of Pb—N and Pb—O happen to fall within this range.
Table 1.
The main bond lengths (nm), bond angles (°), and dihedral angles (°) of the optimized complexes Probe-Pb, Probe-Hg, Probe-Cr, Probe-Cd and Probe-As.
| complex | Probe-Pb | Probe-Hg | Probe-Cr | Probe-Cd | Probe-As |
|---|---|---|---|---|---|
| N1-X | 0.2603 | 0.3015 | 0.1945 | 0.2691 | 0.2066 |
| N2-X | 0.2603 | 0.3014 | 0.1970 | 0.2841 | 0.3037 |
| O1-X | 0.2576 | 0.2910 | 0.2143 | 0.2727 | 0.2710 |
| O2-X | 0.2575 | 0.2910 | 0.2355 | 0.2691 | 0.3510 |
| N3-X | 0.2521 | 0.2973 | 0.1929 | 0.2705 | 0.3584 |
| N1-X-N2 | 104.64 | 90.46 | 98.23 | 79.77 | -159.66 |
| N2-X-O2 | 62.81 | 53.63 | 74.69 | 56.80 | 47.0 |
| O1-X-O2 | 130.18 | 106.72 | 110.90 | 106.96 | 102.94 |
| O2-X-N3 | 65.09 | 56.93 | 72.47 | 57.29 | 49.71 |
| N3-X-O1 | 65.09 | 56.94 | 78.03 | 59.27 | 53.22 |
| O1-X-N1 | 62.79 | 53.64 | 78.40 | 59.80 | 68.32 |
| N1-N2-X-O2 | 174.36 | 132.28 | 4.33 | 154.96 | -159.66 |
| N2-O2-X-N3 | -175.09 | 148.06 | -139.77 | − 131.46 | 175.09 |
| O2-N3-X-O1 | 180.00 | 146.46 | 116.70 | -141.73 | -179.49 |
| N3-O1-X-N1 | 175.08 | 148.08 | -111.89 | -160.41 | -166.62 |
| O1-N1-X-N2 | -174.37 | 132.26 | 151.62 | -114.71 | -173.71 |
| N1-N2-O2-O1 | -5.54 | 0.01 | 20.04 | -19.17 | 7.72 |
For Probe—Hg, its Hg—O and Hg—N bond lengths are both longer than the corresponding Pb—O and Pb—N bond lengths, which is also the main reason why the lower—side aromatic ring of the Probe—Hg complex is distorted and deviates from the plane where the two—side aromatic rings and Hg are located. Additionally, the radius of the Cr ion is significantly smaller than that of Pb and Hg atoms. When the Probe—Cr complex is formed, due to the formation of Cr—N and Cr—O bonds, the aromatic rings where the coordinating atoms O and N are located will be squeezed towards the center, making it almost impossible for Probe—Cr to remain in one plane, thus lacking the possibility of generating fluorescence. Finally, the radius of As is even smaller and it cannot form effective coordination bonds with all the coordinating atoms, resulting in the loss of rigidity of the Probe—As complex. This will make Probe—As lose the possibility of generating fluorescence and thus not possess the fundamental conditions for being a fluorescent probe46.
WIBs
WIBs is a concept used to measure the relative strength of a chemical bond. In traditional covalent bonds, the bond order reflects the number of shared electron pairs between two atoms. For example, the bond order of a hydrogen molecule ( H − H ) is 1, indicating that two hydrogen atoms share one pair of electrons. For a double bond, such as the carbon—carbon bond in ethylene ( C = C ), its bond order is 2 because two carbon atoms share two pairs of electrons; in a triple bond, like the carbon—carbon bond in acetylene ( C≡C ), the bond order is 3, meaning three pairs of electrons are shared. Generally speaking, the higher the bond order, the shorter the bond length, and there is a positive correlation between bond order and bond energy. A higher bond order often implies that the molecule has greater stability47,48.
Table 2 shows the WIBs complexes formed by Probe with Pb, Hg, Cr, Cd, and As. Obviously, in the Probe—Cr complex, the bond orders of N—Cr and O—Cr are higher than those of other complexes, and correspondingly their coordination bond lengths are the shortest. Since Probe has a relatively large space but forms relatively short coordination bonds, this is exactly the reason why the plane where Probe—Cr is located in Fig. 2 is distorted. The bond orders of all the coordination bonds at the same positions in Probe—Pb are greater than those of all the coordination bonds except Probe—Cr. This indicates that the coordination effect between Probe and Pb ions is stronger, while the coordination effects with Hg, Cd, and As are relatively weaker.
Table 2.
Wiberg bond indices of the heavy metal complexes compared to the heme.
| complex | Probe-Pb | Probe-Hg | Probe-Cr | Probe-Cd | Probe-As |
|---|---|---|---|---|---|
| N1-X | 0.4229 | 0.1566 | 0.8558 | 0.2388 | 0.0000 |
| N2-X | 0.3651 | 0.1536 | 0.8098 | 0.2546 | 0.0740 |
| N3-X | 0.3646 | 0.1535 | 0.7899 | 0.2332 | 0.7853 |
| O1-X | 0.1765 | 0.1030 | 0.3793 | 0.1375 | 0.0105 |
| O2-X | 0.1766 | 0.1029 | 0.2982 | 0.1504 | 0.0800 |
Infrared vibration spectrum
Figure 3 shows the infrared vibration spectra of Probe and the complexes formed by Probe with Pb, Hg, Cd, Cr and As (Probe—Pb, Probe—Hg, Probe—Cr, Probe—Cd and Probe—As) after structural optimization. Due to the differences in the radii of various metal ions, the formed complexes exhibit distinct fingerprint peaks. As Probe binds to each heavy—metal ion, the vibration absorption peaks at 3085 and 3215 cm⁻1 disappear. There are no imaginary frequencies in Probe, Probe—Pb, Probe—Hg, Probe—Cr, Probe—Cd and Probe—As, indicating that the complexes in all optimal structures are in a stable state. The structural parameters and WIBs bond orders in this state can serve as important bases for Probe to recognize heavy—metal ions, and are also the structural prerequisites for the analysis of frontier molecular orbital energy levels, binding energies and ultraviolet—visible absorption. This also shows that our analysis of structural parameters and WIBs is carried out based on all molecules being in optimal and stable structures.
Fig. 3.
Infrared vibration spectra of the probe and the complexes formed by the probe with various metal ions.
Natural charge
The reason why natural charges in Natural Bond Orbital (NBO) analysis are adopted in this study is that NBO analysis localizes molecular orbitals, transforming them into bonding and lone—pair electron orbitals that are more in line with chemically intuitive concepts49. Natural charges are atomic charges calculated based on the electron—occupation situation of these localized orbitals. Compared with Mulliken charges, natural charges are more intuitive in chemical meaning and their results are more stable. In coordination compounds, natural charges are helpful for determining which atoms are coordination atoms. Generally speaking, the distribution of natural charges on atoms can reflect the density of their electron clouds. Coordination atoms are usually those with high electron—cloud density (i.e., relatively more negative charges) or low electron—cloud density (i.e., relatively more positive charges). For example, in metal—organic coordination compounds, atoms on organic ligands with lone—pair electrons and relatively negative natural charges (such as nitrogen atoms in nitrogen—containing ligands) are often coordination atoms because they can form coordination bonds with the central metal ions by providing lone—pair electrons.
The static charge situations of the coordination atoms in various complexes are shown in Table 3. It can be clearly seen that after Probe forms various heavy—metal complexes, the negative charges of the coordination atoms are basically reduced to some extent. However, in Probe—Cr, due to the destruction of the Probe planar structure, the negative charge on its N2 atom does not decrease.
Table 3.
The Nature Charge distributions of the complexes compared to the heme.
| complex | N1 | N2 | N3 | O1 | O2 |
|---|---|---|---|---|---|
| Probe | -0.2035 | -0.2020 | -0.2075 | -0.1595 | -0.1536 |
| Probe-Pb | -0.1592 | -0.1592 | -0.1709 | -0.1199 | -0.1199 |
| Probe-Hg | -0.1623 | -0.1623 | -0.1723 | -0.1218 | -0.1218 |
| Probe-Cr | -0.1963 | -0.2089 | -0.1928 | -0.1359 | -0.1399 |
| Probe-Cd | -0.1578 | -0.1574 | -0.1701 | -0.1168 | -0.1165 |
| Probe-As | -0.0702 | -0.0742 | -0.0628 | -0.0868 | -0.0325 |
UV absorption spectrum
Generally speaking, complexes with conjugated systems usually have the ability to absorb wavelengths in the UV light region50. As a ligand containing a conjugated system, it may interact with the central metal ion, thereby changing the energy of electron transitions, resulting in ultraviolet—visible absorption spectra with different characteristic wavelengths. For example, when an organic ligand with a conjugated system coordinates with a metal ion, ligand—to—metal charge—transfer ( LMCT ) or metal—to—ligand charge—transfer ( MLCT ) transitions may occur, and these transitions will produce new absorption peaks in the ultraviolet—visible absorption spectrum, which can serve as a key basis for detecting heavy metal ions using the ligand molecule51. There is a certain relationship between the stability of complexes and the ultraviolet—visible absorption spectrum. More stable complexes have relatively stable structures in solution, and the positions and intensities of the characteristic peaks in their absorption spectra are also relatively stable. When the ligand Probe forms a complex, if the complex exhibits unstable phenomena such as dissociation or structural rearrangement, its ultraviolet—visible absorption spectrum will change, such as broadening of the absorption peak, weakening of the intensity, or shift of the position.
As shown in Fig. 4, when Probe forms complexes with various heavy metals, their ultraviolet—visible absorption spectra all change significantly. Especially for Probe—Pb, Probe—Cd, and Probe—Hg, their absorption intensities increase significantly and obvious red—shift phenomena occur. Their maximum absorption peaks are red—shifted from the original 344 nm to 630 nm, 364 nm, and 500 nm respectively. The reason for the red—shift is that after Probe forms Probe—Pb, Probe—Cd, and Probe—Hg, the planes where the heavy metal ions are located are basically coincident with the conjugated plane of the aromatic ring, enhancing the delocalization of the entire molecule. However, in Probe—Hg, the lower aromatic ring of the aromatic ring flips upward and is separated from the planar system of the molecule, so the range of red—shift that occurs is relatively small. The planarity of Probe—Pb is significantly higher than that of Probe—Cd. Pb is located right in the middle of the Probe—Pb planar molecular system, having the most efficient conjugacy and electron delocalization, which also makes the ultraviolet—visible absorption intensity of Probe—Pb the strongest and the wavelength the longest. Figure 4 also shows that after Probe forms the complexes Probe—Pb, Probe—Hg, and Probe—Cd, the ultraviolet—visible absorption peaks change significantly, indicating that Probe can be effectively used to detect heavy metal ions such as lead, mercury, and cadmium.
Fig. 4.

The probe and the ultraviolet—visible absorption spectrum of the probe in the same aqueous solvent.
Fluorescence intensity
The conjugated system has a significant impact on molecular fluorescence26,27. The larger the conjugated system, the greater the delocalization range of π electrons, making π—π* transitions easier to occur, thereby enhancing fluorescence intensity and causing a red shift in wavelength. Electron—donating substituents can enhance fluorescence, while electron—withdrawing substituents can weaken or even quench fluorescence. Planar structures help stabilize the conjugated system, promote the overlap of π-electron clouds, and thus enhance fluorescence; non-planar structures, on the other hand, tend to weaken fluorescence. Rigid structures can reduce non—radiative transitions, enhance fluorescence, and protect the conjugated system and planarity, further improving fluorescence properties52.
The emission fluorescence spectra of Probe and the complexes formed by the Probe with various heavy metal ions are shown in Fig. 5. It is evident that Probe-Pb exhibits the most intense fluorescence, demonstrating the longest optimal emission wavelength at 785.6 nm. The complex displays ideal planarity, with the Pb atom positioned at the center and maintaining excellent symmetry. This structural characteristic is crucial for fluorescence generation, as it facilitates efficient π-electron delocalization and π-π* transitions, thereby producing strong fluorescence intensity. What’ more, when the bond lengths between the metal ions (Pb, Hg, Cd) and the coordinating atoms (N and O) fall within the range of 0.25–0.26 nm, the metal atom is centrally located, and the probe’s planarity is preserved. This is observed in Probe-Pb, where Pb-N and Pb–O bond lengths are within this range, contributing to its strong fluorescence and stability. Following Probe-Pb, Probe-Hg and Probe-Cd show their optimal fluorescence wavelengths at 627.2 nm and 711.2 nm, respectively. The fluorescence intensity of Probe—Hg is stronger than that of Probe—Cd, which may be related to the fact that both aromatic rings on either side of Probe in Probe—Hg are completely in the same plane, while there is a certain angle between the aromatic rings on either side of Probe in Probe—Cd (this can be deduced from Fig. 2 and Table 1). However, compared to Probe—Hg, Probe—Cd’s fluorescence has undergone a significant red shift. This is because all aromatic rings of Probe in Probe—Cd can basically be in a relatively conjugated system, while the lower aromatic ring of Probe in Probe—Hg is significantly bent upward and cannot participate in forming a large conjugated system. Probe itself has a certain degree of conjugation, so it has a certain ability to emit fluorescence. The formation of Probe—Cr and Probe—As will disrupt the molecular conjugated system or planarity, so these two complexes do not exhibit significant fluorescence emission53.
Fig. 5.

The fluorescence spectra of the probe and the complexes formed by the probe with various heavy metal ions.
Binding energy
The molecular binding energies of the complexes (Probe—Pb, Probe—Hg, Probe—Cr, Probe—Cd, and Probe—As) formed by the Probe with heavy metal ions (Pb, Hg, Cr, Cd, and As) are shown in Table 4. The binding energy for complexes involving N—heterocyclic carbene palladium, among others, is determined through the application of Eq. (1). This methodological approach not only quantifies the strength of the interaction but also provides insights into the structural stability and reactivity of these complexes. (In this formula, ΔE(ab) represents the binding energy of the Probe to the heavy metal ion, E(ab) represents the energy after Probe recognizes and binds to the heavy metal, and Ea and Eb represent the energies of the probe molecule and the heavy metal ion, respectively.)
Table 4.
Binding energy (kJ/mol) of the probe to the metals ions.
| complex | Probe-Pb | Probe-Hg | Probe-Cr | Probe-Cd | Probe-As |
|---|---|---|---|---|---|
| binding energy | -238.1965 | -171.8786 | -556.5541 | -217.5236 | -208.6801 |
As shown in Table 4, it is obvious that the absolute value of the binding energy of Probe—Cr (- 556.5541 kJ/mol) is significantly the largest, indicating that Probe has the strongest binding force to Cr ions. This is consistent with the fact that in the Probe—Cr complex shown in Fig. 2, the bond orders of N—Cr and O—Cr are both higher than those in other complexes, and the coordination bond length formed between the Probe and Cr in Table 1 is the shortest. The absolute value of the binding energy of Probe—Pb (- 238.1965 kJ/mol) ranks second, which means that Probe has a distinct advantage in the binding energy to lead ions. The structure of Probe is not distorted, and Probe—Pb has a rigid planar structure. The characteristics of the Pb ion radius make it exactly located in the center of all coordination atoms of Probe, thus ensuring the overall binding of the whole molecule and having the best conditions for molecular fluorescence emission.
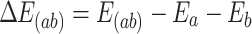 |
1 |
Frontier molecular orbital energy level
Frontier molecular orbital energy levels can be divided into the highest occupied molecular orbital (HOMO) energy level and the lowest unoccupied molecular orbital (LUMO) energy level. Among them, the HOMO energy level reflects the ability of a molecule to donate electrons, while the LUMO energy level reflects the ability of a molecule to accept electrons54,55. In a chemical reaction, the electrons in the HOMO of a molecule are relatively easy to lose, and the LUMO energy level of the central metal ion is low, so it is easy to accept the electrons provided by the ligand to form a coordination bond. Within the same molecule, the energy difference between the highest and lowest molecular orbital energy levels, that is, the energy gap value (obtained by subtracting ELUMO from EHOMO), can directly reflect the ease of activation of the molecule. At the same time, it also indicates that the larger the energy gap value of a molecule, the more difficult it is for electrons to jump within the molecule, the relatively more stable the molecular structure is, less likely to undergo chemical reactions, and thus the higher the stability of the molecule54.
In the research, the frontier molecular orbital energy levels ( EHOMO and ELUMO ) and energy gap values (ΔEH—L) of the complexes formed by the probe with all heavy metal ions are shown in Table 5. Although Probe—Pb has the best UV—visible absorption performance among all the complexes and the highest fluorescence emission intensity, in terms of the stability of the complexes formed after capturing heavy metal ions, Probe—Hg is significantly more stable than other complexes, followed by Probe—As. This indicates that Probe—Hg has good chemical stability, which may be related to the special position of Hg ions in the Probe plane. On one hand, Hg is located at the very center of the aromatic rings on both sides of Probe, providing a natural and stable coordination result; on the other hand, the lower aromatic ring of Probe folds upward, creating asymmetry in the reaction, making the central Hg ion and its coordinating atoms more susceptible to attack, and the activation energy required for the reaction is lower, specifically—171.8786 kJ/mol.
Table 5.
Energy values of ELUMO, EHOMO, and ΔEH-L of the complexes (eV) compared to the heme.
| complex | Probe-Pb | Probe-Hg | Probe-Cr | Probe-Cd | Probe-As |
|---|---|---|---|---|---|
| ELUMO | -0.8207 | -2.4547 | -1.2901 | -1.1938 | -1.9336 |
| EHOMO | 0.7883 | 0.8808 | 0.5837 | 0.4493 | 0.4131 |
| ΔEH-L | 1.609 | 3.3356 | 1.8738 | 1.6430 | 2.3467 |
In terms of optical properties, the energy gap is related to the absorption and emission spectra of molecules. The magnitude of the energy gap determines the energy range of photons that a molecule can absorb. The smaller the energy gap, the longer (lower—energy) wavelengths of light the molecule can absorb, which is basically consistent with the results in UV—visible absorption spectra. For example, among the three complexes with relatively strong UV—visible absorption spectra, Probe—Pb, Probe—Cd, and Probe—Hg, the relationship of their energy gap values is ΔEH—L ( Probe—Hg ) > ΔEH—L( Probe—Cd ) > ΔEH—L ( Probe—Pb ), and the order of the optimal absorption peak wavelengths in UV is Probe—Pb > Probe—Cd > Probe—Hg. Satisfyingly, the ΔEH—L( Probe-Cr ) is the largest among those except for Probe-Hg. Moreover, the binding energy of Probe-Cr is much stronger than that when it binds with other ions. This indicates that this probe has a relatively significant advantage in capturing and treating Cr ions.
Conclusion
Heavy metal pollution poses significant potential hazards to human health and the ecological environment. This study employs DFT calculations to systematically investigate the recognition and detection mechanisms of a quinoline derivative-based fluorescent molecular probe (Probe) for five typical toxic heavy metal ions (Pb, Hg, Cr, Cd, As). The findings reveal that the probe exhibits remarkable selectivity and sensitivity in recognizing and binding heavy metal ions, particularly in the detection of lead (Pb) ions. In this case, the probe forms a complex (Probe—Pb) with a rigid planar structure, demonstrating the strongest fluorescence emission intensity and the longest absorption wavelength (785.6 nm). Furthermore, the probe shows the highest binding energy ( -556.5541 kJ/mol) with chromium (Cr) ions, indicating its significant advantage in capturing and processing chromium ions. After the probe captures the five toxic heavy metal ions, its infrared vibration spectrum, ultraviolet—visible absorption spectrum, and emitted molecular fluorescence spectrum all change quite significantly. More importantly, the ultraviolet—visible absorption spectra and emitted molecular fluorescence spectra produced by Probe—Pb, Probe—Hg, and Probe—Cd are significantly different from those produced by Probe itself. Moreover, there are obvious differences among Probe—Pb, Probe—Hg, and Probe—Cd in terms of ultraviolet—visible absorption spectra and emitted molecular fluorescence spectra, which also proves that the Probe can be used to detect Pb, Hg, and Cd ions. More importantly, in the ultraviolet—visible absorption spectrum and molecular fluorescence spectrum produced by Probe—Pb, the absorption wavelength is the longest, the absorption ability is the strongest, and at the same time, the molecular fluorescence wavelength and intensity are also the highest. Given that the sensitivity of fluorescence detection is much higher than that of ultraviolet—visible absorption detection, this indicates that this Probe can be used as a fluorescence probe for Pb, Hg, and Cd ions, and it is especially most effective when used as a fluorescence probe for Pb ions. What’s more interesting is that the study has confirmed the natural advantage of this probe in capturing and treating Cr ions. The structural parameters, including planarity, bond lengths, WIBs, and natural charge distribution, play a critical role in determining the fluorescence, stability, and binding properties of the probe-metal complexes. Probe-Pb, with its perfect planarity, optimal bond lengths, and strong coordination, exhibits the best fluorescence and UV–visible absorption properties. In contrast, Probe-Cr, despite having the strongest binding energy, lacks fluorescence due to structural distortion. These findings provide a robust foundation for designing more efficient and selective probes for heavy metal ion detection and environmental monitoring. If a Probe molecule is used as a base molecule for microstructure modification, it may be used for the capture treatment and fluorescence detection of multiple heavy metal ions. This will provide critical guidance for the development of probes capable of rapidly and accurately detecting low concentrations of heavy metal ions, thereby facilitating the timely identification and prevention of threats to public health posed by heavy metal contamination.
Acknowledgements
This work was supported by the Construction of Chenzhou Industrial—factor Science and Technology Innovation Service Platform [ 2022sfq51 ], the research projects funded the Chenzhou Technology Innovation Capability Development Programme [ grant number 2021JCYJ03 and 2021JCYJ06 ], the Project of Natural Science Youth Foundation of Hunan Province [ grant number 2023JJ40530], the excellent Youth Project of Hunan Provincial Department of Education [ grant number 23B0778 ], the key laboratories of the Biomedical Microbiology Group in universities in Hunan Province, the Hunan Province Pharmacy Application Characteristic Discipline [ 2018-469 ], and the Xiangnan University Application Characteristic Discipline.
Author contributions
Writing original draft, funding acquisition, Wenbo Lan; writing review and editing, formal analysis, Yanbin Meng; funding acquisition, Methodology, Liping He; writing—review and editing, funding acquisition, Xiaofeng Wang; original draft preparation, formal analysis, Qianru Li; funding acquisition, Methodology, Software, Xianghe Kong. All authors have read and agreed to the published version of the manuscript.
Funding
The research projects funded the Chenzhou Technology Innovation Capability Development Programme, 2021JCYJ03,2021JCYJ06, the excellent Youth Project of Hunan Provincial Department of Education, 23B0778, the key laboratories of the Biomedical Microbiology Group in universities in Hunan Province, the Hunan Province Pharmacy Application Characteristic Discipline, 2018-469,2018-469,2018-469,2018-469,2018-469, the Xiangnan University Application Characteristic Discipline,the Construction of Chenzhou Industrial—factor Science and Technology Innovation Service Platform, 2022sfq51, the Project of Natural Science Youth Foundation of Hunan Province, 2023JJ40530.
Data availability
Upon reasonable request, the corresponding author can provide the data that support the findings of this study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaofeng Wang, Email: wangxf20161123@126.com.
Liping He, Email: hlipingm@163.com.
Xianghe Kong, Email: kongxh@usc.edu.cn.
References
- 1.Zou, H. et al. Heavy metal pollution and ecological risk assessment: A study on Linli County soils based on self-organizing map and positive factorization approaches. J. Cent. South Univ.31, 1371–1382. 10.1007/s11771-024-5624-5 (2024). [Google Scholar]
- 2.Wang, H., Zhang, H. & Xu, R. Heavy metal pollution characteristics and health evaluation of farmland soil in a gold mine slag area of Luoyang in China. Int. J. Agric. Biol. Eng.10.25165/j.ijabe.20211405.6536 (2021). [Google Scholar]
- 3.Amadi, C. N., Igweze, Z. N. & Orisakwe, O. E. Heavy metals in miscarriages and stillbirths in developing nations. Middl. East Fertil. Soc. J.22, 91–100. 10.1016/j.mefs.2017.03.003 (2017). [Google Scholar]
- 4.Gonzalez, K. A. et al. Electrochemical sensing of heavy metals in biological media: A review. Electroanalysis10.1002/elan.202300098 (2023). [Google Scholar]
- 5.Doan, E. et al. Toxic element (As, Cd, Pb and Hg) biodistribution and blood biomarkers in Barbaresca sheep raised in Sicily: One Health preliminary study. Environ. Sci. Pollut. Res.31, 43903–43912. 10.1007/s11356-024-34060-9 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Kaminsky, R. F. Protective role of quercetinum in mercury exposure cardiotoxity. Curr. Issues Pharm. Med. Sci.32, 63–66. 10.2478/cipms-2019-0012 (2019). [Google Scholar]
- 7.Winiarska-Mieczan, A. & Kwiecień, M. The Effect of Exposure to Cd and Pb in the Form of a Drinking Water or Feed on the Accumulation and Distribution of These Metals in the Organs of Growing Wistar Rats. Biol. Trace Elem. Res.169, 230–236. 10.1007/s12011-015-0414-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein, M. A., Al-Saffar, M. T. & Al-Mallah, K. H. Biochemical and Histopathological Assessment of Zinc Acetate-Induced Nephrotoxicity in Male Albino Rats. J. Appl. Vet. Sci.8, 37–42. 10.2168/javs.2023.218887.1248 (2023). [Google Scholar]
- 9.Knecht, M. R. & Sethi, M. Bio-inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles. Anal. Bioanal. Chem.394, 33–46. 10.1007/s00216-008-2594-7 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Doan, E. et al. Nanocarbon-based Electrochemical Detection of Heavy Metals. Electroanalysis28, 2472–2488. 10.1002/elan.201600173 (2016). [Google Scholar]
- 11.Liu, Z. et al. Magnetic mesoporous silica/ε-polylysine nanomotor-based removers of blood Pb2+. J. Mater. Chem. B10, 131–131. 10.1039/D0TB02270E (2020). [DOI] [PubMed] [Google Scholar]
- 12.Choi, H. S. & Kim, H. D. Development of a Portable Heavy Metal Ion Analyzer Using Disposable Screen-Printed Electrodes. Bull. Korean Chem. Soc.30, 1881–1883. 10.5012/bkcs.2009.30.8.1881 (2009). [Google Scholar]
- 13.Jaishankar, M. et al. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol.7, 60–72. 10.2478/intox-2014-0009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández-Flores, S., Santos-Medrano, G. E. & Rico-Martínez, R. Integral Study of Paramecium caudatum Acute and Chronic Toxicity, Sites of Entry and Distribution, Bioconcentration and Body Burdens of Five Metals. Bull. Environ. Contam. Toxicol.111, 19. 10.1007/s00128-023-03768-8 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim, M. et al. Primary, Secondary Metabolites, Biochemical and Antioxidant Activity of Orthosiphon staminues Benth (Misai Kucing) Under Cadmium Exposure. Ann. Res. Rev. Biol.19, 1–14. 10.9734/ARRB/2017/36413 (2017). [Google Scholar]
- 16.Xie, H. et al. Arsenic is cytotoxic and genotoxic to primary human lung cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen.760, 33–41. 10.1016/j.mrgentox.2013.11.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldoni, M. C. et al. Chromium in exhaled breath condensate and pulmonary tissue of non-small cell lung cancer patients. Int. Arch. Occup. Environ. Health81, 487–493. 10.1007/s00420-007-0242-8 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Hansen, J. C. & Danscher, G. Organic Mercury—An Environmental Threat to the Health of Exposed Societies?. Rev. Environ. Health12, 107–116. 10.1515/REVEH.1997.12.2.107 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Kehrig, H. A. et al. Trophic transfer of methylmercury and trace elements by tropical estuarine seston and plankton. Estuar. Coast. Shelf Sci.85, 36–44. 10.1016/j.ecss.2009.05.027 (2009). [Google Scholar]
- 20.Majhi, A., Venkateswarlu, K. & Sasikumar, P. Coumarin Based Fluorescent Probe for Detecting Heavy Metal Ions. J. Fluoresc.34, 1453–1483. 10.1007/s10895-023-03372-3 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Xu, M. et al. Organic small-molecule fluorescent probe-based detection for alkali and alkaline earth metal ions in biological systems. J. Mater. Chem. B11, 3295–3306 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Che, H., Tian, X. & Chen, W. Simultaneous visual detection of multiple heavy metal ions by a high-throughput fluorescent probe. Mikrochim. Acta10.1007/s00604-023-05882-0 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Huang, S. et al. Computational Design of Two-Photon Fluorescent Probes for a Zinc Ion Based on a Salen Ligand. Inorg. Chem.52, 5702–5713. 10.1021/ic3022062 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Syahputri, Y., Sutanto, S. & Zamzani, R. S. Hg (II) and Cd (II) Heavy Metal Ions Detection Based On Fluorescence Using Zn (II) Metal Ion Complex with Pyrazoline Derivatives Ligand. Helium. J. Sci. Appl. Chem.10.33751/helium.v2i1.5407 (2022). [Google Scholar]
- 25.Xu, W. et al. An Artificial Tongue Fluorescent Sensor Array for Identification and Quantitation of Various Heavy Metal Ions. Anal. Chem.86, 8763–8769. 10.1021/ac501953z (2014). [DOI] [PubMed] [Google Scholar]
- 26.Luo, H. et al. Recent advances in molecular and nanoparticle probes for fluorescent bioanalysis. Nano Res.17, 6443. 10.1007/s12274-024-6659-5 (2024). [Google Scholar]
- 27.Zhou, X. et al. Research progress on fluorescence sensing properties of metal-organic frameworks. J. Mol. Sci.40, 107–114. 10.13563/j.cnki.jmolsci.2023.08.007 (2024). [Google Scholar]
- 28.Weber, G. & Bablouzian, B. Construction and performance of a fluorescence polarization spectrophotometer. J. Biol. Chem.241, 2558. 10.1023/A:1024128115666 (1966). [PubMed] [Google Scholar]
- 29.Vasil’evaNurmukhametovKiseleva, I. A. R. N. N. A. Fine-structure fluorescence of conjugated chain molecules. Opt. Spectrosc.98, 753–760. 10.1134/1.1929062 (2005). [Google Scholar]
- 30.Sun, M. Z. et al. An efficient blue electro-fluorescence material with high electron and balanced carrier mobilities based on effective π-stacking between acceptors. Org. Chem. Front.10, 4878–5488. 10.1039/D3QO01253K (2023). [Google Scholar]
- 31.Nijegorodov, N. I. & Downey, W. S. The Influence of Planarity and Rigidity on the Absorption and Fluorescence Parameters and Intersystem Crossing Rate Constant in Aromatic Molecules. J. Phys. Chem.98, 5639–5643. 10.1021/j100073a011 (1994). [Google Scholar]
- 32.Abramova, A. M. et al. Molecular nature of breakdown of the folic acid under hydrothermal treatment: A combined experimental and DFT study. Sci. Rep.10, 19668. 10.1038/s41598-020-76311-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma, N. & Chaturvedib, R. P. Spectroscopic & Antimicrobial studies of mixed ligand complexes of transition metal(II) ions with Nitro Quinoline & dibenzoyl Methane. Sci. Rev. Chem. Commun.2, 108–114 (2012). [Google Scholar]
- 34.Hu, X. et al. Optimizing side chains for crystal growth from water: A case study of aromatic amide foldamers. Chem. Sci.8, 3741–3749. 10.1039/c7sc00430c (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X. et al. Heteroaggregation behavior of graphene oxide on Zr-based metal–organic frameworks in aqueous solutions: A combined experimental and theoretical study. J. Mater. Chem. A5, 20398–20406. 10.1039/C7TA06462D (2017). [Google Scholar]
- 36.Zahedifar, M. et al. Unexpected regio- and stereoselective [4 + 3] cycloaddition reaction of azomethine ylides with benzylidene thiazolidinediones: Synthesis of pharmacologically active spiroindoline oxazepine derivatives and theoretical study. Mol. Divers.25, 29–43. 10.1007/s11030-019-10022-z (2021). [DOI] [PubMed] [Google Scholar]
- 37.Lan, W. B. et al. Computational insight into complex structures of thorium coordination with N, N’- bis(3-allyl salicylidene)- o-phenylenediamine. J. Mol. Model.22, 224. 10.1007/s00894-016-3089-7 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Rabichi, I. et al. Experimental and Theoretical Investigation of Olive Mill Solid Waste Biochar for Vanillic Acid Adsorption Using DFT/B3LYP Analysis. Water Air Soil Pollut.235, 369. 10.1007/s11270-024-07183-5 (2024). [Google Scholar]
- 39.Isayev, O., Rasulev, B. & Gorb, L. Structure-toxicity relationships of nitroaromatic compounds. Mol. Divers.10, 233–245. 10.1007/s11030-005-9002-4 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Frisch MJ, Trucks GW, Schlegel HB et al. Gaussian 16 Rev. C.01.: Wallingford, CT (2016)
- 41.Aljohani, F. S. et al. Design, structural inspection of new bis(1H-benzo[d]imidazol-2-yl)methanone complexes: Biomedical applications and theoretical implementations via DFT and docking approaches. Inorg. Chem. Commun.148, 110331–110331. 10.1016/j.inoche.2022.110331 (2023). [Google Scholar]
- 42.Aljohani, E. T., Shehata, M. R. & Abu-Dief, A. M. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+, and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl. Organometal. Chem.35, 6169. 10.1002/aoc.6169 (2021). [Google Scholar]
- 43.Klein, E., Lukes, V. & Ilcin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys.336, 51–57. 10.1016/j.chemphys.2007.05.007 (2007). [Google Scholar]
- 44.Ezzat, H. et al. DFT: B3LYP/ LANL2DZ Study for the Removal of Fe, Ni, Cu, As, Cd and Pb with Chitosan. Biointerface Res. Appl. Chem.10, 7002–7010. 10.3263/BRIAC106.70027010 (2020). [Google Scholar]
- 45.Basiuk, V. Interaction of Porphine and its Metal Complexes with C 60 Fullerene: A DFT B3LYP/LANL2MB Study. J. Comput. Theor. Nanosci.2, 370–377. 10.1166/JCTN.2005.206 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Yu, Z., Liu, S. & Zhang, H. Polyaryl substituted imidazobenzothiadiazoles as multifunctional organic fluorescent materials for visualization of latent fingerprints and lysosome staining. Dyes Pigments214, 11213. 10.1016/j.dyepig.2023.111213 (2023). [Google Scholar]
- 47.Harper, L. K., Shoaf, A. L. & Bayse, C. A. Predicting Trigger Bonds in Explosive Materials through Wiberg Bond Index Analysis. ChemPhysChem16, 3886–3892. 10.1002/cphc.201500773 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Alves, E. H. S., Oliveira, D. A. S. & Braga, A. A. C. Palladium(II)-catalyzed annulation ofN-methoxy amides and arynes: Computational mechanistic insights and substituents effects. J. Mol. Model10.1007/s00894-024-05930-3 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Halim, S. A. et al. DFT study, and natural bond orbital (NBO) population analysis of 2-(2-Hydroxyphenyl)-1-azaazulene tautomers and their mercapto analouges. Sci. Rep.10.1038/s41598-023-50660-w (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hriz, K. et al. Synthesis and characterization of new anthracene-based semi-conducting materials. J. Mater. Sci.47, 8067–8075. 10.1007/s10853-012-6699-1 (2012). [Google Scholar]
- 51.Oh, M. & Mirkin, C. A. Chemically tailorable colloidal particles from infinite coordination polymers. Nature438, 651–654. 10.1038/nature04191 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Shen, D. et al. Toward a Highly Sensitive Fluorescence Sensing System of an Amphiphilic Molecular Rod: Facile Synthesis and Significant Solvent-Assisted Photophysical Tunability. Macromolecules44, 1009–1015. 10.1021/ma102311n (2011). [Google Scholar]
- 53.Thierry, B. & Del, G. P. A. Factors influencing the detection of bacterial cells using fluorescence in situ hybridization (FISH): A quantitative review of published reports. FEMS Microbiol. Ecol.44, 3–15. 10.1016/S0168-6496(02)00461-0 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, K. New strategy for lowering the energy levels of one frontier molecular orbital in conjugated molecules and polymers based on Aza-substitution at the isolated HOMO or LUMO. Polym. J.56, 61–70. 10.1038/s41428-023-00843-z (2024). [Google Scholar]
- 55.Camacho-Mendoza, R. L. et al. A new computational model for the prediction of toxicity of phosphonate derivatives using QSPR. Mol. Divers22, 269–280. 10.1007/s11030-018-9819-2 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request, the corresponding author can provide the data that support the findings of this study.




