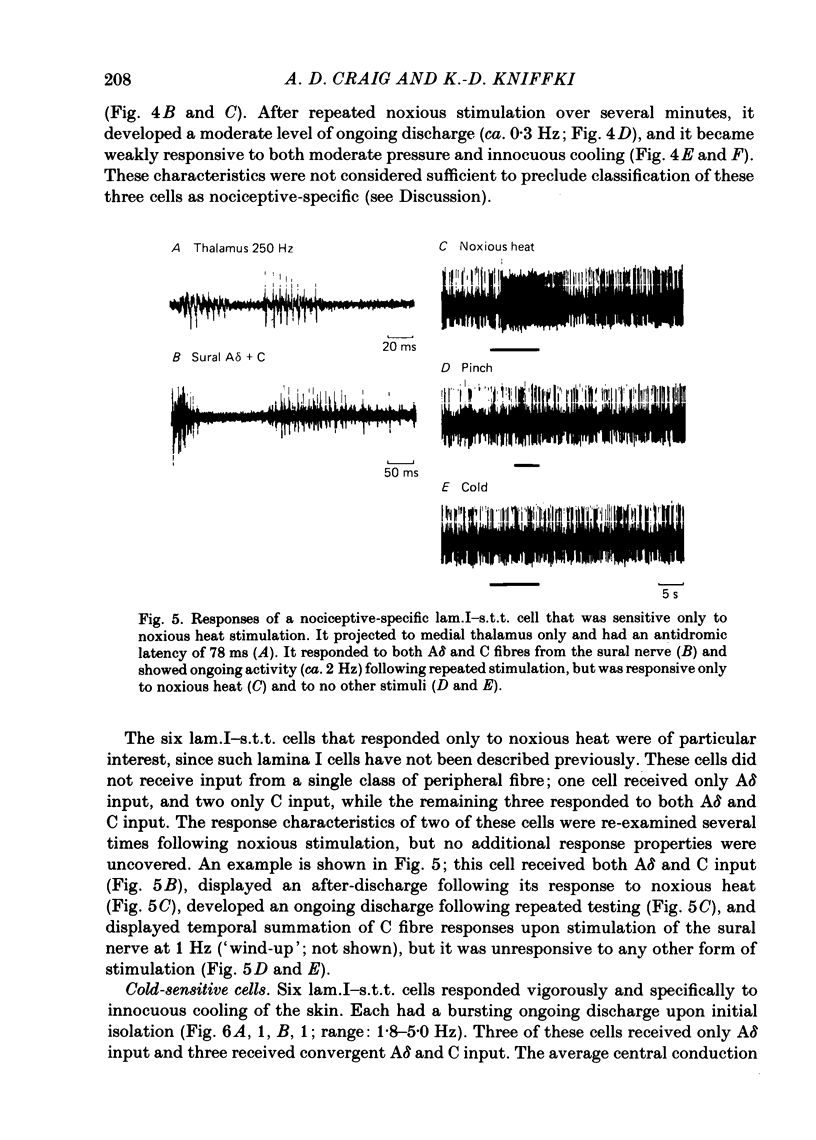
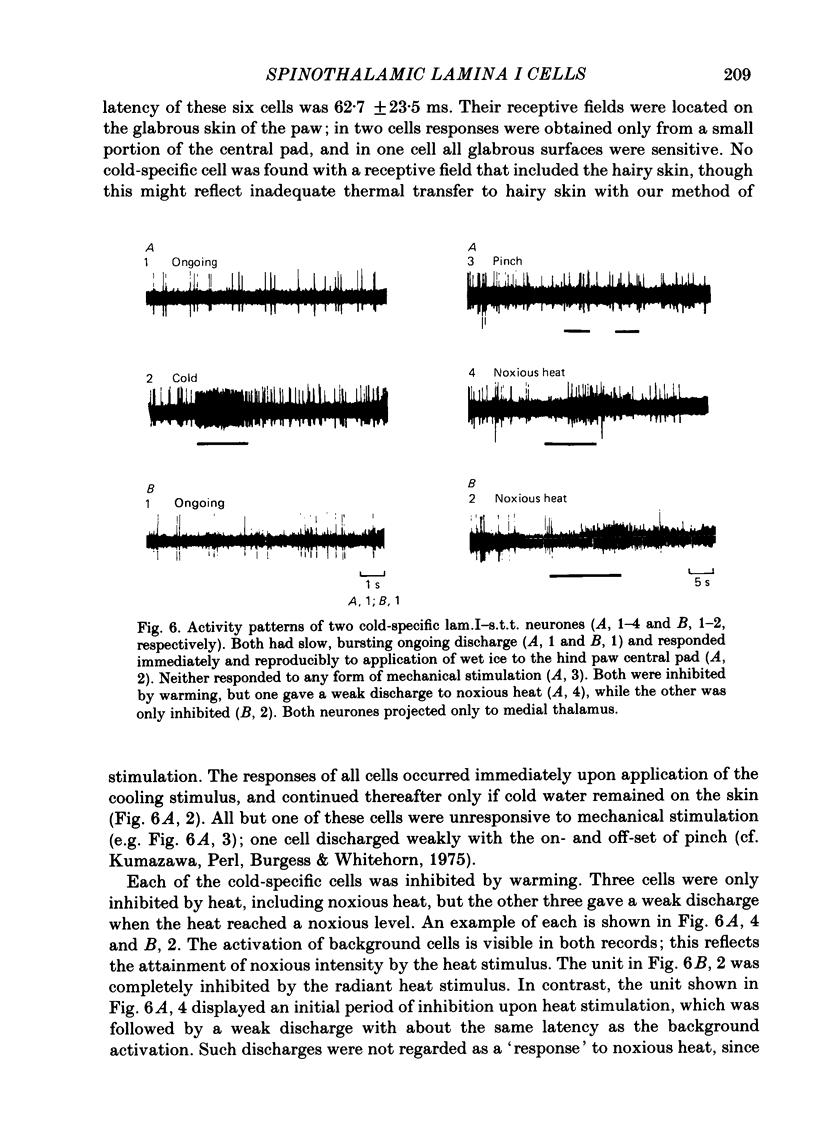
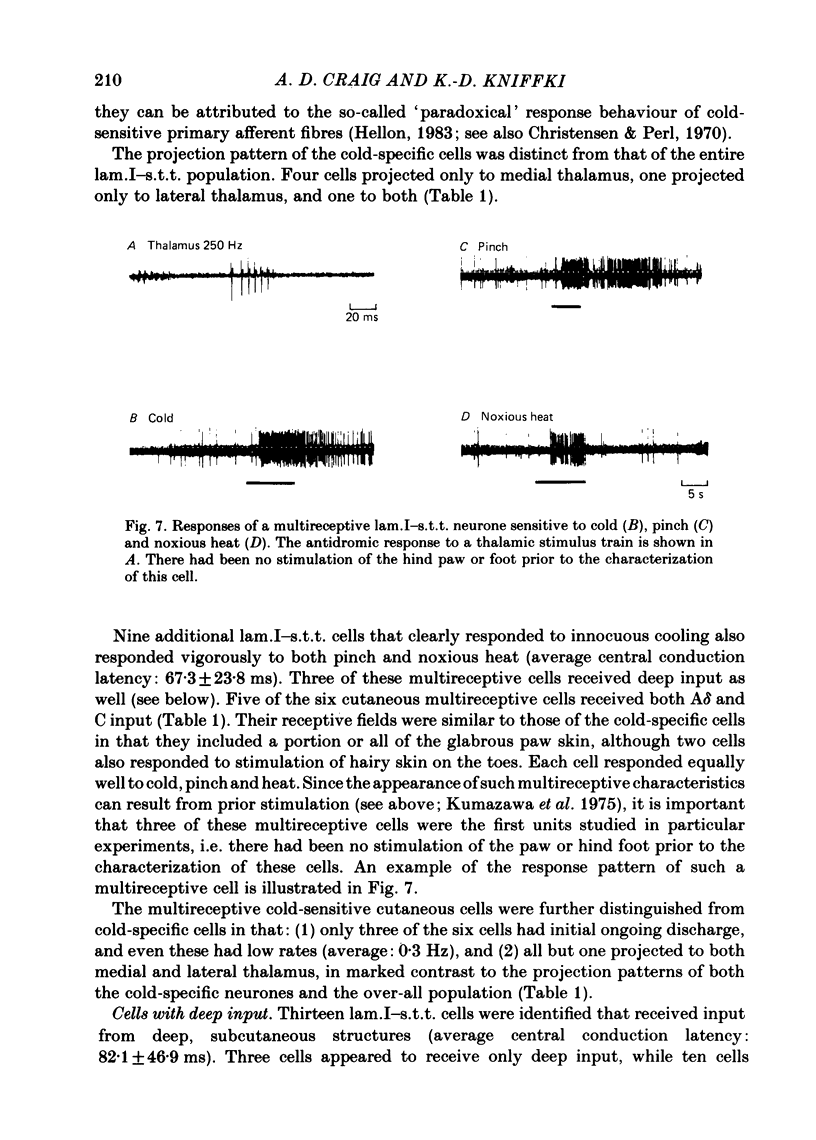
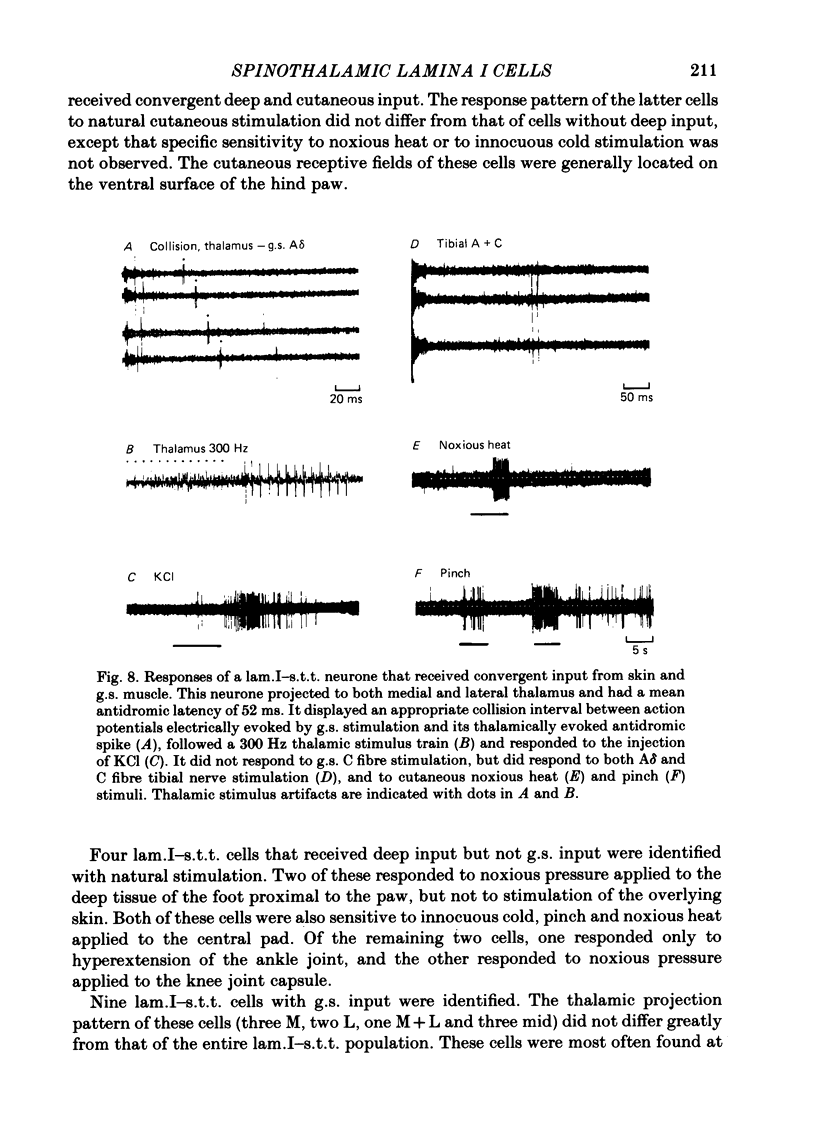
Abstract
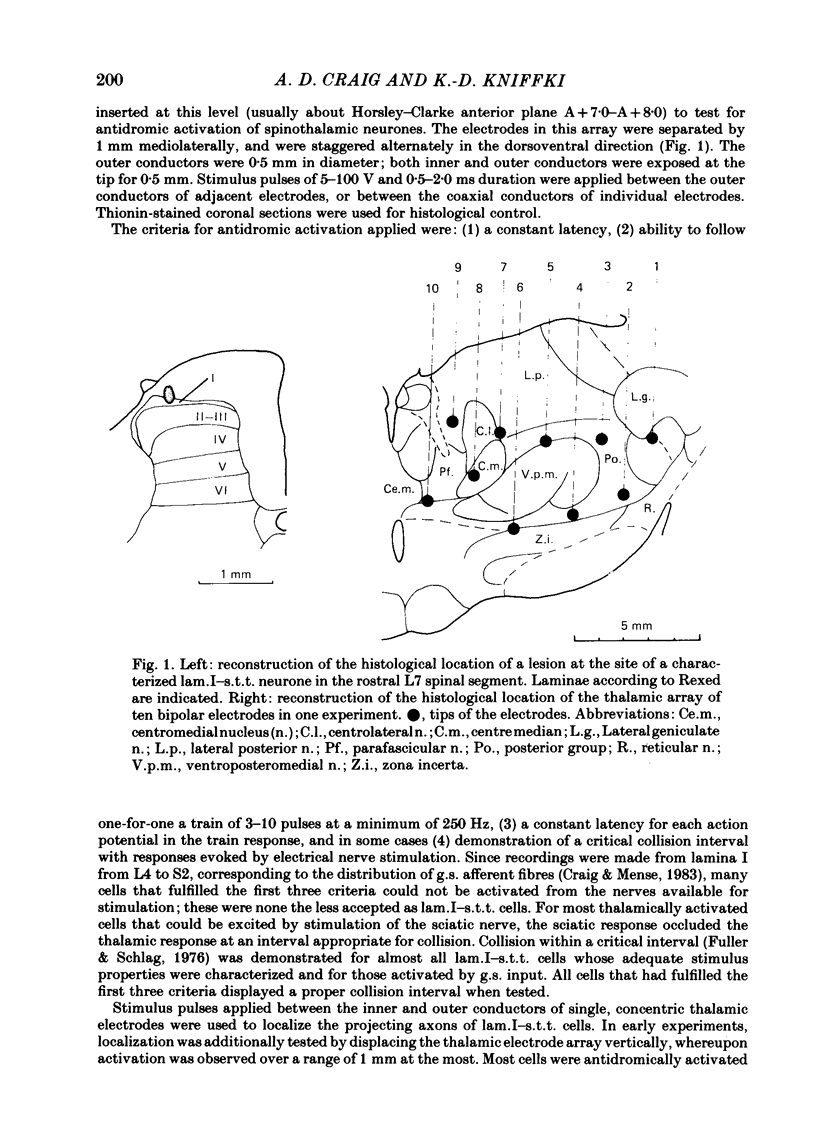
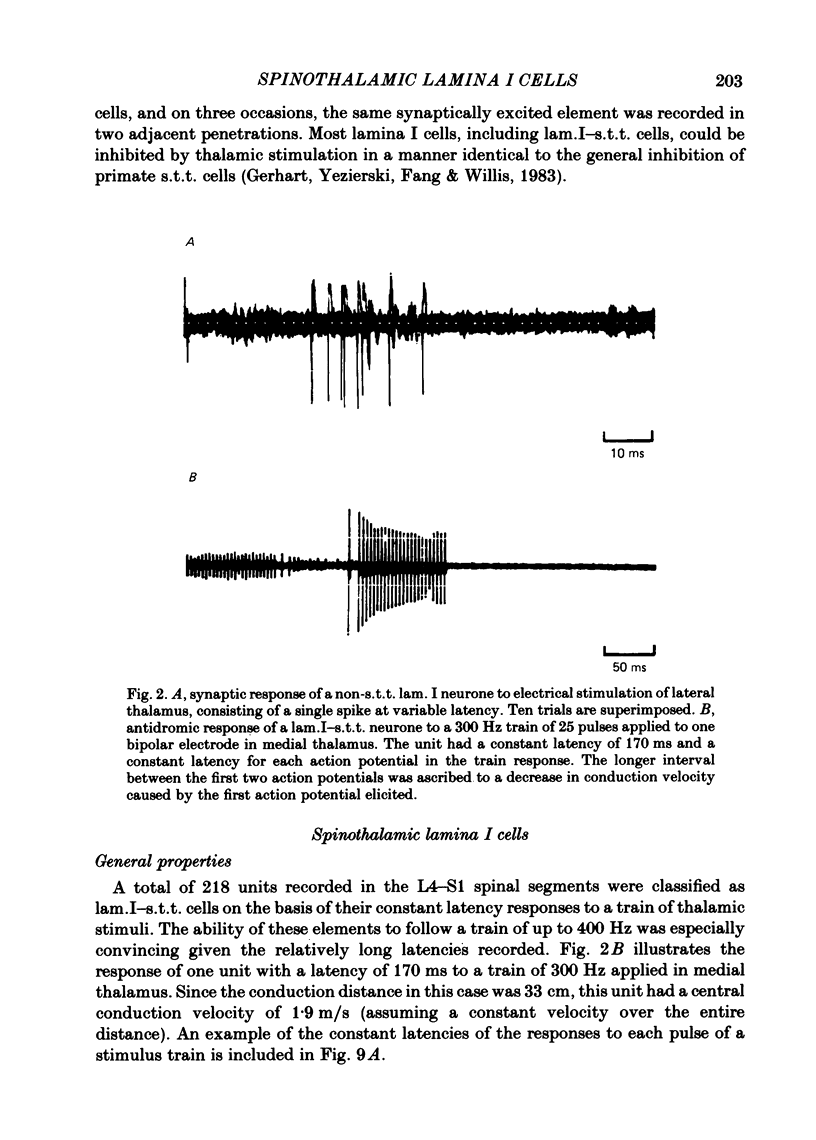
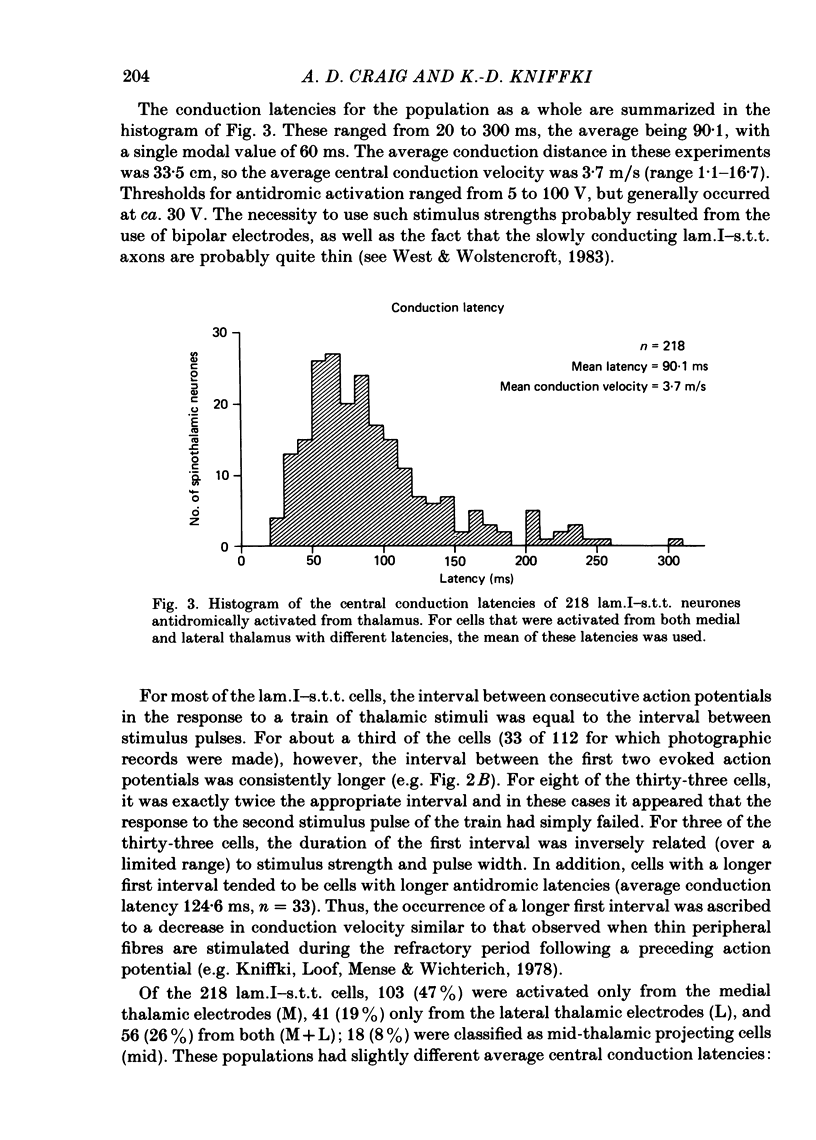
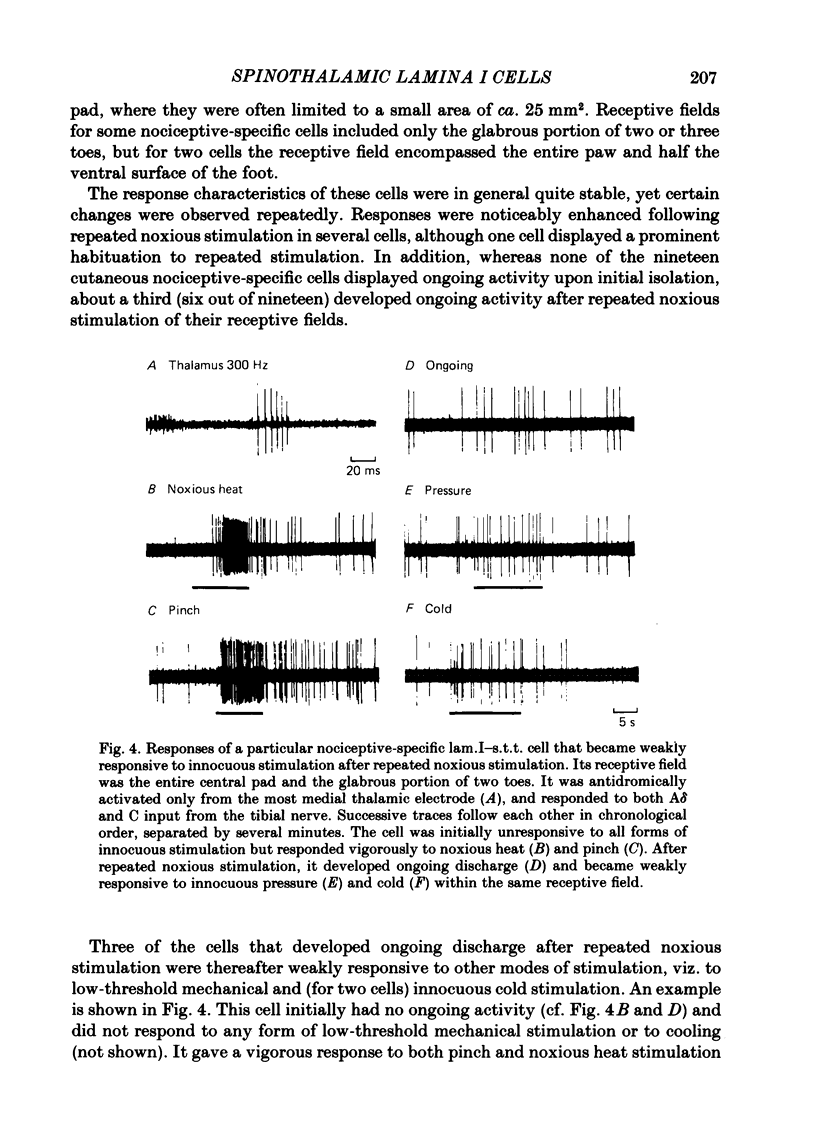
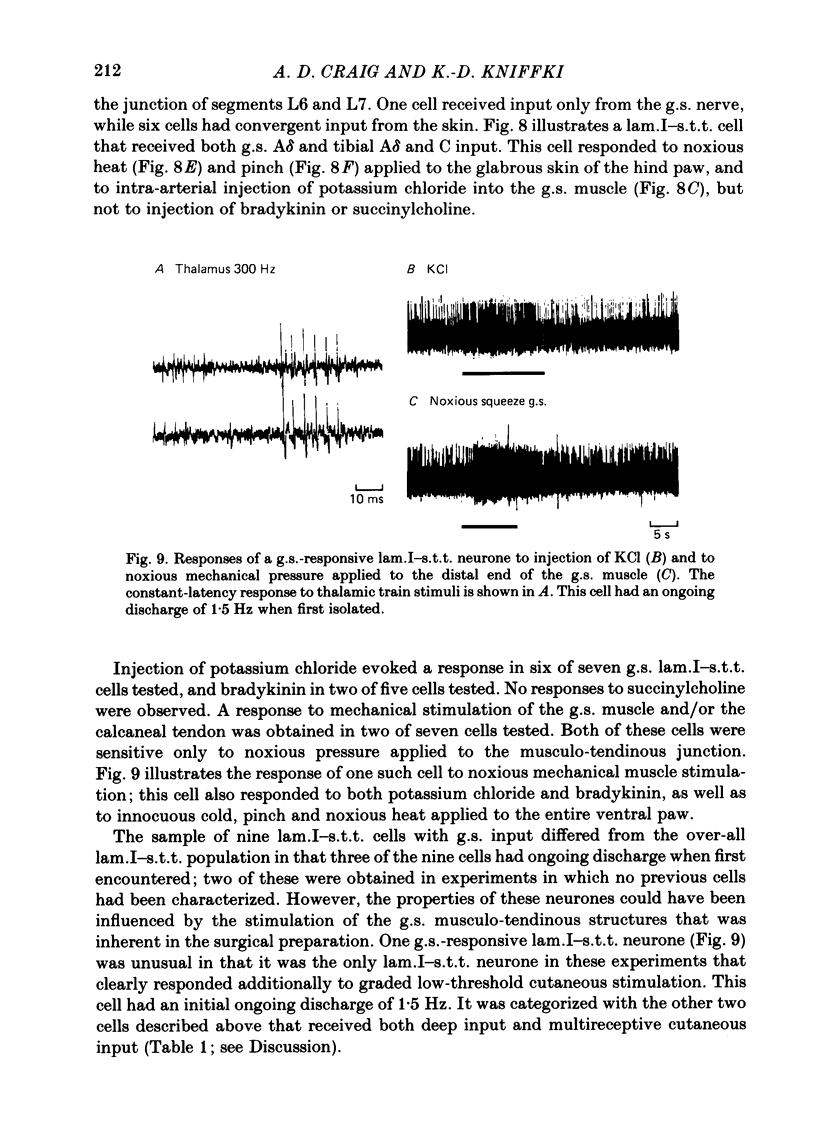
The response characteristics of lamina I neurones recorded extracellularly in the lumbosacral enlargement of chloralose-anaesthetized cats were examined with peripheral nerve electrical stimulation, adequate mechanical and thermal stimulation of hind-limb skin, and algesic mechanical and chemical stimulation of musculotendinous structures, particularly the gastrocnemius-soleus (g.s.) muscle. Antidromic activation from an electrode array that spanned the contralateral thalamus was used to identify lamina I spinothalamic tract (lam.I-s.t.t.) neurones. Recordings were made from a total of 218 lumbosacral lam.I-s.t.t. neurones. Their mean central conduction latency was 90.1 ms (range 20-300 ms), corresponding to a mean conduction velocity of 3.7 m/s (range 1.1-16.7). Neurones responsive only to peripheral A delta fibre stimulation had significantly shorter central conduction latencies (mean = 62.8 ms) than those with both A delta and C fibre input (mean = 81.9 ms) and those with only C fibre input (mean = 134.6 ms). Of these 218 neurones, 103 (47%) projected only to medial thalamus, 41 (19%) only to lateral thalamus, and 56 (26%) to both; 18 (8%) were classified as mid-thalamic projecting cells. About 10% of all cells had ongoing activity when first isolated. Ninety-three lam.I-s.t.t. neurones responded to stimulation of the sciatic nerve. The response characteristics of forty-seven of these were examined with the complete set of stimuli used. Twenty-four non-s.t.t. lamina I neurones were also characterized for comparison. Twenty-eight of the lam.I-s.t.t. neurones tested with the complete set of stimuli responded specifically to either cutaneous noxious (n = 19), cutaneous innocuous cold (n = 6) or algesic musculo-tendinous (n = 3) stimulation. Thirteen neurones responded to cutaneous noxious stimulation, and, in addition, to cold stimulation (n = 6), to deep stimulation (n = 4), or to both (n = 3). Six cells did not respond to any of the natural stimuli employed. All of the cold-specific and many of the multireceptive cold-sensitive neurones had ongoing discharge. The average central conduction latencies of cold-sensitive neurones (65.5 ms) and unresponsive neurones (48.7 ms) were shorter than that of nociceptive neurones (91.2 ms). Two response categories had distinct thalamic projection patterns. The majority of cold-specific neurones projected only to medial thalamus. Almost all multireceptive cold-sensitive neurones projected to both medial and lateral thalamus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP G. H. The relation between nerve fiber size and sensory modality: phylogenetic implications of the afferent innervation of cortex. J Nerv Ment Dis. 1959 Feb;128(2):89–114. [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Berkley K. J. Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol. 1980 Sep 1;193(1):283–317. doi: 10.1002/cne.901930119. [DOI] [PubMed] [Google Scholar]
- Carstens E., Trevino D. L. Laminar origins of spinothalamic projections in the cat as determined by the retrograde transport of horseradish peroxidase. J Comp Neurol. 1978 Nov 1;182(1):161–165. [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Ascending projections of nociceptor-driven Lamina I neurones in the cat. Exp Brain Res. 1979 Mar 9;35(1):135–149. doi: 10.1007/BF00236790. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983 Apr;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr, Burton H. Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol. 1981 Mar;45(3):443–466. doi: 10.1152/jn.1981.45.3.443. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr, Wiegand S. J., Price J. L. The thalamo-cortical projection of the nucleus submedius in the cat. J Comp Neurol. 1982 Mar 20;206(1):28–48. doi: 10.1002/cne.902060105. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett. 1983 Nov 11;41(3):233–238. doi: 10.1016/0304-3940(83)90456-1. [DOI] [PubMed] [Google Scholar]
- Dawson N. J., Hellon R. F. Facilitation and suppression of antidromic invasion by orthodromic impulses in the cat [proceedings]. J Physiol. 1979 Apr;289:57P–58P. [PubMed] [Google Scholar]
- Dostrovsky J. O., Hellon R. F. The representation of facial temperature in the caudal trigeminal nucleus of the cat. J Physiol. 1978 Apr;277:29–47. doi: 10.1113/jphysiol.1978.sp012258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R., Bennett G. J. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Schmidt R. F., Willis W. D. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979 Jan;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller J. H., Schlag J. D. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976 Aug 13;112(2):283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Gerhart K. D., Yezierski R. P., Fang Z. R., Willis W. D. Inhibition of primate spinothalamic tract neurons by stimulation in ventral posterior lateral (VPLc) thalamic nucleus: possible mechanisms. J Neurophysiol. 1983 Feb;49(2):406–423. doi: 10.1152/jn.1983.49.2.406. [DOI] [PubMed] [Google Scholar]
- Gobel S., Falls W. M., Humphrey E. Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: candidates for the terminal axonal arbors of primary neurons with unmyelinated (C) axons. J Neurosci. 1981 Oct;1(10):1163–1179. doi: 10.1523/JNEUROSCI.01-10-01163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda C. N., Mense S., Perl E. R. Neurons in ventrobasal region of cat thalamus selectively responsive to noxious mechanical stimulation. J Neurophysiol. 1983 Mar;49(3):662–673. doi: 10.1152/jn.1983.49.3.662. [DOI] [PubMed] [Google Scholar]
- Kniffki K. D., Mizumura K. Responses of neurons in VPL and VPL-VL region of the cat to algesic stimulation of muscle and tendon. J Neurophysiol. 1983 Mar;49(3):649–661. doi: 10.1152/jn.1983.49.3.649. [DOI] [PubMed] [Google Scholar]
- Light A. R., Perl E. R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979 Jul 15;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J., Sjölund B. H. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983 Nov;50(5):1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- West D. C., Wolstencroft J. H. Strength-duration characteristics of myelinated and non-myelinated bulbospinal axons in the cat spinal cord. J Physiol. 1983 Apr;337:37–50. doi: 10.1113/jphysiol.1983.sp014610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg M., Blomqvist A. The spinomesencephalic tract in the cat: its cells of origin and termination pattern as demonstrated by the intraaxonal transport method. Brain Res. 1984 Jan 16;291(1):1–18. doi: 10.1016/0006-8993(84)90645-0. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Kenshalo D. R., Jr, Leonard R. B. The cells of origin of the primate spinothalamic tract. J Comp Neurol. 1979 Dec 15;188(4):543–573. doi: 10.1002/cne.901880404. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983 Dec 10;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]



