Abstract
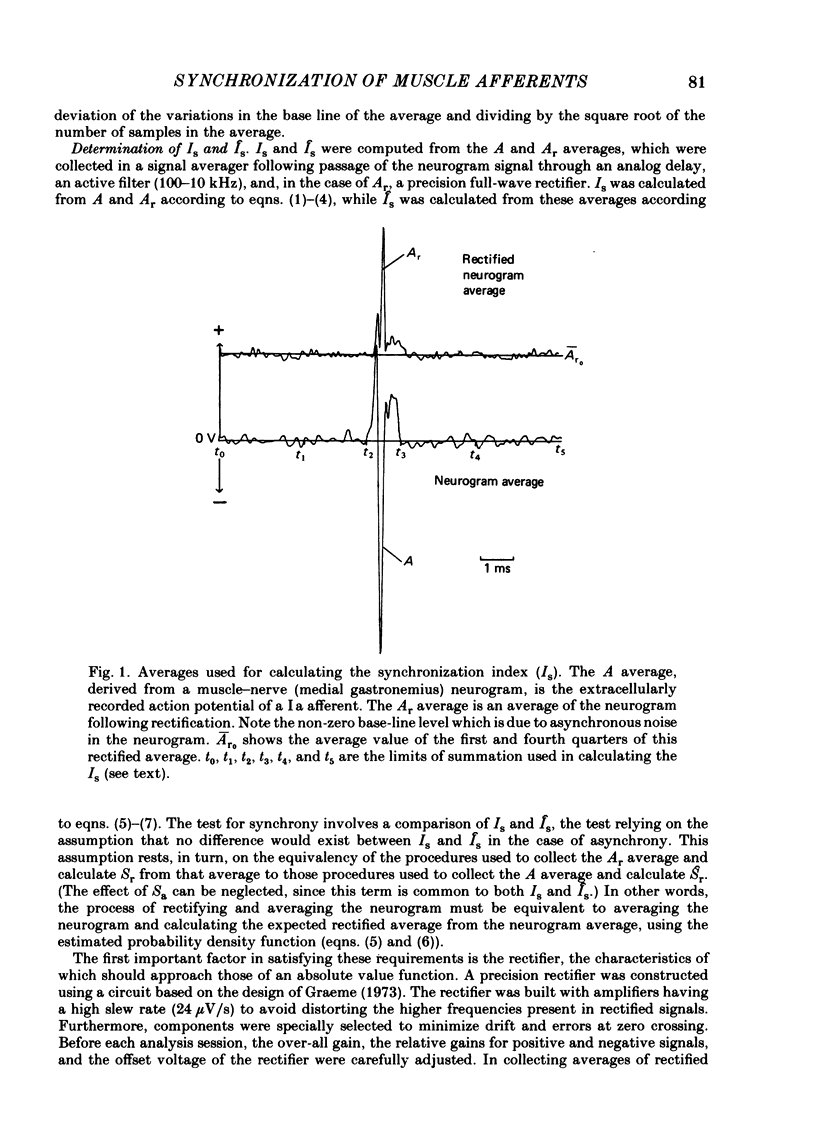
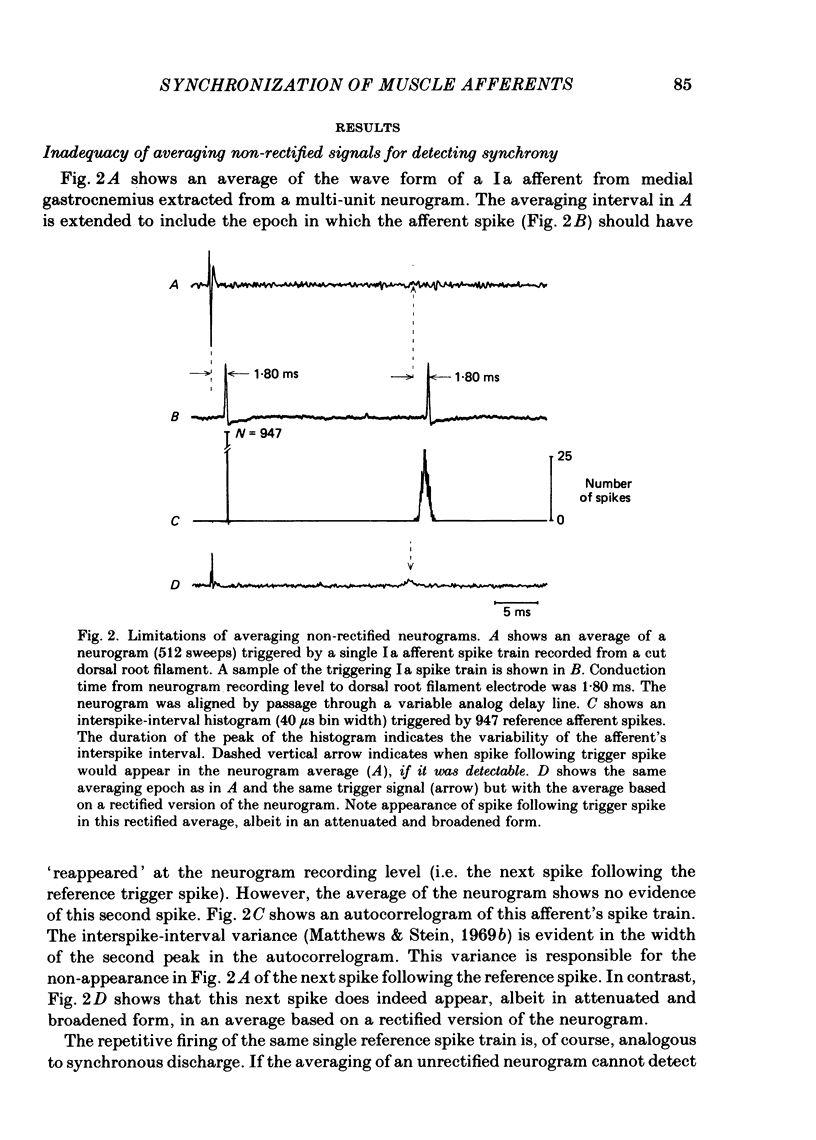
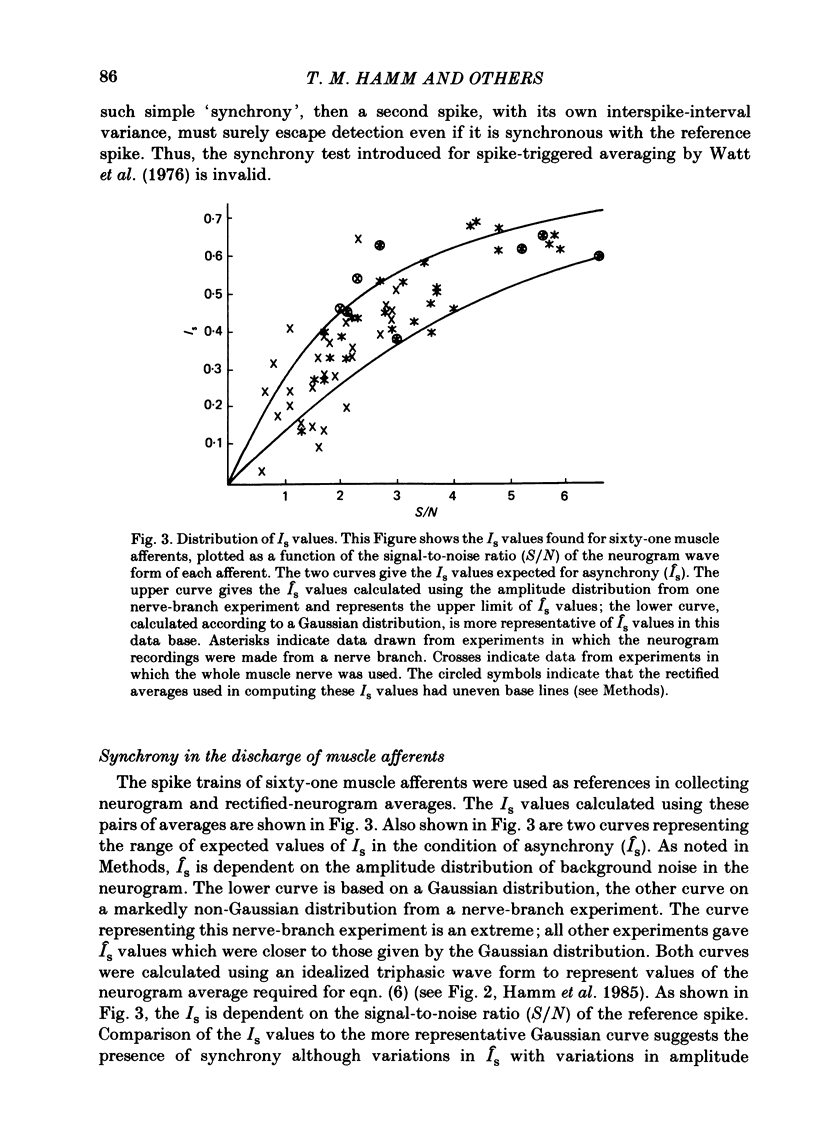
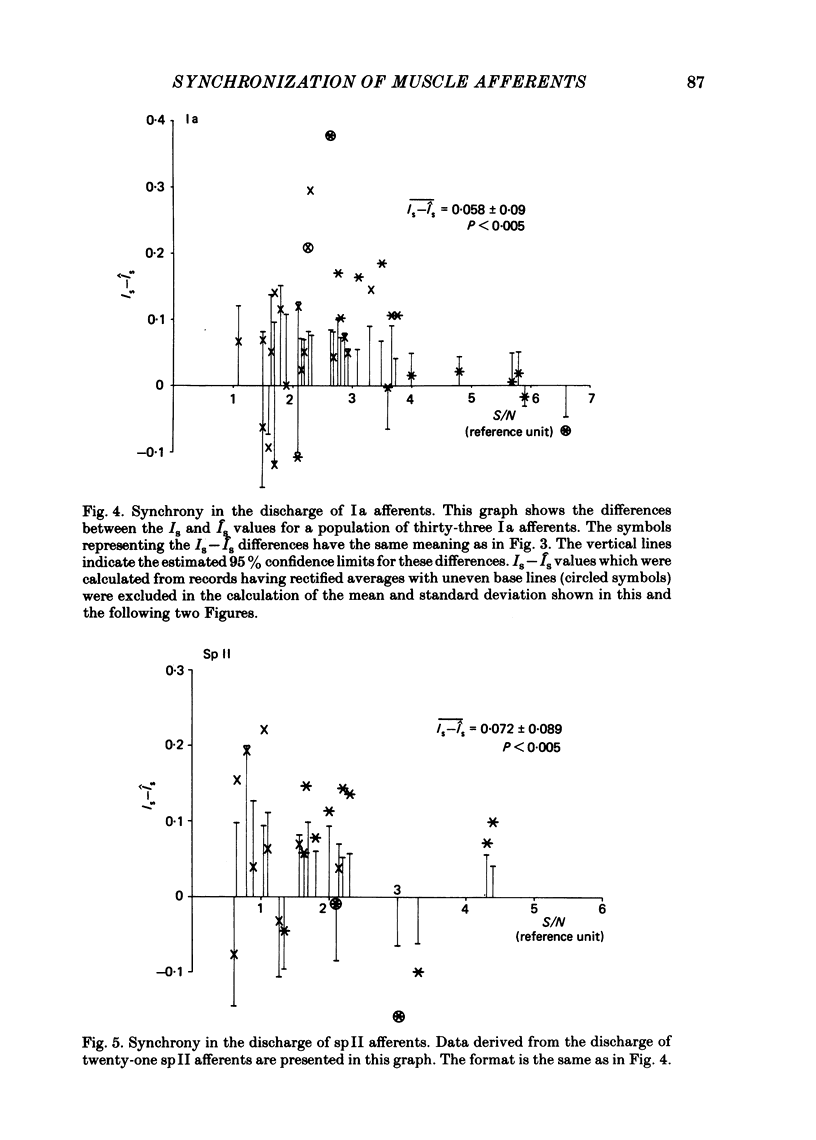
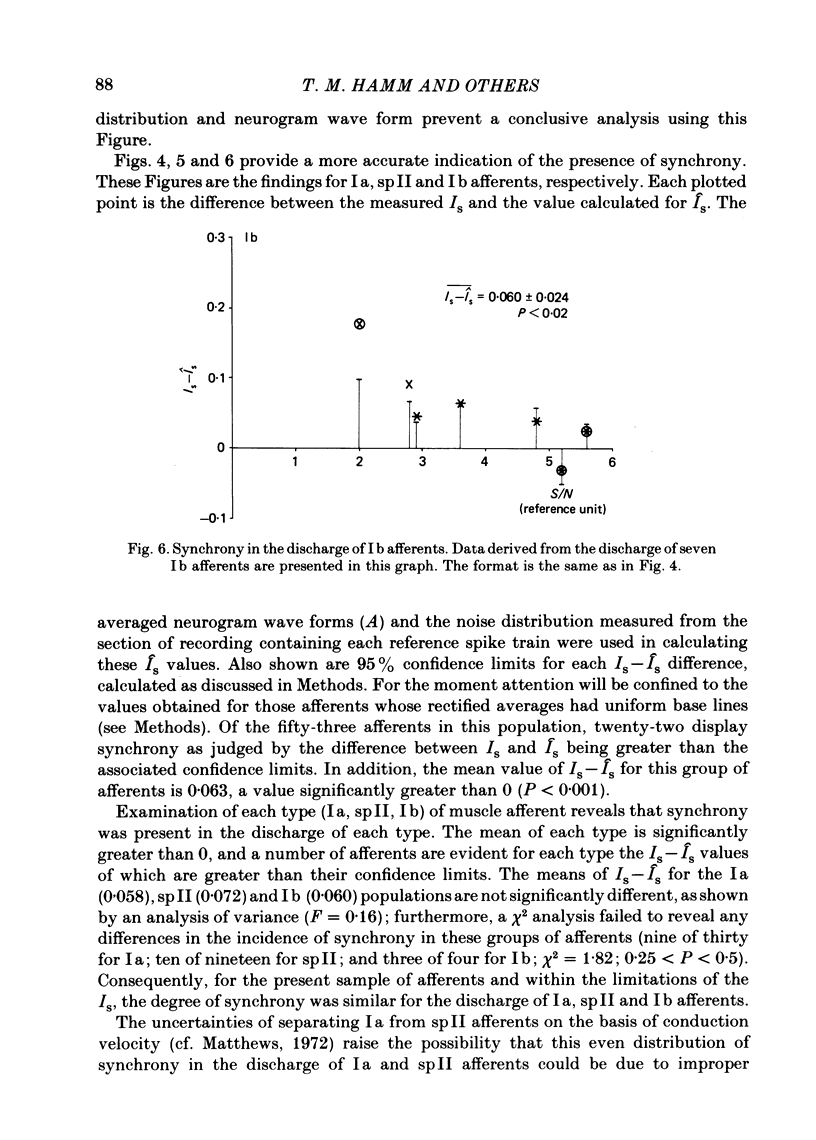
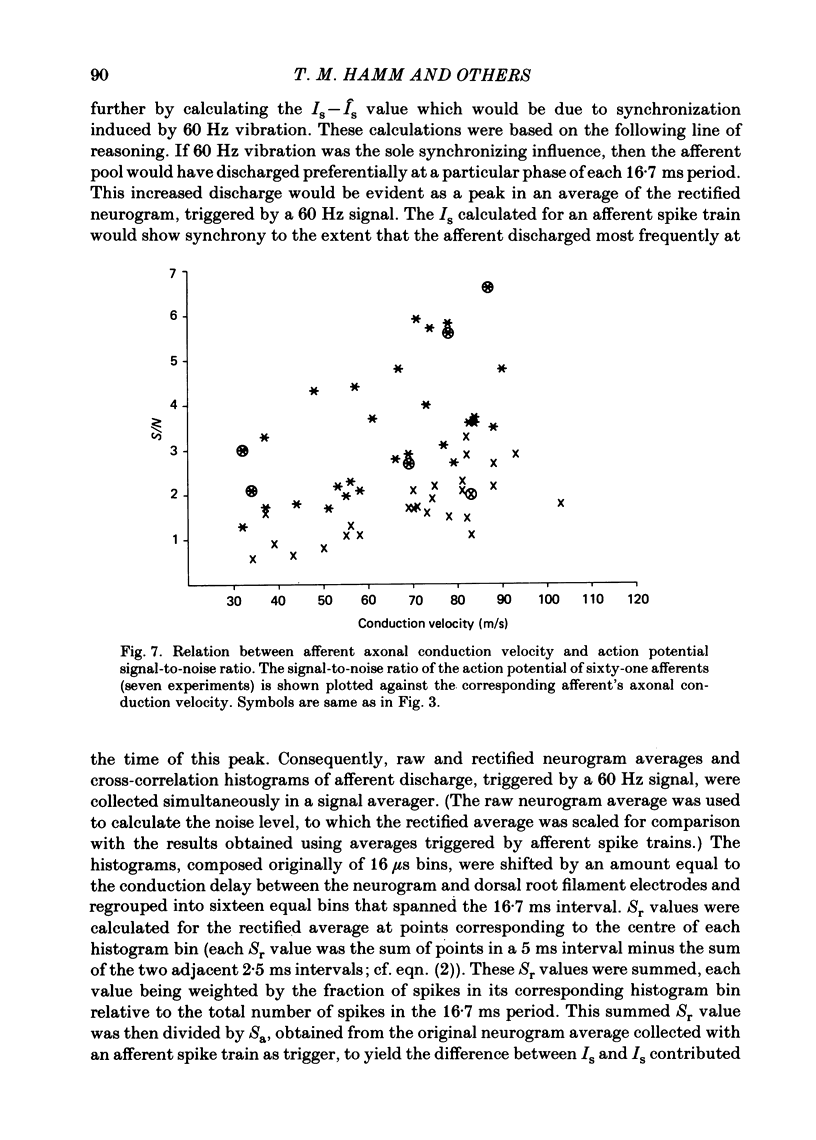
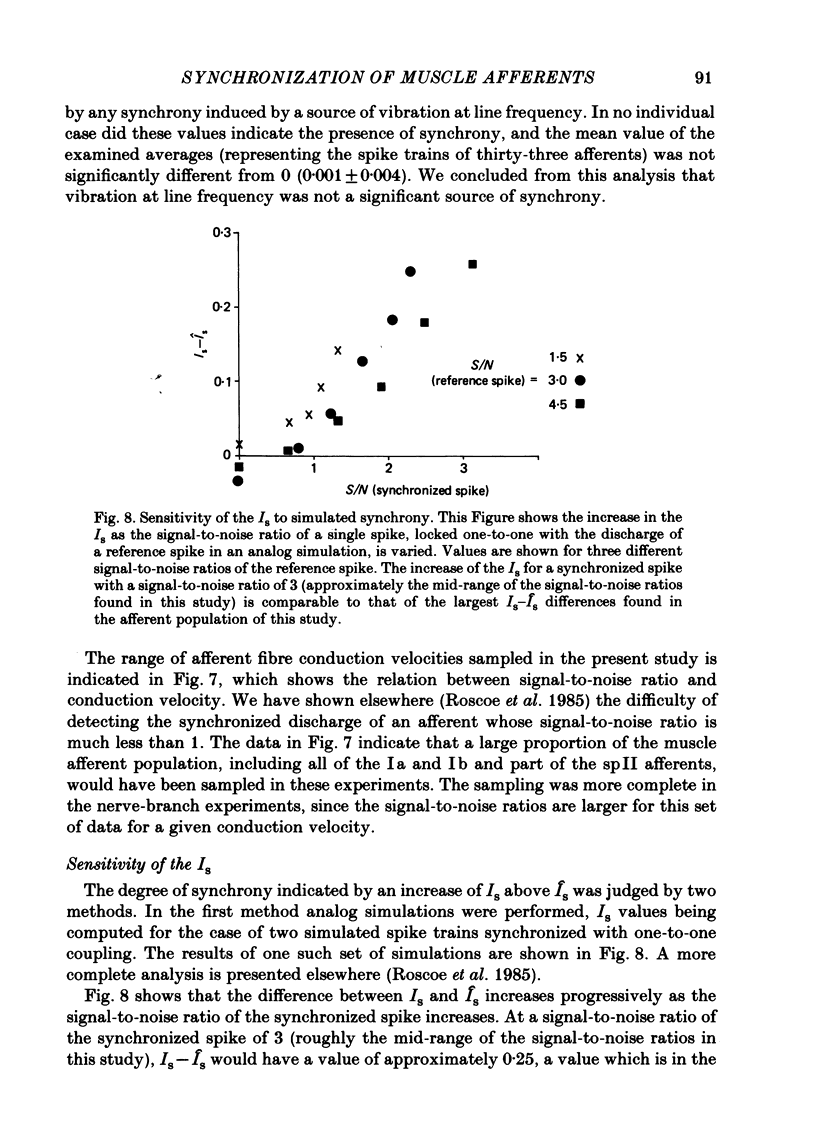
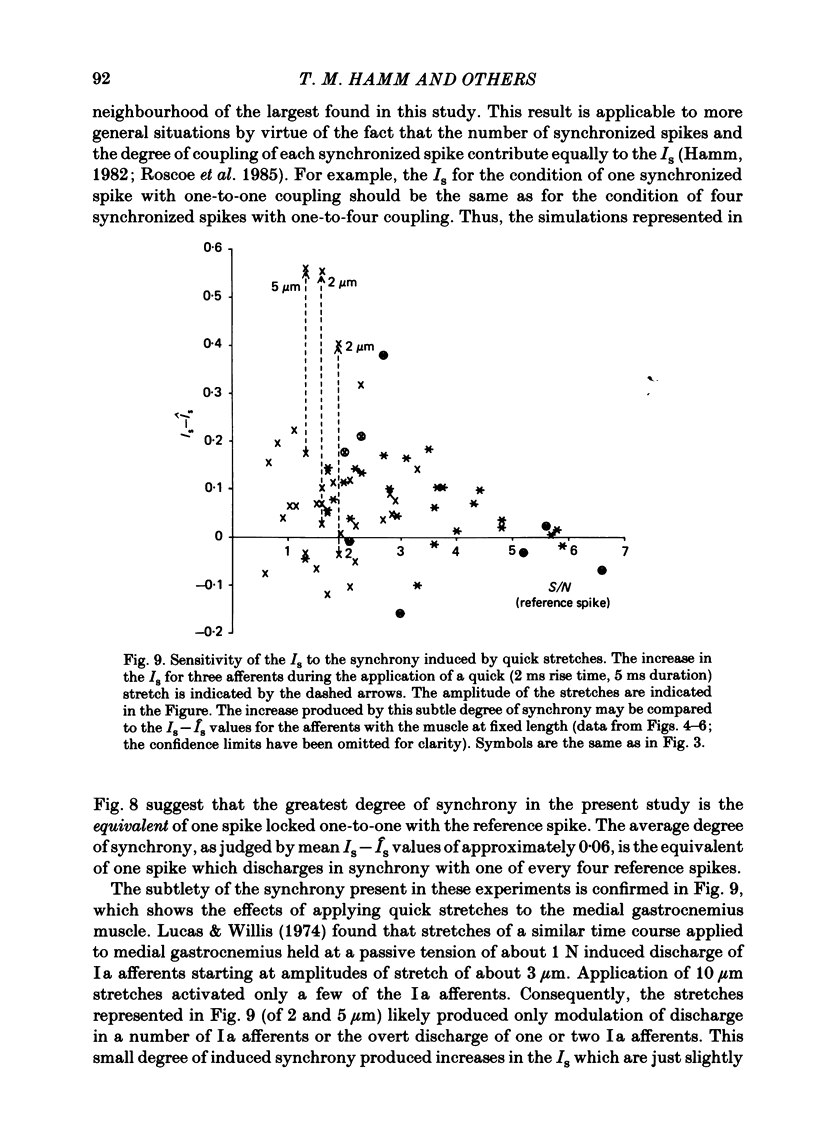
Evidence is presented for the existence of synchrony between the spike trains of muscle afferents of the passive cat medial gastrocnemius muscle held at fixed length. Synchrony between the spike trains of a population of muscle afferents was quantified by means of a synchronization index (Is), derived from spike-triggered averages of the muscle-nerve neurogram and the rectified neurogram. A previously used test based solely upon the neurogram average (Watt, Stauffer, Taylor, Reinking & Stuart, 1976) is shown to be invalid. The differences between experimentally derived Is values and theoretical Is values derived for the condition of asynchrony were compared to estimated confidence limits for those differences. This comparison revealed that twenty-two of fifty-three muscle-afferent spike trains whose rectified averages satisfied certain conditions for interpreting the Is were synchronized with the discharge of other afferents. The form of the rectified averages of another eight afferents suggested that these afferents might also have been synchronized. Synchrony in the discharge of muscle afferents was found in three experiments in which the neurogram was recorded from a single nerve branch to medial gastrocnemius, as well as in the data of experiments in which the whole muscle nerve was used. The degree of synchrony was similar for Ia, spindle group II and Ib afferents. The magnitude of the synchrony found in these experiments was judged by comparison to the results of analog simulations and the increase in Is values resulting from the application of small, quick stretches to the medial gastrocnemius muscle. The degree of synchrony found on average was approximately equivalent to that of a single spike occurring once for every four discharges of the reference spike train. Simulations were performed to determine the distortion of monosynaptic excitatory post-synaptic potentials (e.p.s.p.s) obtained by spike-triggered averaging which would be produced by synchrony between the spike trains of Ia and spindle group II afferents of the magnitude found in this study. These simulations indicate that the apparent amplitude would be increased by approximately 4 microV on average. Both the 10-90% rise time and the half-width would increase, the effects being greater for smaller e.p.s.p.s. Consequently, the synchrony found in this study is of most concern in the study of small post-synaptic potentials, such as those due to spindle group II afferents.
Full text
PDF







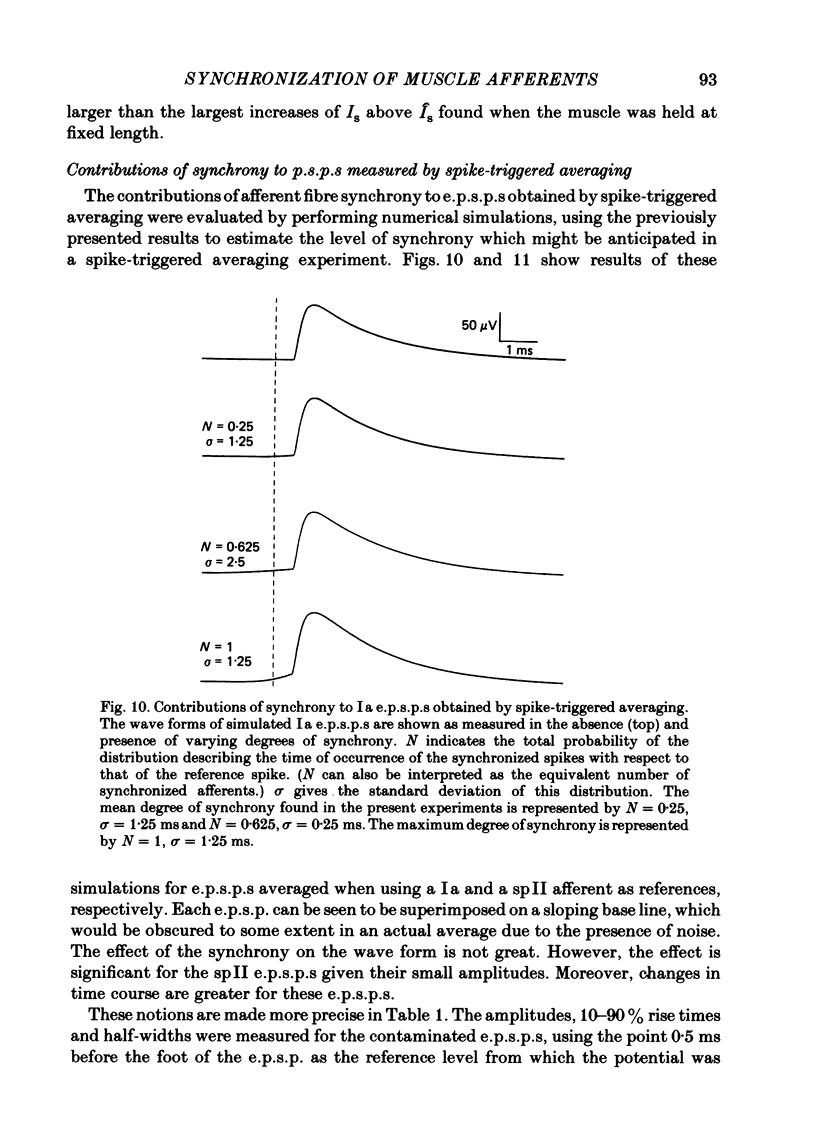
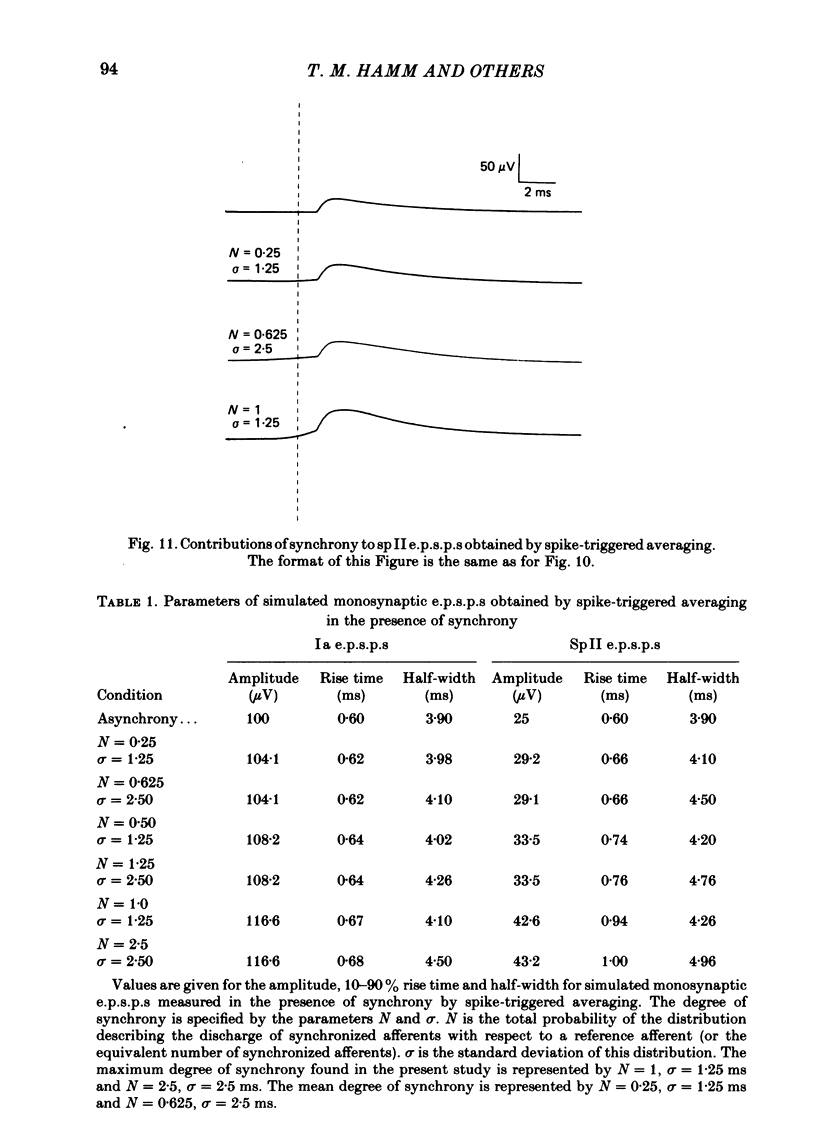








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreassen S., Stein R. B., Oğuztöreli M. N. Application of optimal multichannel filtering to simulated nerve signals. Biol Cybern. 1979 Feb 2;32(1):25–33. doi: 10.1007/BF00337448. [DOI] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stuart D. G. The response of Golgi tendon organs to single motor unit contractions. J Physiol. 1977 Oct;271(2):337–349. doi: 10.1113/jphysiol.1977.sp012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. "Sensory partitioning" of cat medial gastrocnemius muscle by its muscle spindles and tendon organs. J Neurophysiol. 1981 Jul;46(1):32–47. doi: 10.1152/jn.1981.46.1.32. [DOI] [PubMed] [Google Scholar]
- Cussons P. D., Hulliger M., Matthews P. B. Effects of fusimotor stimulation on the response of the secondary ending of the muscle spindle to sinusoidal stretching. J Physiol. 1977 Sep;270(3):835–850. doi: 10.1113/jphysiol.1977.sp011984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Furness P. Increased probability of muscle spindle firing time-locked to the electrocardiogram in rabbits [proceedings]. J Physiol. 1977 Dec;273(2):92P–92P. [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W. Homonymous projection of individual group Ia-fibers to physiologically characterized medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1339–1348. doi: 10.1152/jn.1981.46.6.1339. [DOI] [PubMed] [Google Scholar]
- Fukami Y. Responses of isolated Golgi tendon organs of the cat to muscle contraction and electrical stimulation. J Physiol. 1981 Sep;318:429–443. doi: 10.1113/jphysiol.1981.sp013876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm T. M., Roscoe D. D., Reinking R. M., Stuart D. G. Detection of synchrony in the discharge of a population of neurons. I. Development of a synchronization index. J Neurosci Methods. 1985 Mar;13(1):37–50. doi: 10.1016/0165-0270(85)90042-1. [DOI] [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Analysis of response properties of deefferented mammalian spindle receptors based on frequency response. J Neurophysiol. 1975 May;38(3):663–672. doi: 10.1152/jn.1975.38.3.663. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Collins W. F., 3rd, Mendell L. M. Alpha-motoneuron EPSPs exhibit different frequency sensitivities to single Ia-afferent fiber stimulation. J Neurophysiol. 1983 Apr;49(4):886–901. doi: 10.1152/jn.1983.49.4.886. [DOI] [PubMed] [Google Scholar]
- Katz B., Schmitt O. H. Electric interaction between two adjacent nerve fibres. J Physiol. 1940 Feb 14;97(4):471–488. doi: 10.1113/jphysiol.1940.sp003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler W., Hamm T. M., Enoka R. M., Stuart D. G., Windhorst U. Linear and non-linear summation of alpha-motoneuron potential changes elicited by contractions of homonymous motor units in cat medial gastrocnemius. Brain Res. 1984 Apr 2;296(2):385–388. doi: 10.1016/0006-8993(84)90079-9. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Lucas M. E., Willis W. D. Identification of muscle afferents which activate interneurons in the intermediate nucleus. J Neurophysiol. 1974 Mar;37(2):282–293. doi: 10.1152/jn.1974.37.2.282. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Res. 1977 Feb 25;122(3):551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The regularity of primary and secondary muscle spindle afferent discharges. J Physiol. 1969 May;202(1):59–82. doi: 10.1113/jphysiol.1969.sp008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon B., Burke D. Component of muscle spindle discharge related to arterial pulse. J Neurophysiol. 1981 Oct;46(4):788–796. doi: 10.1152/jn.1981.46.4.788. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973 Jan;228(2):285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single fibre excitatory post-synaptic potentials in triceps surae motoneurones. J Physiol. 1979 Nov;296:329–342. doi: 10.1113/jphysiol.1979.sp013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W., Zengel J. E., Lofton S. A., Fleshman J. W. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1982 Nov;48(5):1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele R. E., Bowman R. J. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970 Jan;33(1):59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stuart D. G. Servo-regulated electromagnetic system for muscle stretch and vibration. Am J Phys Med. 1974 Feb;53(1):1–28. [PubMed] [Google Scholar]
- Roscoe D. D., Hamm T. M., Reinking R. M., Stuart D. G. Detection of synchrony in the discharge of a population of neurons. II. Implementation and sensitivity of a synchronization index. J Neurosci Methods. 1985 Mar;13(1):51–64. doi: 10.1016/0165-0270(85)90043-3. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]
- Sturart D. G., Mosher C. G., Gerlach R. I., Reinking R. M. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–292. doi: 10.1007/BF00816163. [DOI] [PubMed] [Google Scholar]
- Trott J. R. The effect of low amplitude muscle vibration on the discharge of fusimotor neurones in the decerebrate cat. J Physiol. 1976 Mar;255(3):635–649. doi: 10.1113/jphysiol.1976.sp011300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


