Abstract
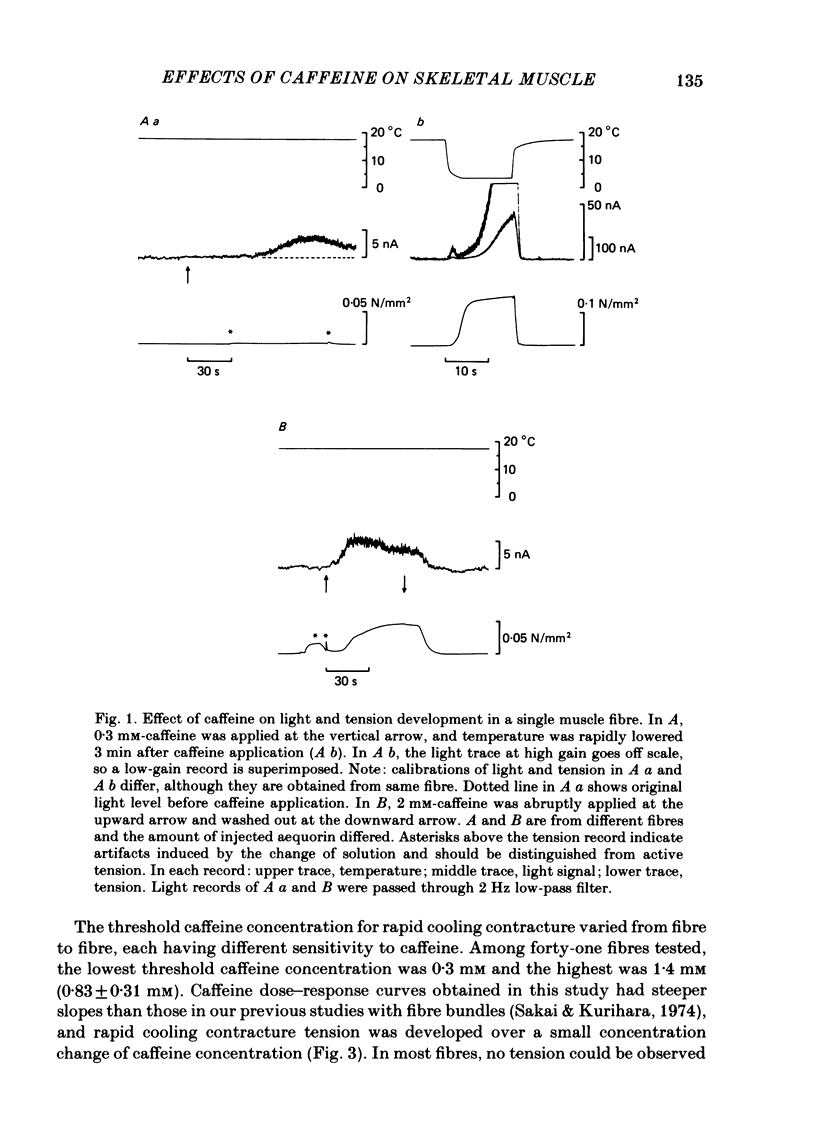
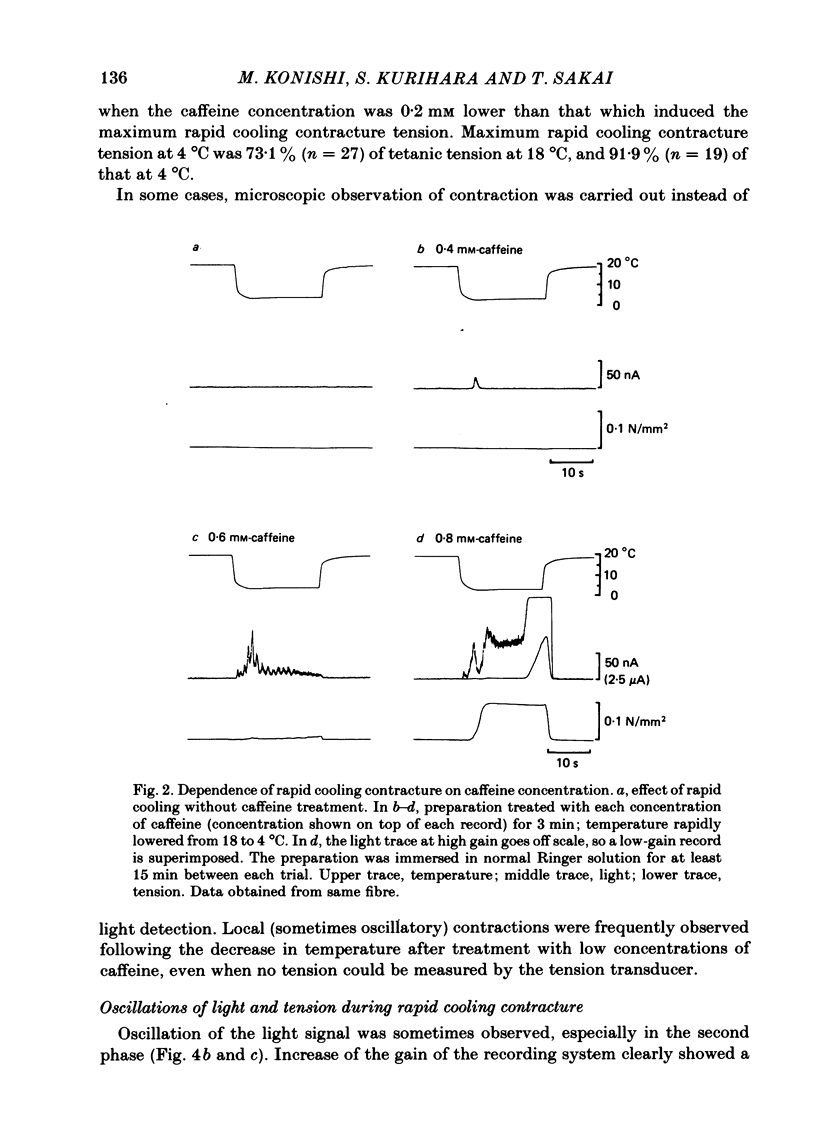
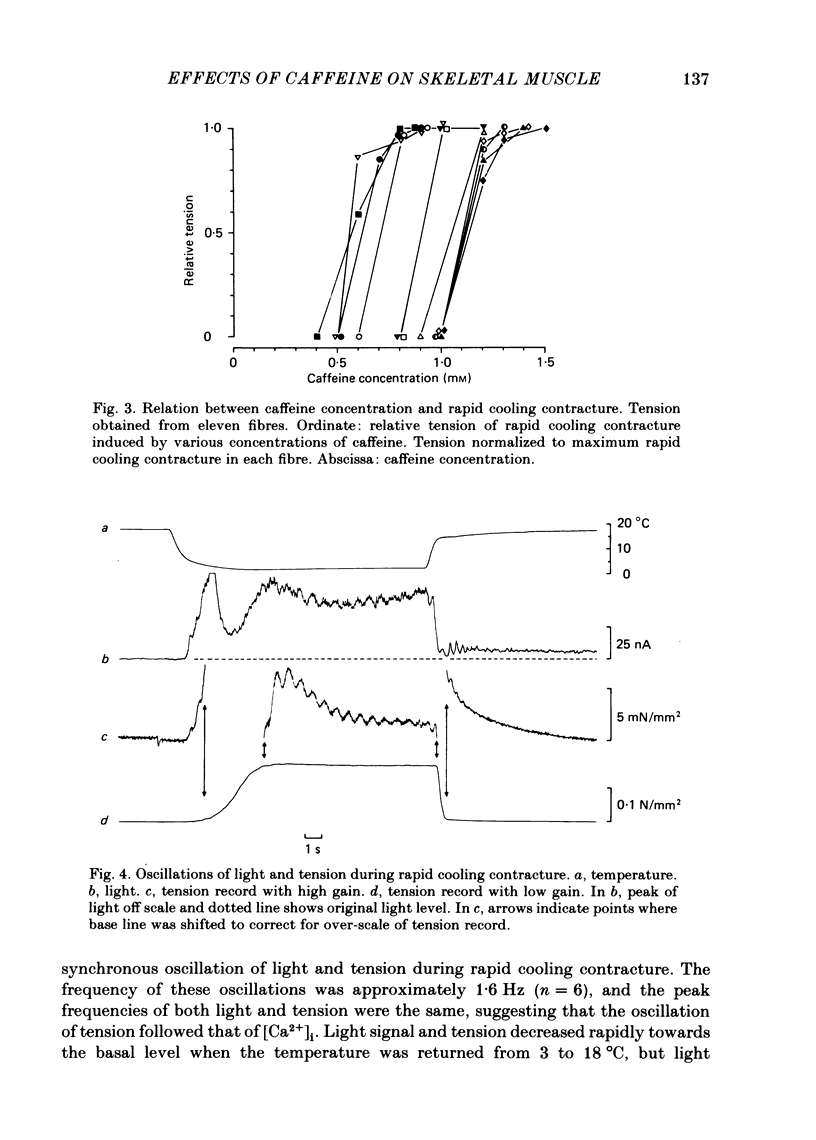
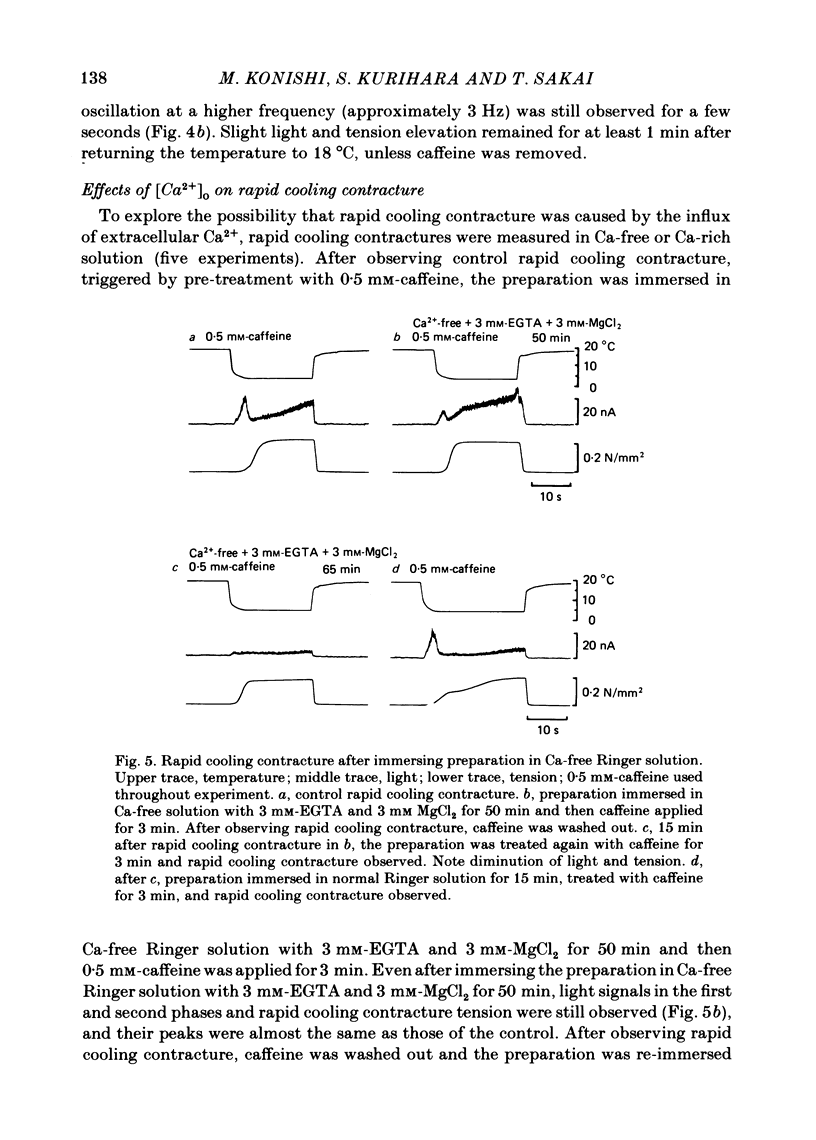
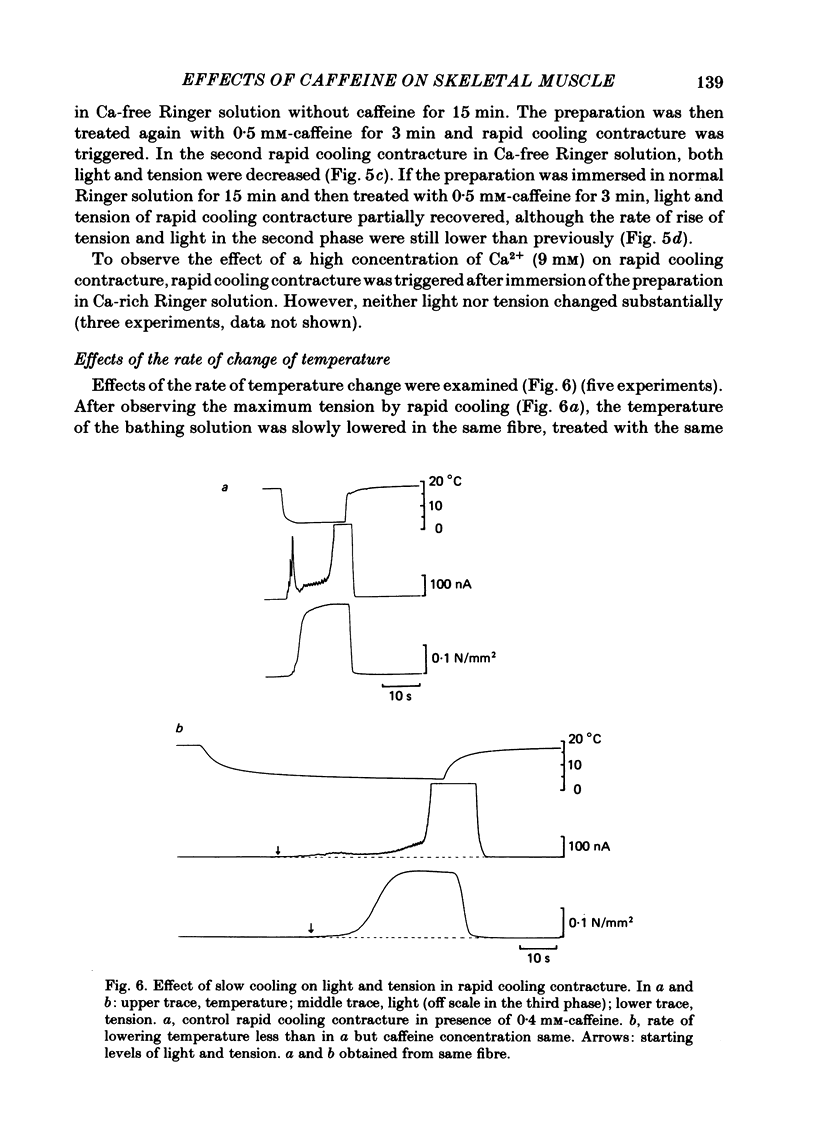
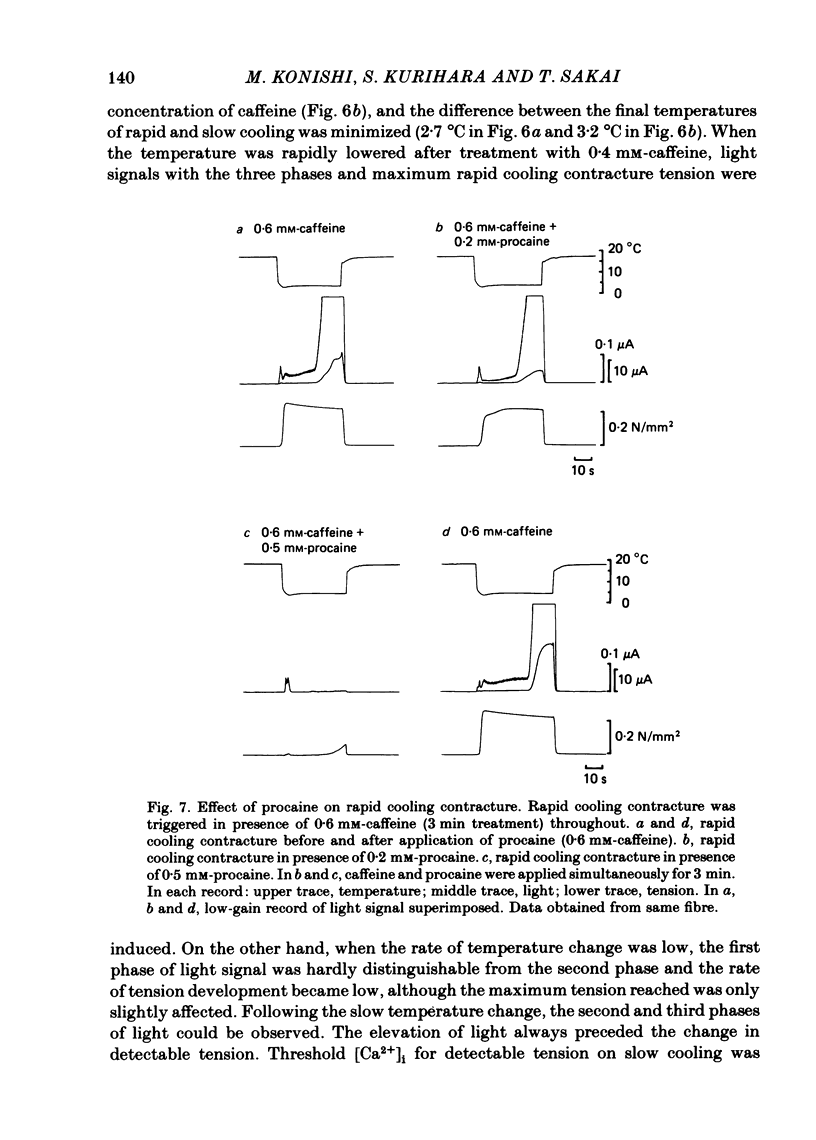
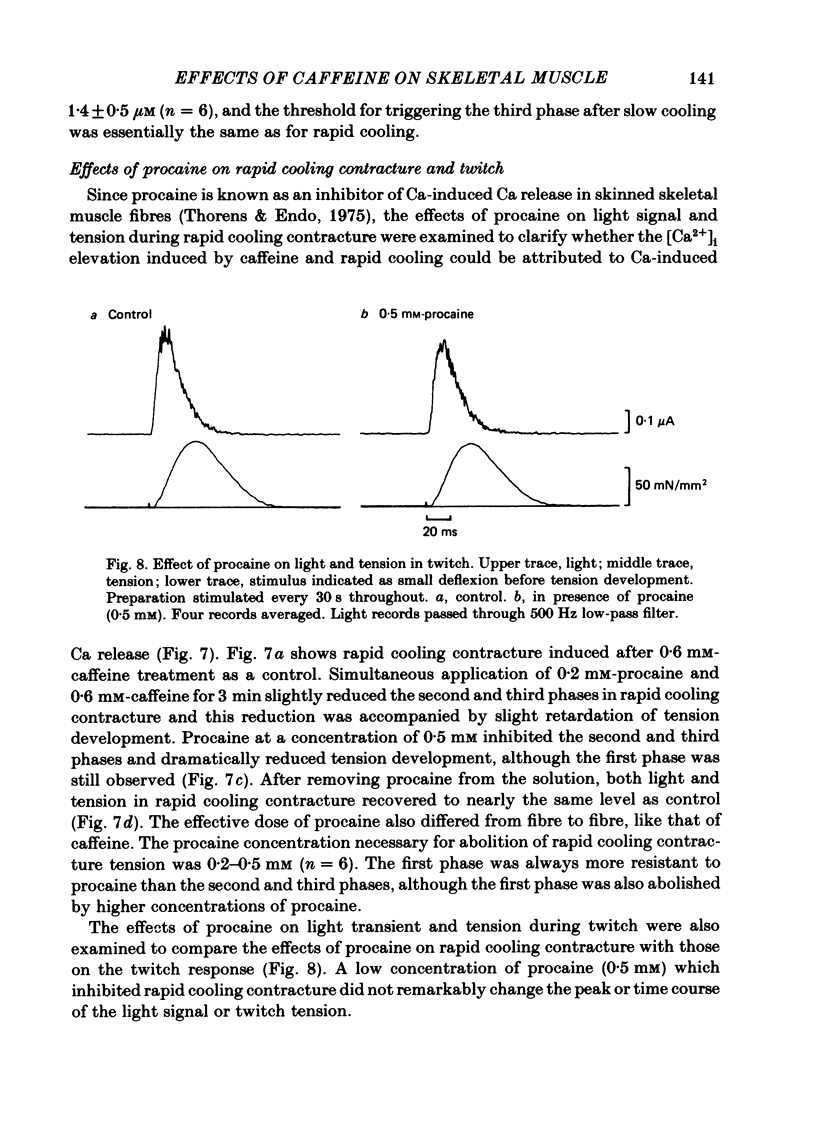
In a single skeletal muscle fibre treated with concentrations of caffeine below threshold for caffeine contracture, rapid lowering of the temperature of the bathing solution from 18 degrees C to below 5 degrees C induced a contracture (rapid cooling contracture). Intracellular Ca2+ concentration ([Ca2+]i) was recorded during rapid cooling contracture using aequorin. Low concentrations of caffeine often caused a slight elevation of the light signal in resting muscle without detectable tension. During rapid cooling contracture, the change in light signal occurred in three phases. The first phase was a transient change of [Ca2+]i accompanying slight tension. During the second phase, the light signal slowly increased as cooling produced maximum tension development. The third phase was an additional light signal induced after the second phase, even though the tension was saturated. The second and third phases were more sensitive to low concentrations of procaine (0.2-0.5 mM) than the first phase. Synchronous oscillations of light and tension were often observed during the second phase. The light signal during rapid cooling contracture was only slightly affected by long incubation in Ca-free or Ca-rich solutions. These results are interpreted as follows. A low concentration of caffeine elevates cytoplasmic resting Ca2+ level without tension development. The oscillations of light and tension often observed in the second phase might represent a cyclic release of Ca2+ from the sarcoplasmic reticulum (s.r.). The third phase is considered to be due to a massive Ca2+ release by a Ca-induced Ca-release mechanism which might be similar to that in skinned fibres. The second phase is probably essential for generation of rapid cooling contracture tension and the third phase represents an excess Ca2+ for tension development.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S. The effects of drugs on calcium uptake and calcium release by mitochondria and sarcoplasmic reticulum of frog skeletal muscle. Biochem Pharmacol. 1974 Jan 1;23(1):89–101. doi: 10.1016/0006-2952(74)90316-5. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brandt N. R., Caswell A. H., Brunschwig J. P. ATP-energized Ca2+ pump in isolated transverse tubules of skeletal muscle. J Biol Chem. 1980 Jul 10;255(13):6290–6298. [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A., McLaughlin S. G. Release of bound sodium in single muscle fibers. Can J Physiol Pharmacol. 1967 Jul;45(4):655–667. doi: 10.1139/y67-078. [DOI] [PubMed] [Google Scholar]
- Inesi G., Watanabe S. Temperature dependence of ATP hydrolysis and calcium uptake by fragmented sarcoplasmic membranes. Arch Biochem Biophys. 1967 Sep;121(3):665–671. doi: 10.1016/0003-9861(67)90051-3. [DOI] [PubMed] [Google Scholar]
- Kovács L., Szücs G. Effect of caffeine on intramembrane charge movement and calcium transients in cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:559–578. doi: 10.1113/jphysiol.1983.sp014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbaraci N. M., Nastuk W. L. Action of caffeine in excitation-contraction coupling of frog skeletal muscle fibres. J Physiol. 1982 Apr;325:195–211. doi: 10.1113/jphysiol.1982.sp014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara S., Konishi M., Miyagishima T., Sakai T. Effects of enflurane on excitation-contraction coupling in frog skeletal muscle fibers. Pflugers Arch. 1984 Dec;402(4):345–352. doi: 10.1007/BF00583934. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Konishi M., Sakai T. Changes in [Ca2+]i induced by rapid cooling of single skeletal muscle fibres treated with low concentration of caffeine. Adv Exp Med Biol. 1984;170:565–568. doi: 10.1007/978-1-4684-4703-3_50. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan A., Wilson T. L., Reeves R. B. Intracellular pH in cold-blooded vertebrates as a function of body temperature. Respir Physiol. 1976 Oct;28(1):29–47. doi: 10.1016/0034-5687(76)90083-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Calcium-induced calcium release from sarcoplasmic reticulum vesicles. J Biochem. 1981 Sep;90(3):749–755. doi: 10.1093/oxfordjournals.jbchem.a133529. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. The influence of hydrogen ion concentration on calcium binding and release by skeletal muscle sarcoplasmic reticulum. J Gen Physiol. 1972 Jan;59(1):22–32. doi: 10.1085/jgp.59.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Ebashi S. Ca-releasing action of beta, gamma-methylene adenosine triphosphate on fragmented sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1149–1157. doi: 10.1093/oxfordjournals.jbchem.a131370. [DOI] [PubMed] [Google Scholar]
- Sakai T., Geffner E. S., Sandow A. Caffeine contracture in muscle with disrupted transverse tubules. Am J Physiol. 1971 Mar;220(3):712–717. doi: 10.1152/ajplegacy.1971.220.3.712. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Somlyo A. P. Calcium and magnesium contents and volume of the terminal cisternae in caffeine-treated skeletal muscle. J Cell Biol. 1984 Aug;99(2):558–568. doi: 10.1083/jcb.99.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


