Abstract
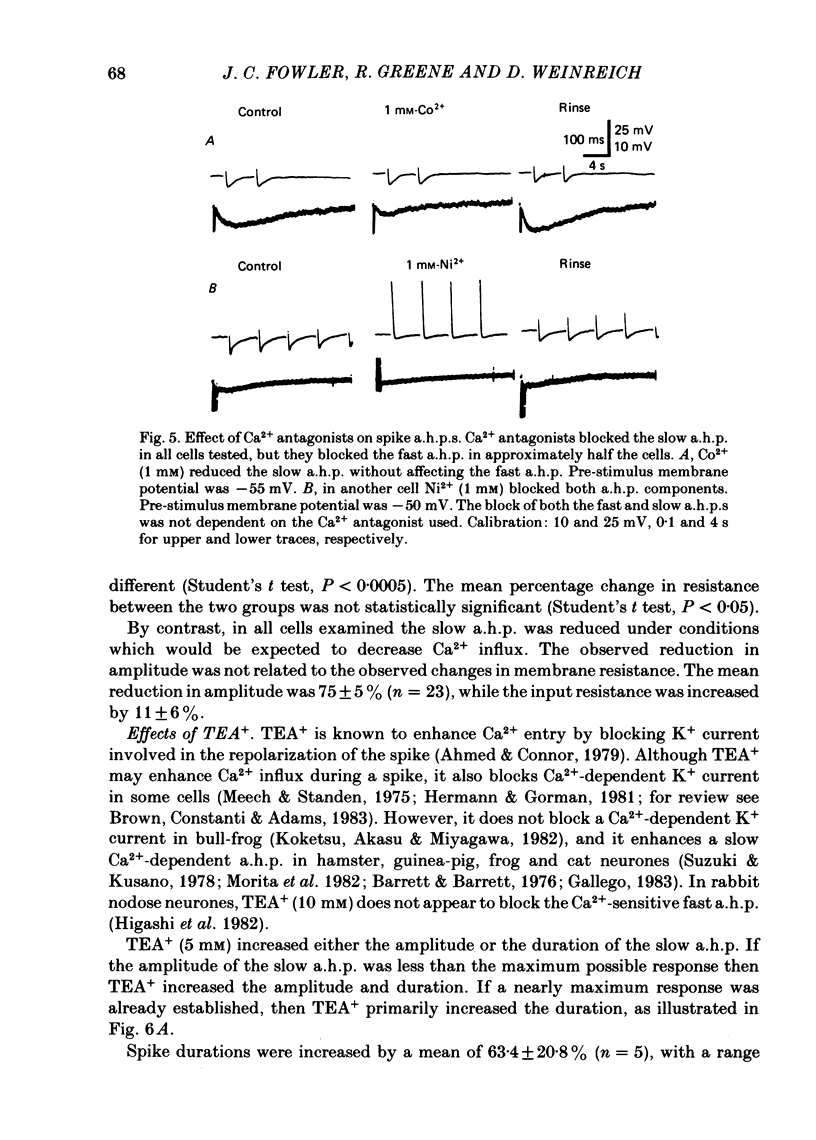
Intracellular recordings were made from rabbit nodose neurones in vitro. Two temporally distinct spike after-hyperpolarizations (a.h.p.s) were identified in a subpopulation of C-type neurones. The fast a.h.p. after a single spike lasted no longer than 500 ms, while the slow a.h.p. persisted for seconds. Both a.h.p.s. were increased in amplitude in low K+ (0.56 mM) solutions and decreased in amplitude in high K+ (11.2 mM) solutions, and both were reversed at hyperpolarized membrane potentials. The slow a.h.p. was reduced in low Ca2+ (0.22 mM), in the presence of Ca2+ antagonists (Ni2+, 1 mM; Cd2+, 100 microM; or Co2+, 1 mM) and was enhanced in tetraethylammonium (5 mM). In approximately half of the cells tested, the fast a.h.p. was reduced in low Ca2+ and in the presence of the Ca2+ antagonists. In the remaining cells the fast a.h.p. was insensitive to these procedures. Prostaglandin (PGE1, 1-10 micrograms/ml) reduced the slow a.h.p. in all cells tested. Neither the Ca2+-sensitive nor the Ca2+-insensitive fast a.h.p. was affected by the prostaglandin. It is concluded that there is a subpopulation of C-type nodose neurones possessing a slow a.h.p. which is due to a Ca2+-dependent K+ current. This subpopulation of neurones can further be divided on the basis of the presence of a Ca2+-sensitive fast a.h.p. Furthermore, PGE1 pharmacologically separates the fast and slow a.h.p.s by selectively blocking the slow one. The blockage by the PGE1 is most probably not due to a reduction in Ca2+ influx.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J Physiol. 1983 Sep;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Ca-activated potassium current in vertebrate sympathetic neurons. Cell Calcium. 1983 Dec;4(5-6):407–420. doi: 10.1016/0143-4160(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Walsh J. H., Yamamura H. I., Burks T. F. Neuropeptides in sensory neurons. Life Sci. 1982 May 31;30(22):1857–1866. doi: 10.1016/0024-3205(82)90465-9. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Ginzel K. H., Baker D. G., Banzett R. B., Morrison M. A. Stimulation of 'irritant' receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976 Dec 2;264(5585):451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- Coleridge J. C., Coleridge H. M. Afferent C-fibers and cardiorespiratory chemoreflexes. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):251–260. doi: 10.1164/arrd.1977.115.S.251. [DOI] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C. Membrane and action potential characteristics of A and C nodose ganglion cells studied in whole ganglia and in tissue slices. J Neurophysiol. 1978 Sep;41(5):1217–1232. doi: 10.1152/jn.1978.41.5.1217. [DOI] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helke C. J., Jacobowitz D. M., Thoa N. B. Capsaicin and potassium evoked substance P release from the nucleus tractus solitarius and spinal trigeminal nucleus in vitro. Life Sci. 1981 Oct 26;29(17):1779–1785. doi: 10.1016/0024-3205(81)90188-0. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Hartung K. Ca2+ activated K+ conductance in molluscan neurones. Cell Calcium. 1983 Dec;4(5-6):387–405. doi: 10.1016/0143-4160(83)90016-7. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Shinnick-Gallagher P., Gallagher J. P. Morphine enhances and depresses Ca2+-dependent responses in visceral primary afferent neurons. Brain Res. 1982 Nov 11;251(1):186–191. doi: 10.1016/0006-8993(82)91291-4. [DOI] [PubMed] [Google Scholar]
- Jaffe R. A., Sampson S. R. Analysis of passive and active electrophysiologic properties of neurons in mammalian nodose ganglia maintained in vitro. J Neurophysiol. 1976 Jul;39(4):802–815. doi: 10.1152/jn.1976.39.4.802. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. Substance P in the vagal sensory ganglia: localization in cell bodies and pericellular arborizations. J Comp Neurol. 1980 Sep 15;193(2):549–564. doi: 10.1002/cne.901930216. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Akasu T., Miyagawa M. Identification of gK systems activated by [Ca2+]. Brain Res. 1982 Jul 15;243(2):369–372. doi: 10.1016/0006-8993(82)90263-3. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., MacDermott A. B., Weight F. F. Detection of intracellular Ca2+ transients in sympathetic neurones using arsenazo III. 1983 Jul 28-Aug 3Nature. 304(5924):350–352. doi: 10.1038/304350a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kusano K. Hyperpolarizing potentials induced by Ca-mediated K-conductance increase in hamster submandibular ganglion cells. J Neurobiol. 1978 Sep;9(5):367–392. doi: 10.1002/neu.480090504. [DOI] [PubMed] [Google Scholar]


