Abstract
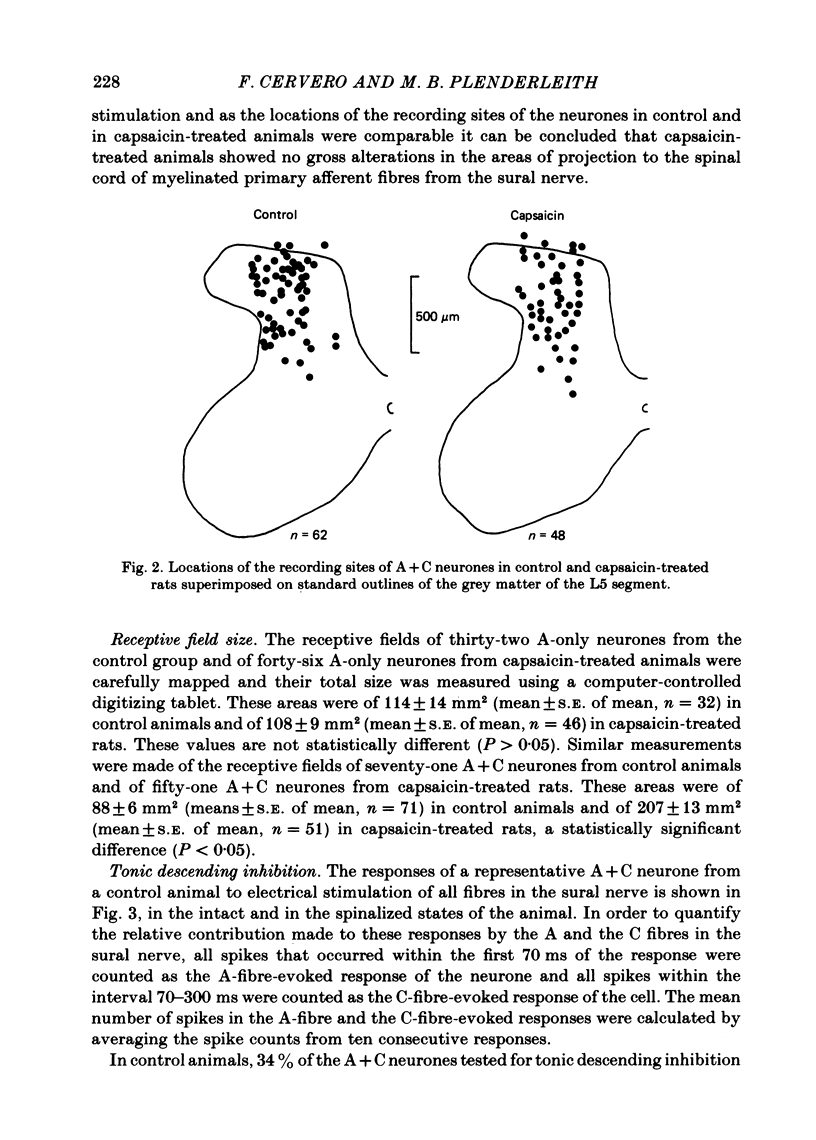
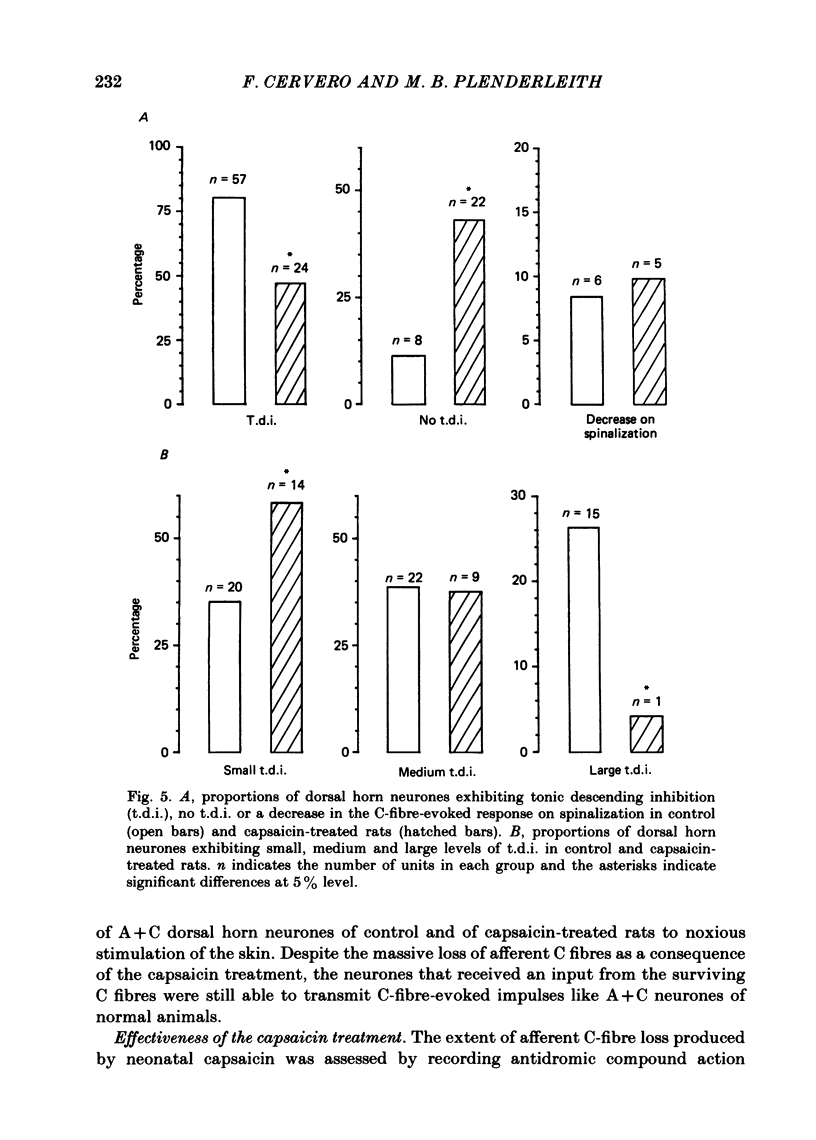
Single unit electrical activity has been recorded from dorsal horn neurones in the lumbar cord of rats anaesthetized with sodium pentobarbitone. Three groups of animals were used: normal adult rats, adult rats that had been treated at birth with capsaicin (50 mg kg-1 s.c.) and adult rats that had been injected at birth with the drug vehicle only. Rats treated at birth with capsaicin showed a substantial reduction in the number of afferent C fibres as indicated by the virtual absence of C waves in the compound action potentials evoked in the sural nerve by antidromic stimulation of the L4-L6 dorsal roots. No significant differences were found in any of the parameters measured between the vehicle treated and the untreated animals. Therefore, rats from these two groups are referred to as control animals. All dorsal horn neurones studied were driven by electrical stimulation of the A fibres in the ipsilateral sural nerve and had cutaneous receptive fields in the ipsilateral hind limb. Two groups of neurone were distinguished: those receiving an input from A fibres only (A only) and those neurones that could also be driven by sural C fibres (A + C). In the control group, 56% of the neurones were A only and 44% were A + C. In capsaicin-treated rats these proportions were significantly different: 78% and 22% respectively. No differences were found in receptive field sizes of A-only neurones between those recorded in control rats and those from capsaicin-treated animals. However, a large and significant increase in receptive field size of A + C neurones was observed in capsaicin-treated rats compared to their counterparts in normal animals. In control rats 80% of the A + C neurones showed tonic descending inhibition of their C-fibre-evoked responses as assessed by reversible spinalization. In capsaicin-treated rats this proportion fell to 47% of the A + C neurones. The magnitude of the tonic descending inhibition was also reduced in the fewer A + C neurones of capsaicin-treated rats that were subjected to it. Only 4% of A + C neurones with tonic descending inhibition in capsaicin-treated rats were powerfully inhibited compared to 26% in control animals. The mean number of spikes evoked by C-fibre stimulation of the sural nerve in A + C neurones of control and of capsaicin-treated rats was not significantly different between these two groups of animals in the intact and in the spinalized states.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck S. H., Miller M. S., Burks T. F. Depletion of primary afferent substance P by capsaicin and dihydrocapsaicin without altered thermal sensitivity in rats. Brain Res. 1982 Feb 4;233(1):216–220. doi: 10.1016/0006-8993(82)90945-3. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cervero F., McRitchie H. A. Neonatal capsaicin and thermal nociception: a paradox. Brain Res. 1981 Jun 29;215(1-2):414–418. doi: 10.1016/0006-8993(81)90527-8. [DOI] [PubMed] [Google Scholar]
- Cervero F., Plenderleith M. B. Dorsal root potentials are unchanged in adult rats treated at birth with capsaicin. J Physiol. 1984 Dec;357:357–368. doi: 10.1113/jphysiol.1984.sp015504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F., Shouenborg J., Sjölund B. H., Waddell P. J. Cutaneous inputs to dorsal horn neurones in adult rats treated at birth with capsaicin. Brain Res. 1984 May 28;301(1):47–57. doi: 10.1016/0006-8993(84)90401-3. [DOI] [PubMed] [Google Scholar]
- Coimbra A., Sodré-Borges B. P., Magalhães M. M. The substantia gelatinosa Rolandi of the rat. Fine structure, cytochemistry (acid phosphatase) and changes after dorsal root section. J Neurocytol. 1974 Jun;3(2):199–217. doi: 10.1007/BF01098389. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T., Johnson S. M. Supraspinal inhibition of the excitation of dorsal horn neurones by impulses in unmyelinated primary afferents: lack of effect by strychnine and bicuculline. Brain Res. 1981 Apr 6;210(1-2):231–241. doi: 10.1016/0006-8993(81)90896-9. [DOI] [PubMed] [Google Scholar]
- Duggan A. W. Pharmacology of descending control systems. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):375–391. doi: 10.1098/rstb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Gregor M., Zimmermann M. Characteristics of spinal neurones responding to cutaneous myelinated and unmyelinated fibres. J Physiol. 1972 Mar;221(3):555–576. doi: 10.1113/jphysiol.1972.sp009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker H. O., Iggo A., Zimmermann M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975 Jun;1(2):147–165. doi: 10.1016/0304-3959(75)90099-8. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Scadding J. W., Skingle M., Tyers M. B. Effects of neonatal administration of capsaicin on nociceptive thresholds in the mouse and rat. J Pharm Pharmacol. 1981 Mar;33(3):183–185. doi: 10.1111/j.2042-7158.1981.tb13748.x. [DOI] [PubMed] [Google Scholar]
- Holzer P., Jurna I., Gamse R., Lembeck F. Nociceptive threshold after neonatal capsaicin treatment. Eur J Pharmacol. 1979 Oct 15;58(4):511–514. doi: 10.1016/0014-2999(79)90327-3. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Kelly J. S., Emson P. C., Kimmel J. R., Miller R. J., Wu J. Y. An immunohistochemical study of neuronal populations containing neuropeptides or gamma-aminobutyrate within the superficial layers of the rat dorsal horn. Neuroscience. 1981;6(10):1883–1898. doi: 10.1016/0306-4522(81)90029-4. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Király E. Distribution of chemosensitive primary sensory afferents in the central nervous system of the rat. J Comp Neurol. 1980 Apr 15;190(4):781–792. doi: 10.1002/cne.901900409. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Molony V. Fine glass micro-electrodes for recording from small neurones in the spinal cord of the cat [proceedings]. J Physiol. 1978 Nov;284:27P–28P. [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P., Iversen L. L., Emson P. C. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6(10):1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Iversen L. L., Goedert M., Chapman D., Hunt S. P. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983 Feb;3(2):399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. I., Vincent S. R., Staines W. A., Fibiger H. C., Reisine T. D., Yamamura H. I. Neurotoxic action of capsaicin on spinal substance P neurons. Brain Res. 1980 Mar 31;186(2):435–444. doi: 10.1016/0006-8993(80)90987-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Coimbra A. Capsaicin causes selective damage to type I synaptic glomeruli in rat substantia gelatinosa. Brain Res. 1984 Jan 9;290(2):380–383. doi: 10.1016/0006-8993(84)90961-2. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Functional and topographical properties of field potentials evoked in rat dorsal horn by cutaneous C-fibre stimulation. J Physiol. 1984 Nov;356:169–192. doi: 10.1113/jphysiol.1984.sp015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J., Sjölund B. H. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983 Nov;50(5):1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Fitzgerald M., Nussbaumer J. C., Van der Loos H., Devor M. Somatotopic maps are disorganized in adult rodents treated neonatally with capsaicin. Nature. 1982 Feb 25;295(5851):691–693. doi: 10.1038/295691a0. [DOI] [PubMed] [Google Scholar]


