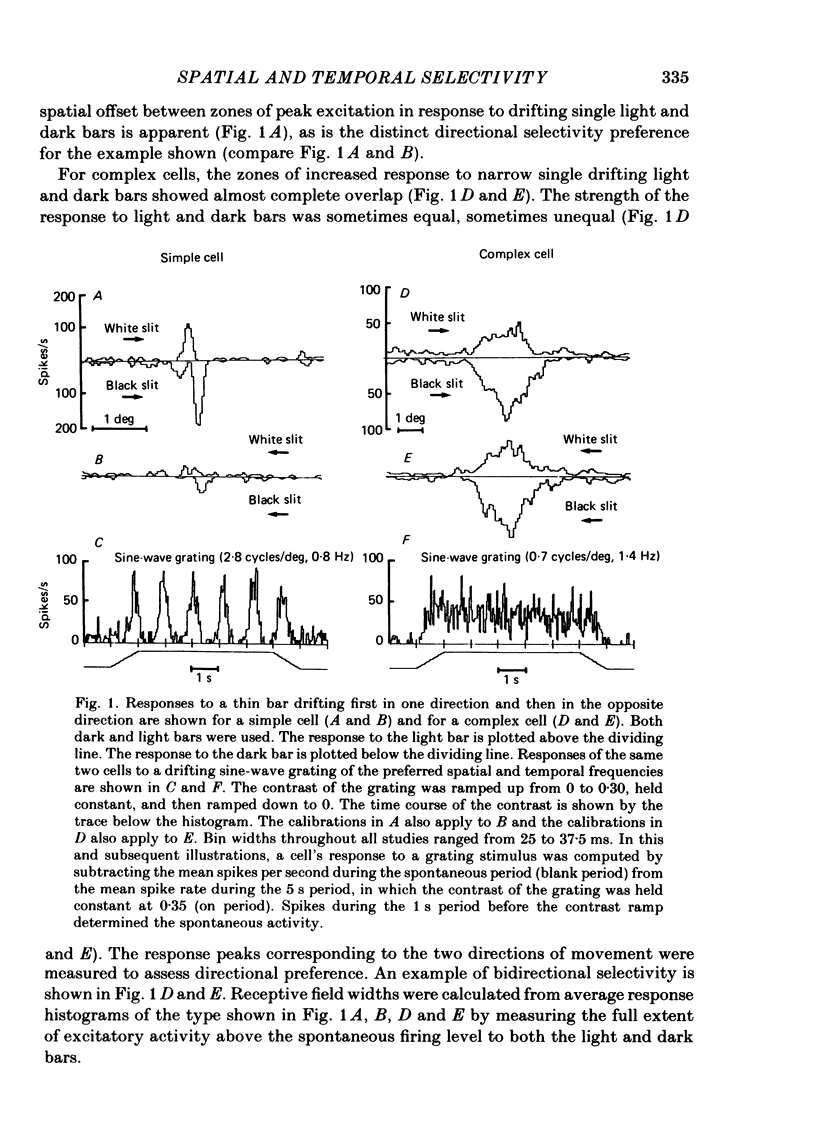
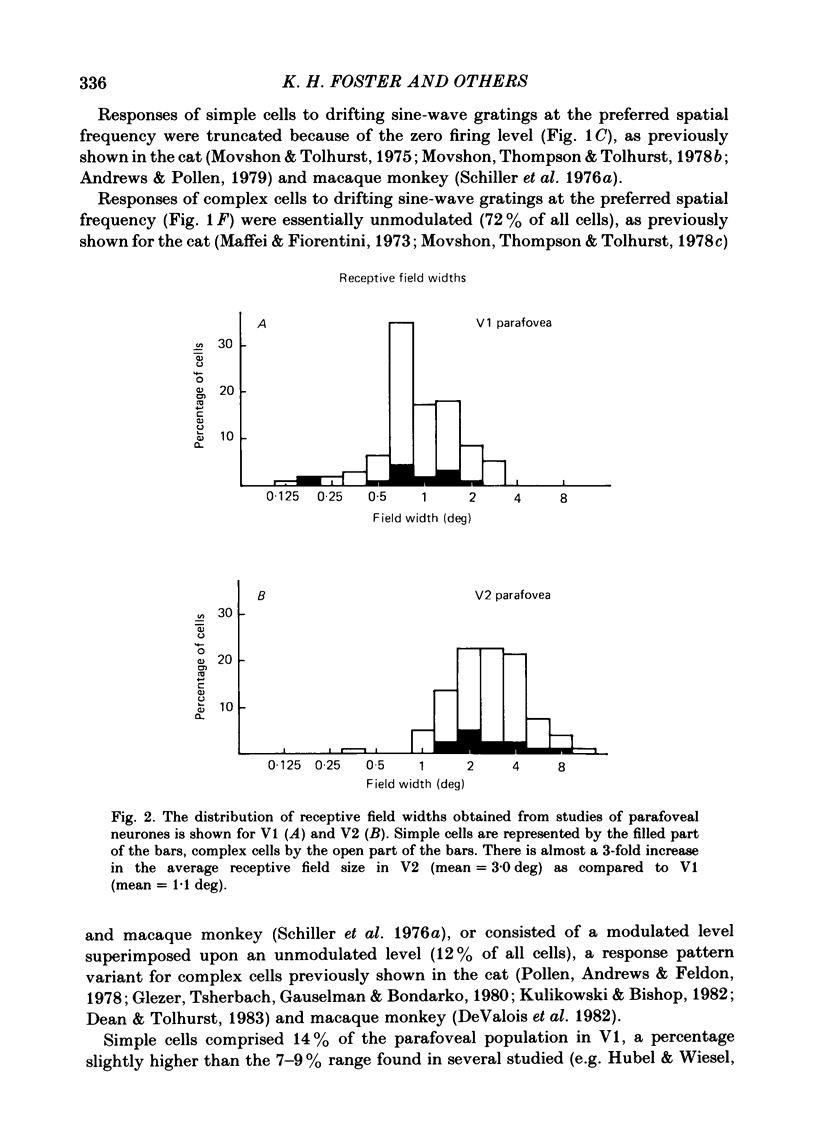
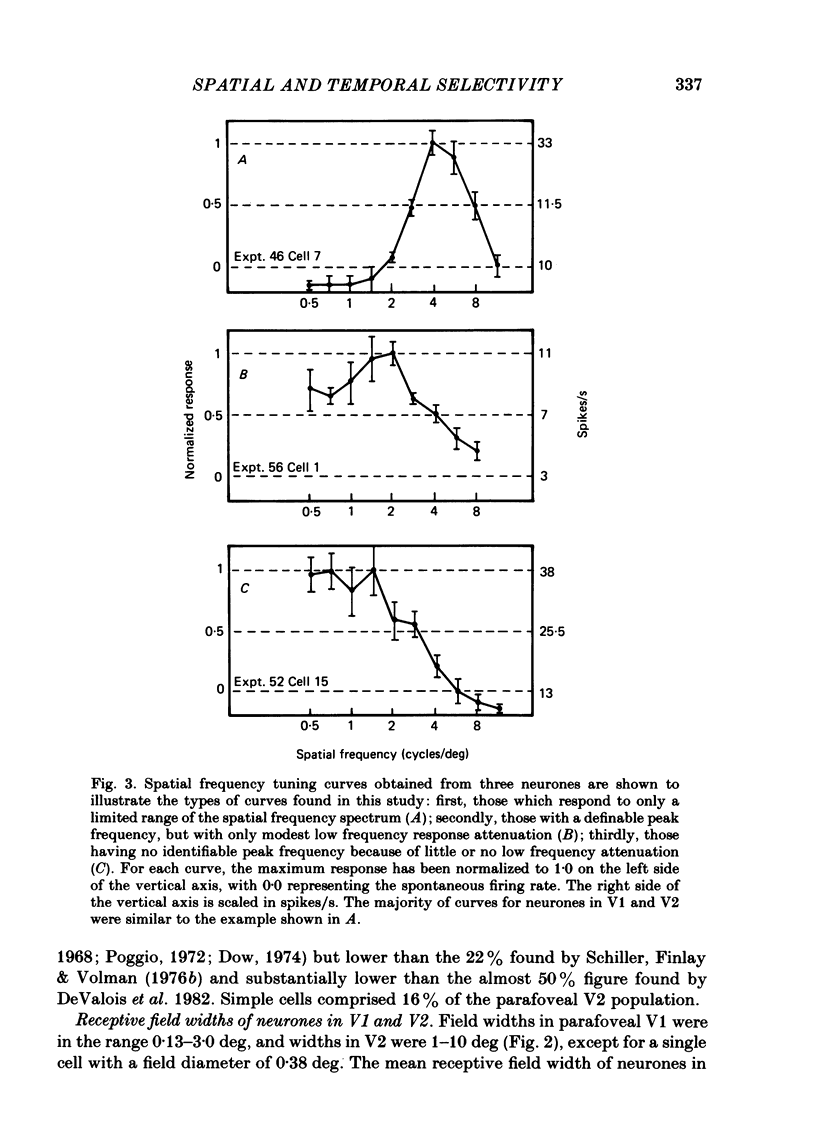
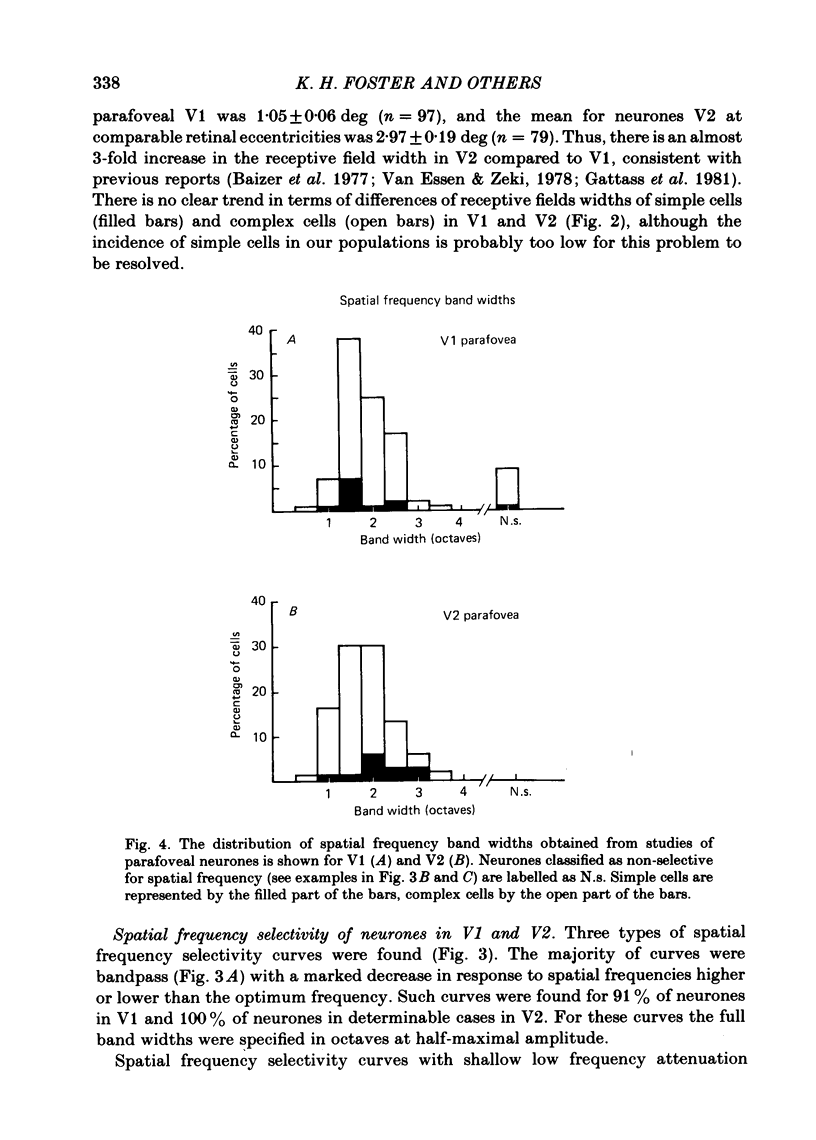
Abstract
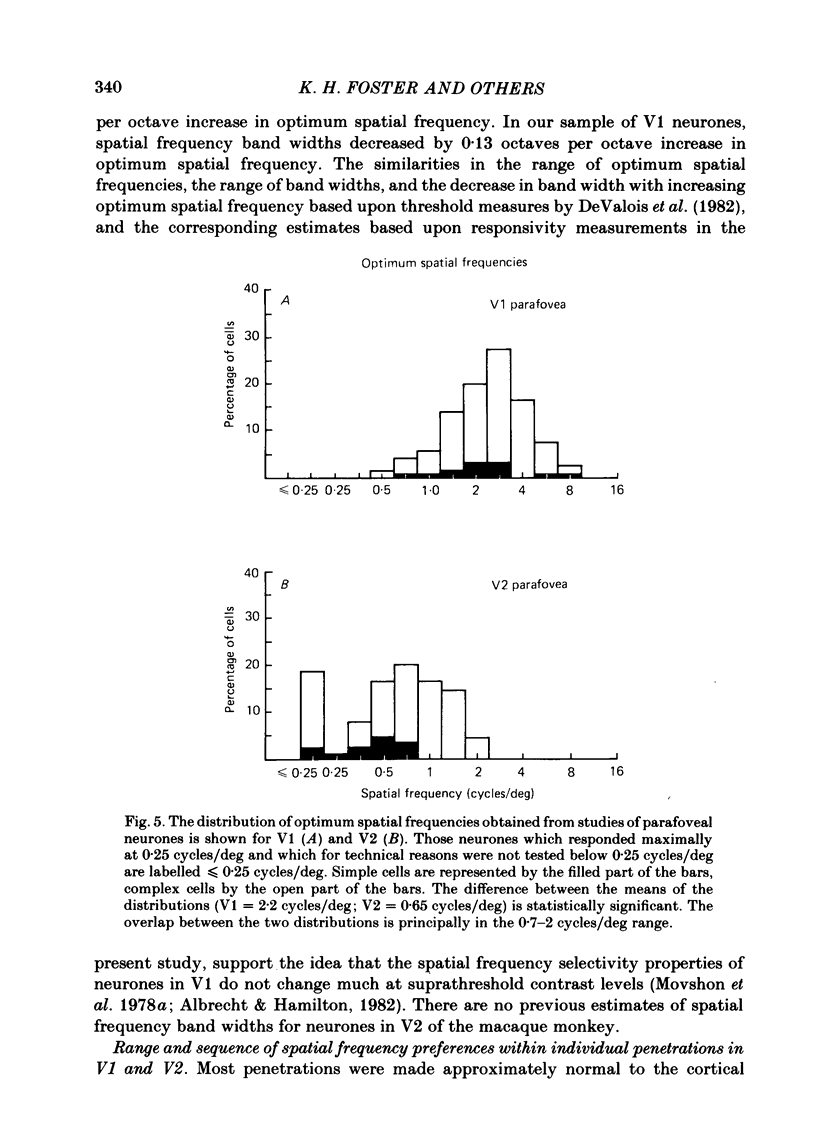
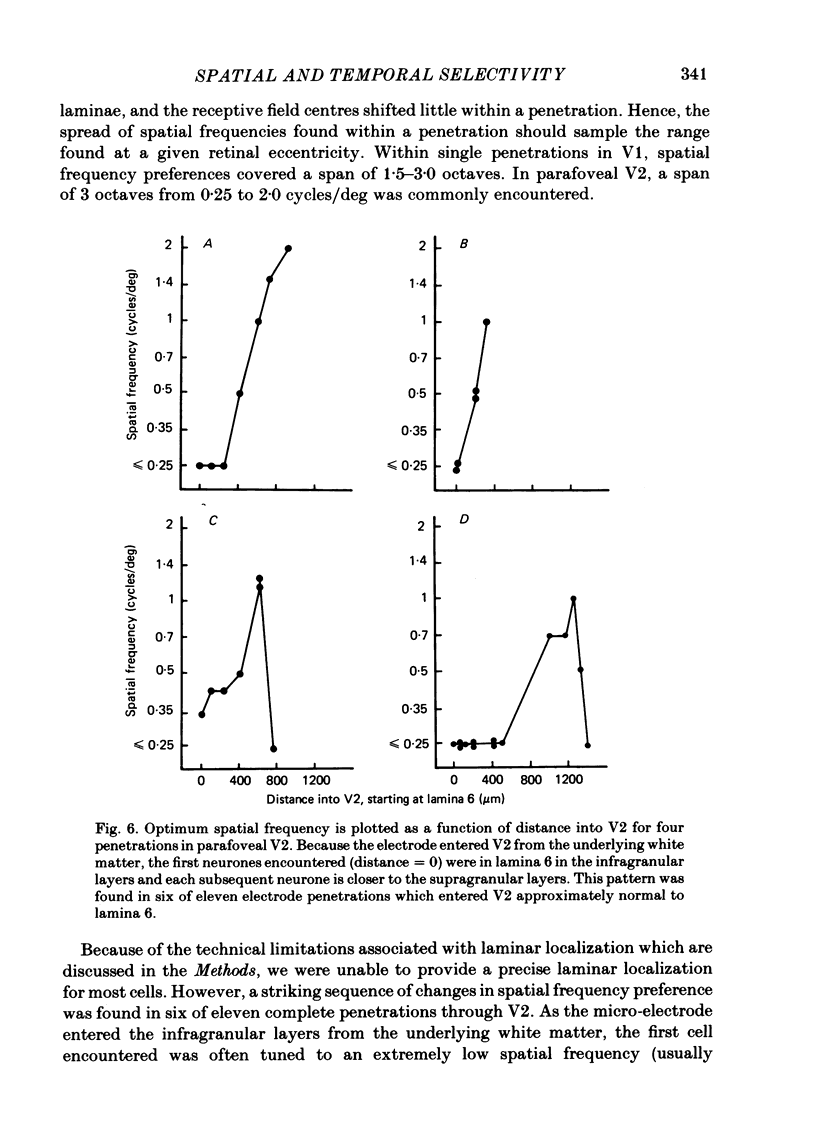
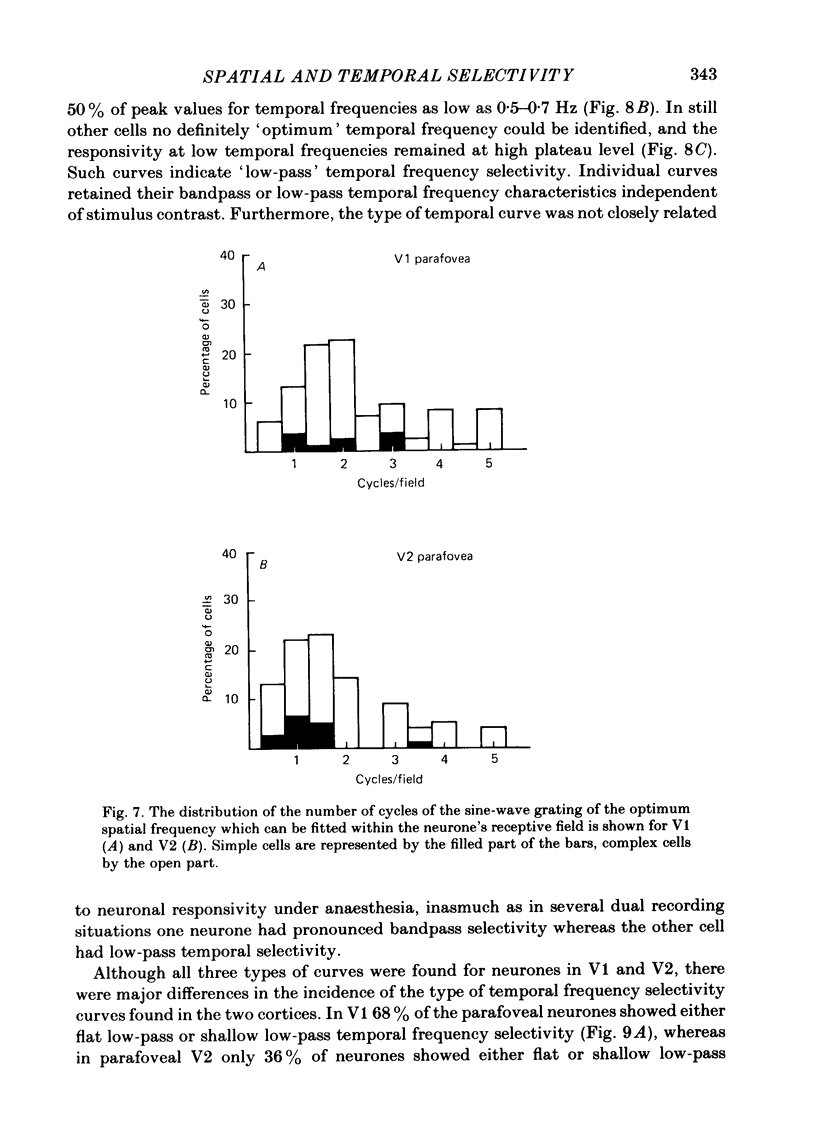
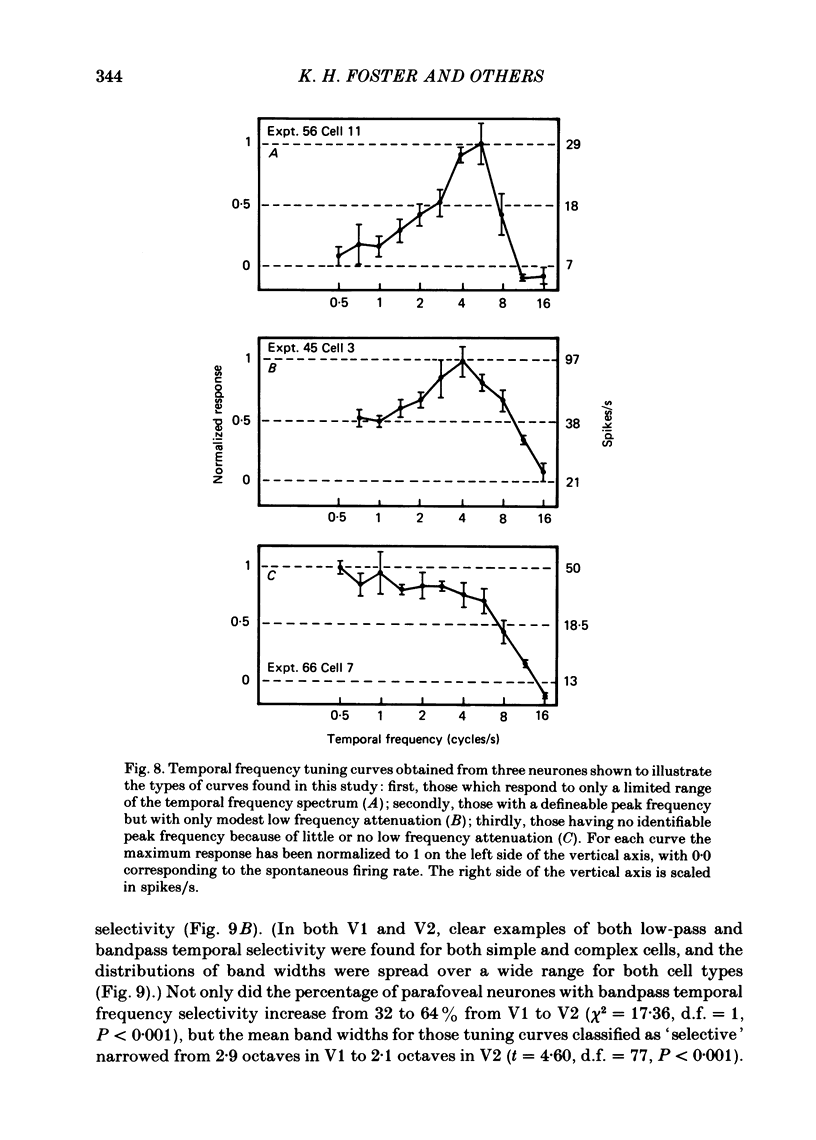
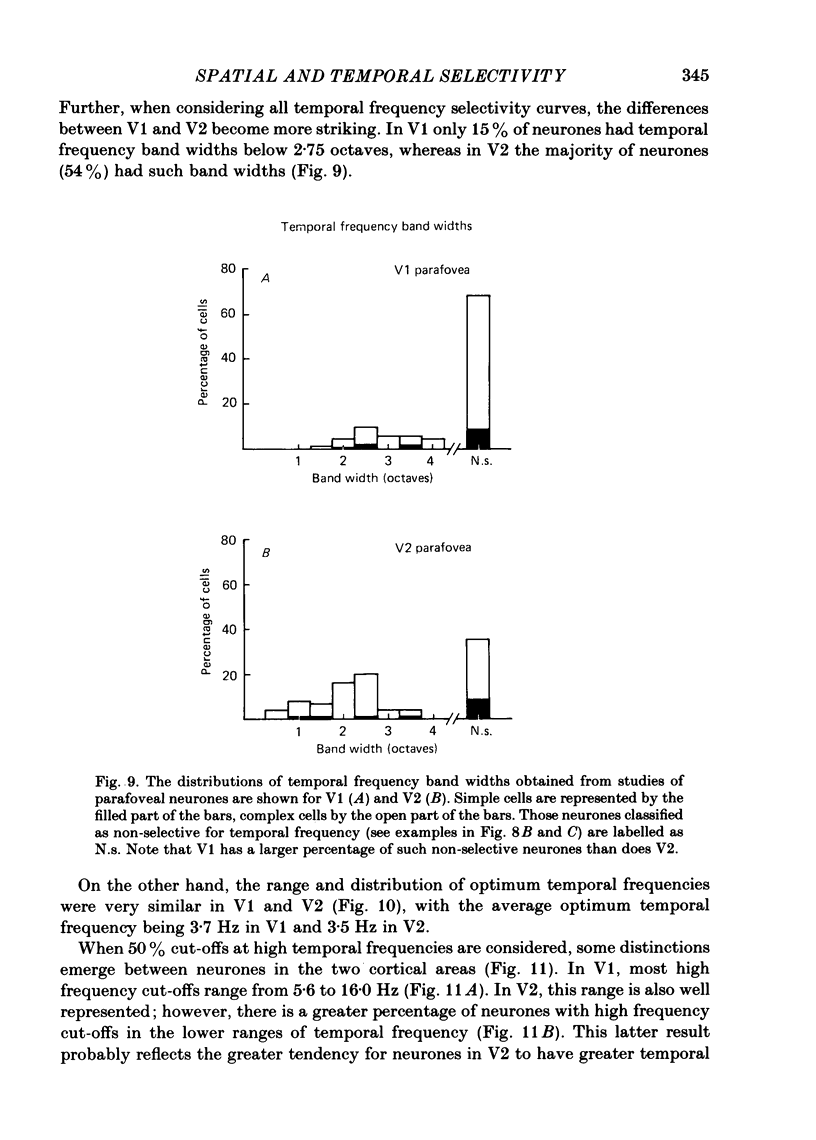
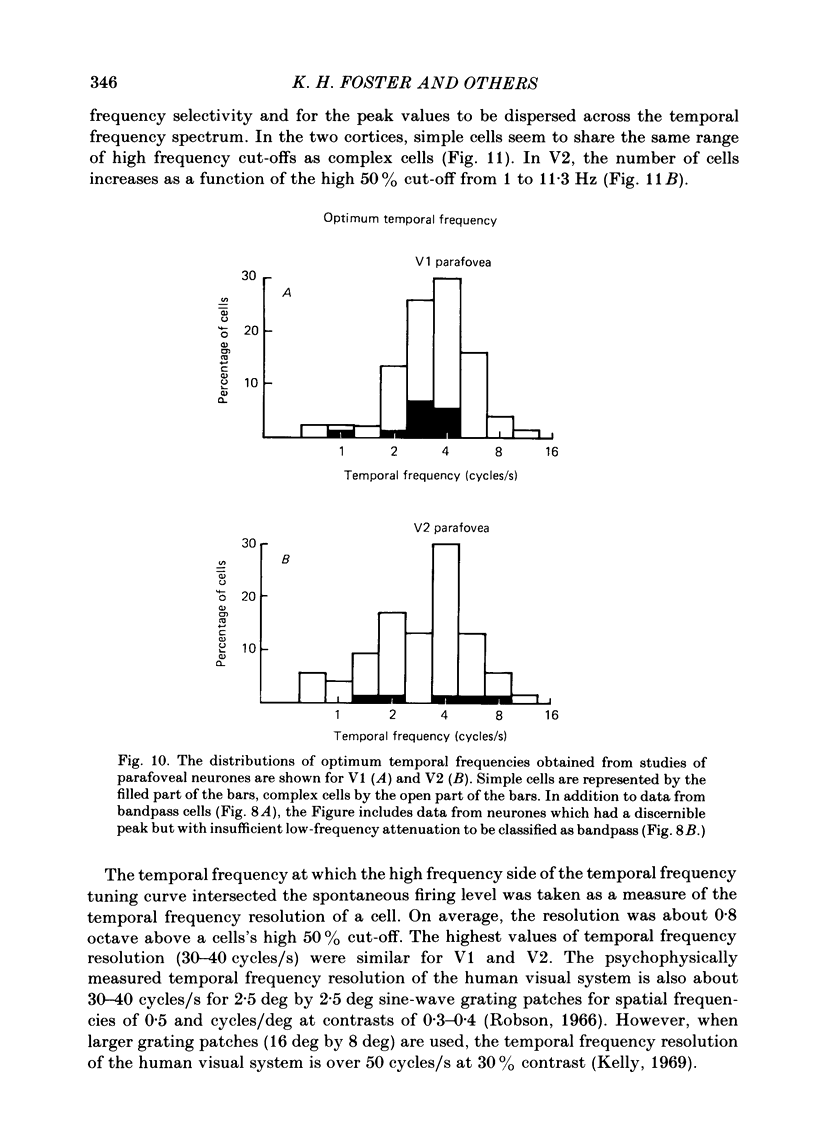
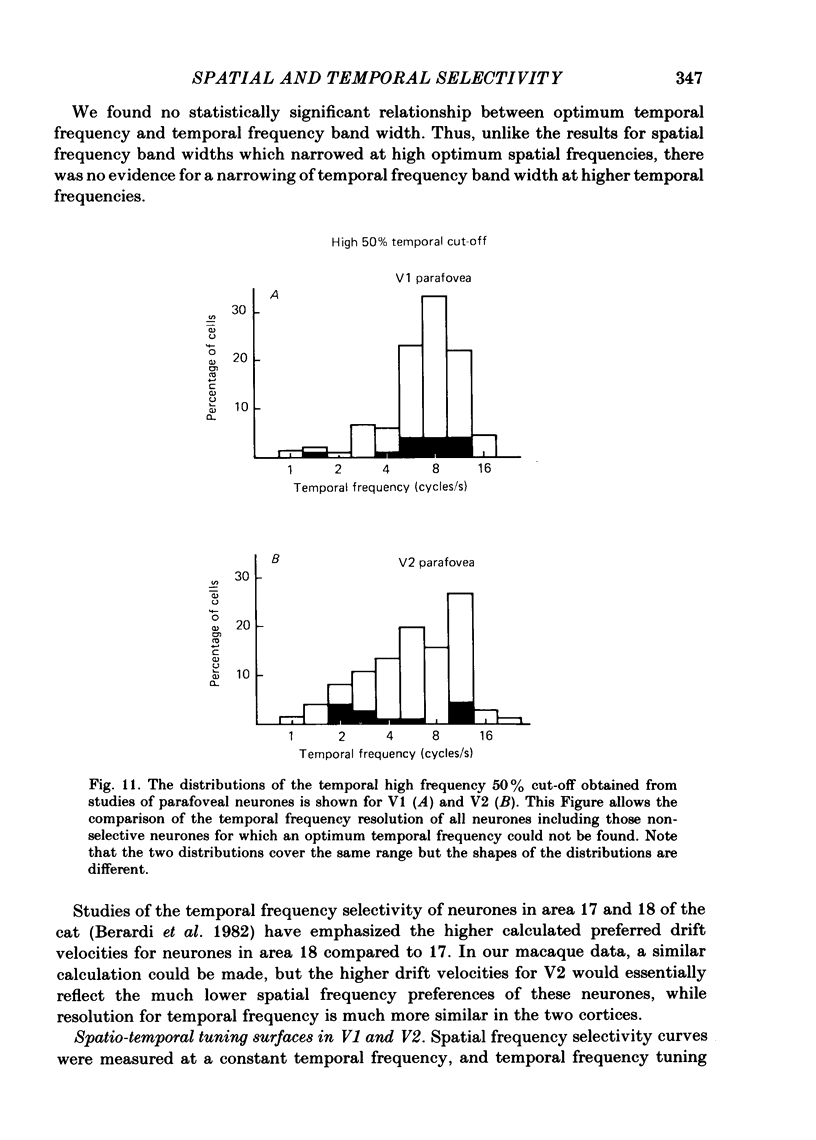
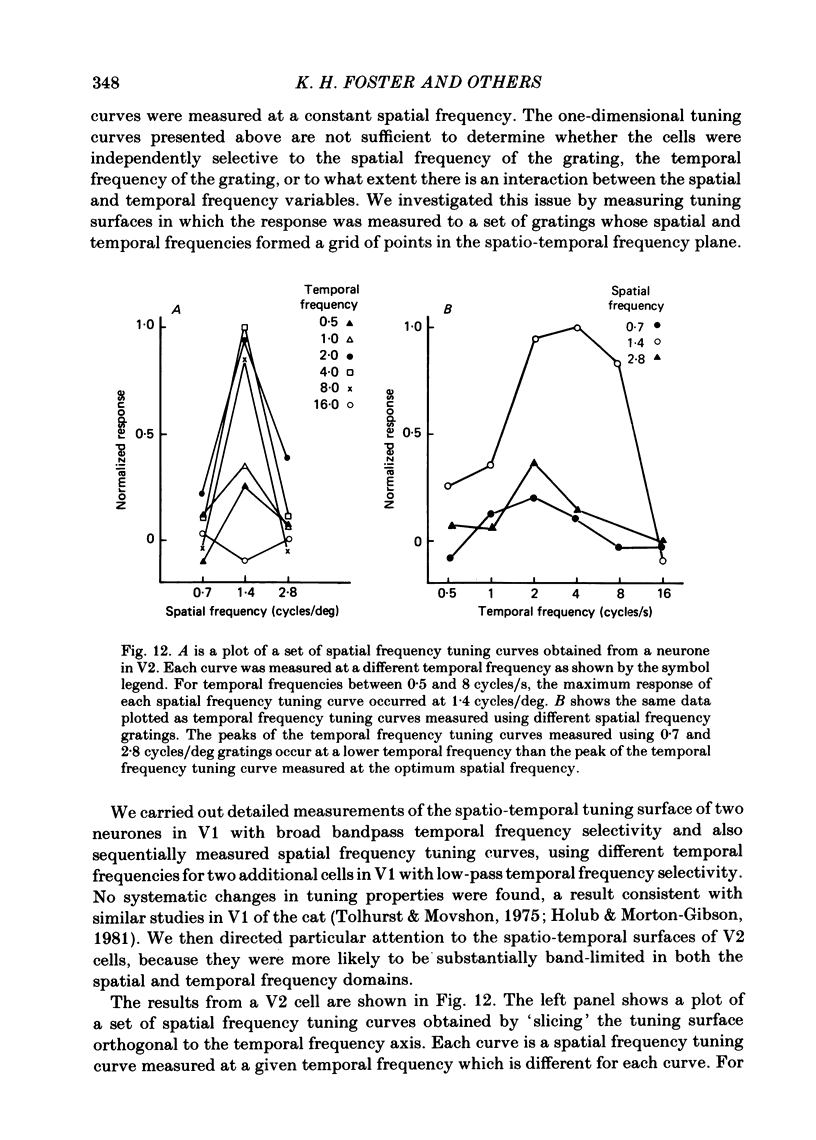
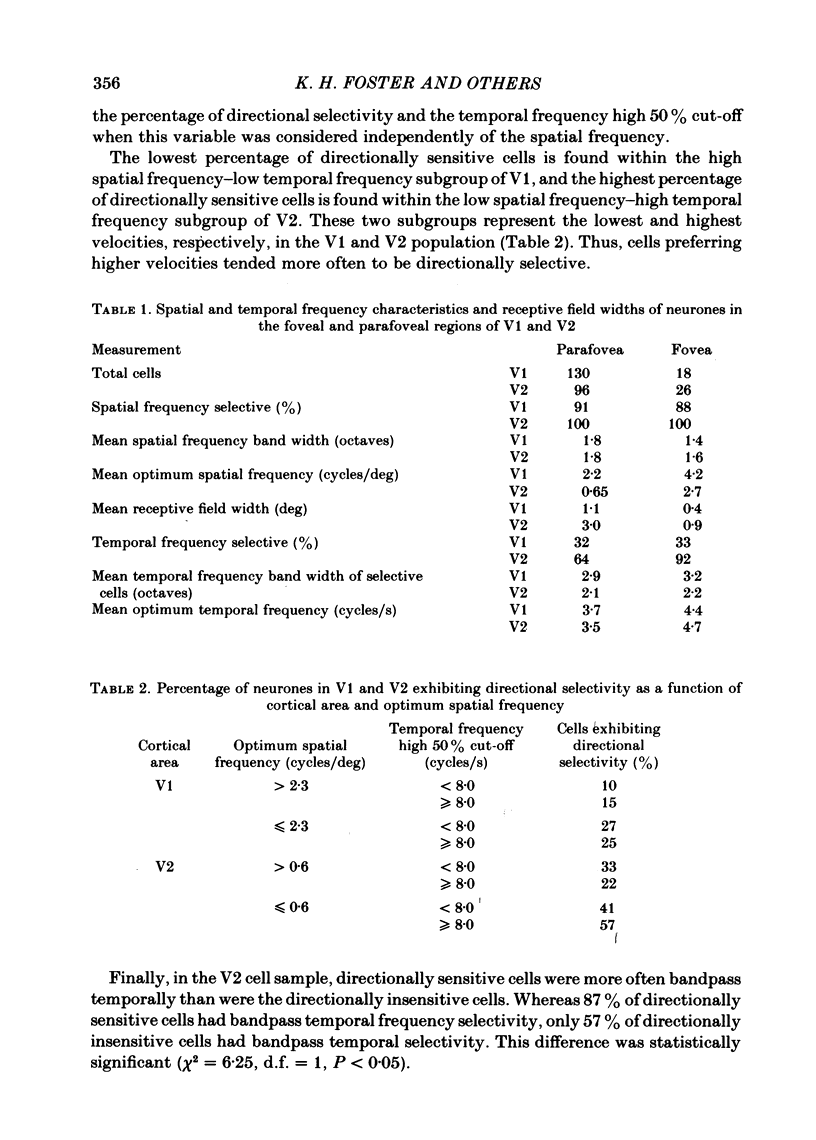
The spatial and temporal frequency selectivity of 148 neurones in the striate cortex, V1, and of 122 neurones in the second visual cortical area, V2, of the macaque monkey were studied using sine-wave gratings of suprathreshold contrast drifting over the receptive field at the preferred orientation and direction. Neurones in V1 and V2 were selective for different but partially overlapping ranges of the spatial frequency spectrum. At retinal eccentricities of 2-5 deg from the fovea, the spatial frequency preferences for neurones ranged from 0.5 to 8.0 cycles/deg in V1 and from 0.2 to 2.1 cycles/deg in V2 and were on average almost 2 octaves lower in V2 than in V1. Spatial frequency full band widths in the two cortical areas were in the range 0.8-3.0 octaves, with a mean value of 1.8 octaves, in the parafoveal representation of both V1 and V2, and 1.4 and 1.6 octaves respectively in the foveal representation of V1 and V2. Most neurones in V1 and some in V2 responded well at temporal frequencies up to 5.6-8.0 Hz before their responses dropped off at still higher frequencies. In V1, 68% of the neurones exhibited low-pass temporal tuning characteristics and 32% were very broadly tuned, with a mean temporal frequency full band width of 2.9 octaves. However, in V2 only 30% of the neurones showed low-pass temporal selectivity and 70% of the cells had bandpass temporal characteristics, with a mean full band width of 2.1 octaves. In V2 the minimal overlap of bandpass tuning curves across the temporal frequency spectrum suggests that there are at least two distinct bandpass temporal frequency mechanisms as well as neurones with low-pass temporal frequency tuning at each spatial frequency. A matrix of spatial and temporal frequency combinations was employed as stimuli for neurones with bandpass temporal frequency selectivity in both V1 and V2. The resultant spatio-temporal surfaces provided evidence that a neurone's preference for spatial frequency is essentially independent of the test temporal frequency; however, in V2 there was some tendency for temporal frequency peaks to shift slightly towards lower frequencies when non-optimum values of spatial frequency either above or below the preferred value were tested. Neurones with pronounced directional selectivity were encountered over a wide range of spatial frequencies, although in both cortical areas there was a tendency for an increased incidence of directional selectivity among neurones which were selective for lower spatial frequencies and higher temporal frequencies.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht D. G., Hamilton D. B. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982 Jul;48(1):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Allman J. M., Kaas J. H. The organization of the second visual area (V II) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974 Aug 16;76(2):247–265. doi: 10.1016/0006-8993(74)90458-2. [DOI] [PubMed] [Google Scholar]
- Andrews B. W., Pollen D. A. Relationship between spatial frequency selectivity and receptive field profile of simple cells. J Physiol. 1979 Feb;287:163–176. doi: 10.1113/jphysiol.1979.sp012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditi A. R., Anderson P. A., Movshon J. A. Monocular and binocular detection of moving sinusoidal gratings. Vision Res. 1981;21(3):329–336. doi: 10.1016/0042-6989(81)90160-7. [DOI] [PubMed] [Google Scholar]
- Baizer J. S., Robinson D. L., Dow B. M. Visual responses of area 18 neurons in awake, behaving monkey. J Neurophysiol. 1977 Sep;40(5):1024–1037. doi: 10.1152/jn.1977.40.5.1024. [DOI] [PubMed] [Google Scholar]
- Berardi N., Bisti S., Cattaneo A., Fiorentini A., Maffei L. Correlation between the preferred orientation and spatial frequency of neurones in visual areas 17 and 18 of the cat. J Physiol. 1982 Feb;323:603–618. doi: 10.1113/jphysiol.1982.sp014094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker D. O., Tulunay-Keesey U. Sensitivity to countermodulating gratings following spatiotemporal adaptation. J Opt Soc Am. 1983 Apr;73(4):427–435. doi: 10.1364/josa.73.000427. [DOI] [PubMed] [Google Scholar]
- Bullier J., Kennedy H. Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Exp Brain Res. 1983;53(1):168–172. doi: 10.1007/BF00239409. [DOI] [PubMed] [Google Scholar]
- Burr D. C., Ross J. Contrast sensitivity at high velocities. Vision Res. 1982;22(4):479–484. doi: 10.1016/0042-6989(82)90196-1. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Harting J. K. Organization of pulvinar afferents to area 18 in the squirrel monkey: evidence for stripes. Brain Res. 1978 Mar 17;143(1):155–161. doi: 10.1016/0006-8993(78)90759-x. [DOI] [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois K. K., Tootell R. B. Spatial-frequency-specific inhibition in cat striate cortex cells. J Physiol. 1983 Mar;336:359–376. doi: 10.1113/jphysiol.1983.sp014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H., Snodderly D. M. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974 Jan;14(1):75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Dean A. F., Tolhurst D. J. On the distinctness of simple and complex cells in the visual cortex of the cat. J Physiol. 1983 Nov;344:305–325. doi: 10.1113/jphysiol.1983.sp014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Fleming J., Gross C. G. Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res. 1980 Feb 17;184(1):41–55. doi: 10.1016/0006-8993(80)90586-7. [DOI] [PubMed] [Google Scholar]
- Dow B. M. Functional classes of cells and their laminar distribution in monkey visual cortex. J Neurophysiol. 1974 Sep;37(5):927–946. doi: 10.1152/jn.1974.37.5.927. [DOI] [PubMed] [Google Scholar]
- Essen D. C., Zeki S. M. The topographic organization of rhesus monkey prestriate cortex. J Physiol. 1978 Apr;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R., Gross C. G., Sandell J. H. Visual topography of V2 in the macaque. J Comp Neurol. 1981 Oct 1;201(4):519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Glezer V. D., Tsherbach T. A., Gauselman V. E., Bondarko V. M. Linear and non-linear properties of simple and complex receptive fields in area 17 of the cat visual cortex. A model of the field. Biol Cybern. 1980;37(4):195–208. doi: 10.1007/BF00337038. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R., Ogren M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978 Nov 1;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Lee B. B., Vidyasagar T. R. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983 Apr;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub R. A., Morton-Gibson M. Response of Visual Cortical Neurons of the cat to moving sinusoidal gratings: response-contrast functions and spatiotemporal interactions. J Neurophysiol. 1981 Dec;46(6):1244–1259. doi: 10.1152/jn.1981.46.6.1244. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. H., Burbeck C. A. Critical problems in spatial vision. Crit Rev Biomed Eng. 1984;10(2):125–177. [PubMed] [Google Scholar]
- Kulikowski J. J., Bishop P. O. Silent periodic cells in the cat striate cortex. Vision Res. 1982;22(1):191–200. doi: 10.1016/0042-6989(82)90182-1. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Marcelja S., Bishop P. O. Theory of spatial position and spatial frequency relations in the receptive fields of simple cells in the visual cortex. Biol Cybern. 1982;43(3):187–198. doi: 10.1007/BF00319978. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Hendrickson A. E., Ogren M. P., Tobin E. A. Anatomical organization of primate visual cortex area VII. J Comp Neurol. 1981 Oct 10;202(1):19–45. doi: 10.1002/cne.902020104. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res. 1976;16(10):1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., van Essen D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983 Dec;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren M. P., Hendrickson A. E. The distribution of pulvinar terminals in visual areas 17 and 18 of the monkey. Brain Res. 1977 Dec 2;137(2):343–350. doi: 10.1016/0006-8993(77)90344-4. [DOI] [PubMed] [Google Scholar]
- Poggio G. F. Spatial properties of neurons in striate cortex of unanesthetized macaque monkey. Invest Ophthalmol. 1972 May;11(5):368–377. [PubMed] [Google Scholar]
- Pollen D. A., Andrews B. W., Feldon S. E. Spatial frequency selectivity of periodic complex cells in the visual cortex of the cat. Vision Res. 1978;18(6):665–682. doi: 10.1016/0042-6989(78)90146-3. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Pandya D. N. Cortical connections of the occipital lobe in the rhesus monkey: interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Res. 1981 May 18;212(2):249–270. doi: 10.1016/0006-8993(81)90461-3. [DOI] [PubMed] [Google Scholar]
- Sakitt B., Barlow H. B. A model for the economical encoding of the visual image in cerebral cortex. Biol Cybern. 1982;43(2):97–108. doi: 10.1007/BF00336972. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. III. Spatial frequency. J Neurophysiol. 1976 Nov;39(6):1334–1351. doi: 10.1152/jn.1976.39.6.1334. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. The effect of striate cortex cooling on area 18 cells in the monkey. Brain Res. 1977 May 6;126(2):366–369. doi: 10.1016/0006-8993(77)90734-x. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Klein S., Dawson B. M., Spillmann L. Low spatial-frequency channels in human vision: adaptation and masking. Vision Res. 1982;22(2):225–233. doi: 10.1016/0042-6989(82)90122-5. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J., Movshon J. A. Spatial and temporal contrast sensitivity of striate cortical neurones. Nature. 1975 Oct 23;257(5528):674–675. doi: 10.1038/257674a0. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., De Valois R. L., Jacobs G. H. Functional organization of the second cortical visual area in primates. Science. 1983 May 13;220(4598):737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Jacobson S. Areal and laminar distribution of some pulvinar cortical efferents in rhesus monkey. J Comp Neurol. 1976 Oct 1;169(3):371–392. doi: 10.1002/cne.901690307. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Robson J. G. Discrimination at threshold: labelled detectors in human vision. Vision Res. 1981;21(7):1115–1122. doi: 10.1016/0042-6989(81)90014-6. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Thompson P. G., Murphy B. J., Nachmias J. Summation and discrimination of gratings moving in opposite directions. Vision Res. 1980;20(4):341–347. doi: 10.1016/0042-6989(80)90020-6. [DOI] [PubMed] [Google Scholar]
- Weller R. E., Kaas J. H. Retinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeys. J Comp Neurol. 1983 Nov 1;220(3):253–279. doi: 10.1002/cne.902200302. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., McFarlane D. K., Phillips G. C. Spatial frequency tuning of orientation selective units estimated by oblique masking. Vision Res. 1983;23(9):873–882. doi: 10.1016/0042-6989(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Wilson H. R. Spatiotemporal characterization of a transient mechanism in the human visual system. Vision Res. 1980;20(5):443–452. doi: 10.1016/0042-6989(80)90035-8. [DOI] [PubMed] [Google Scholar]


