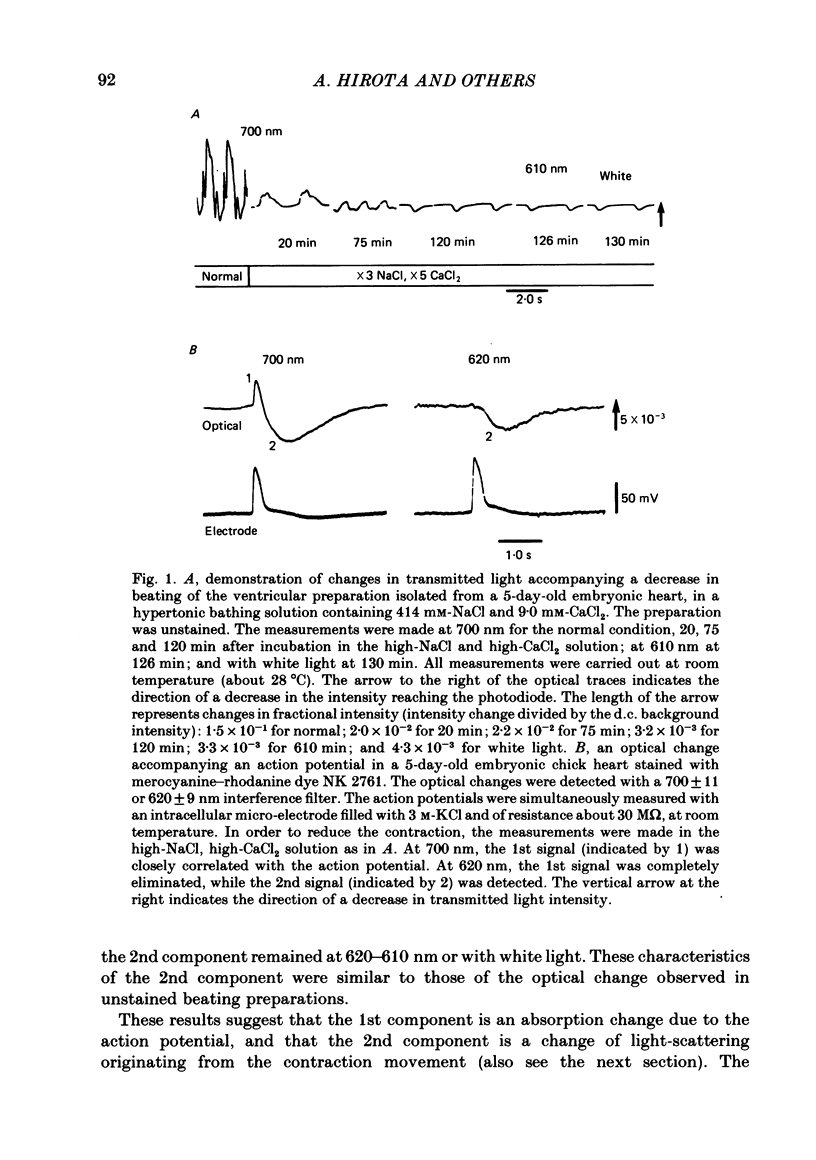
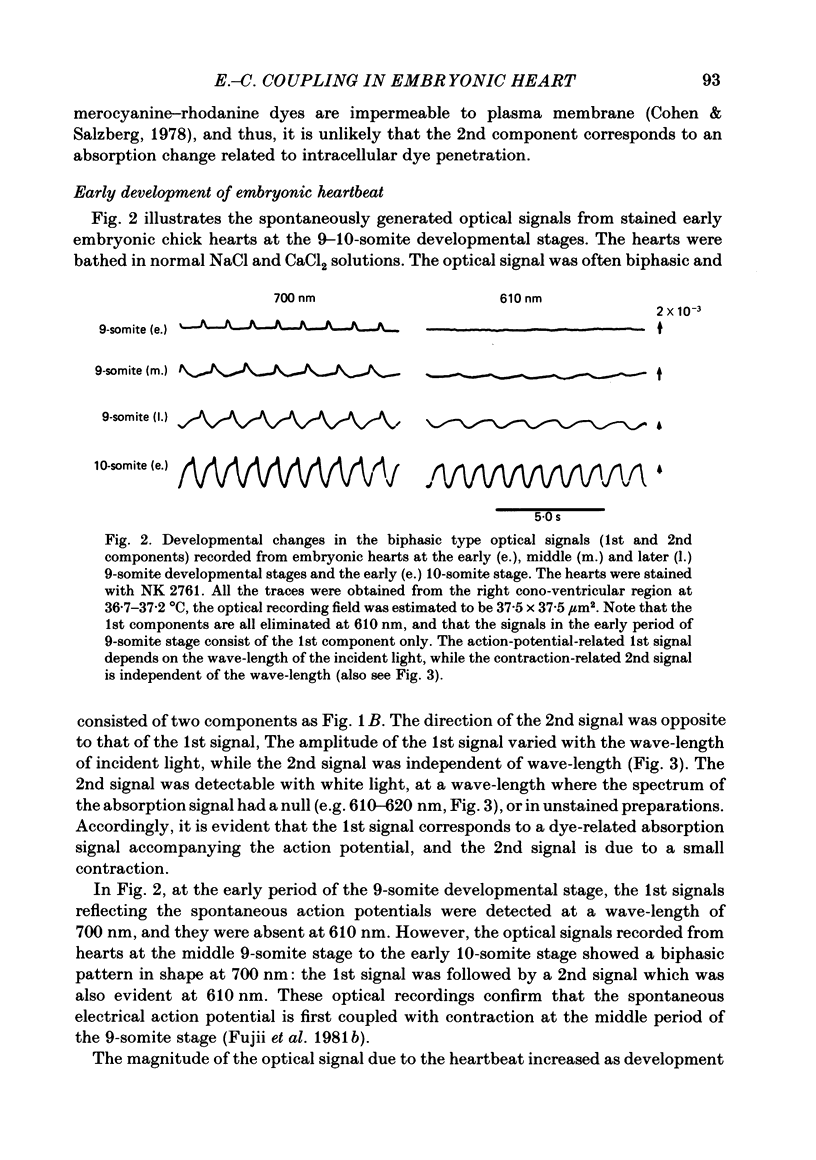
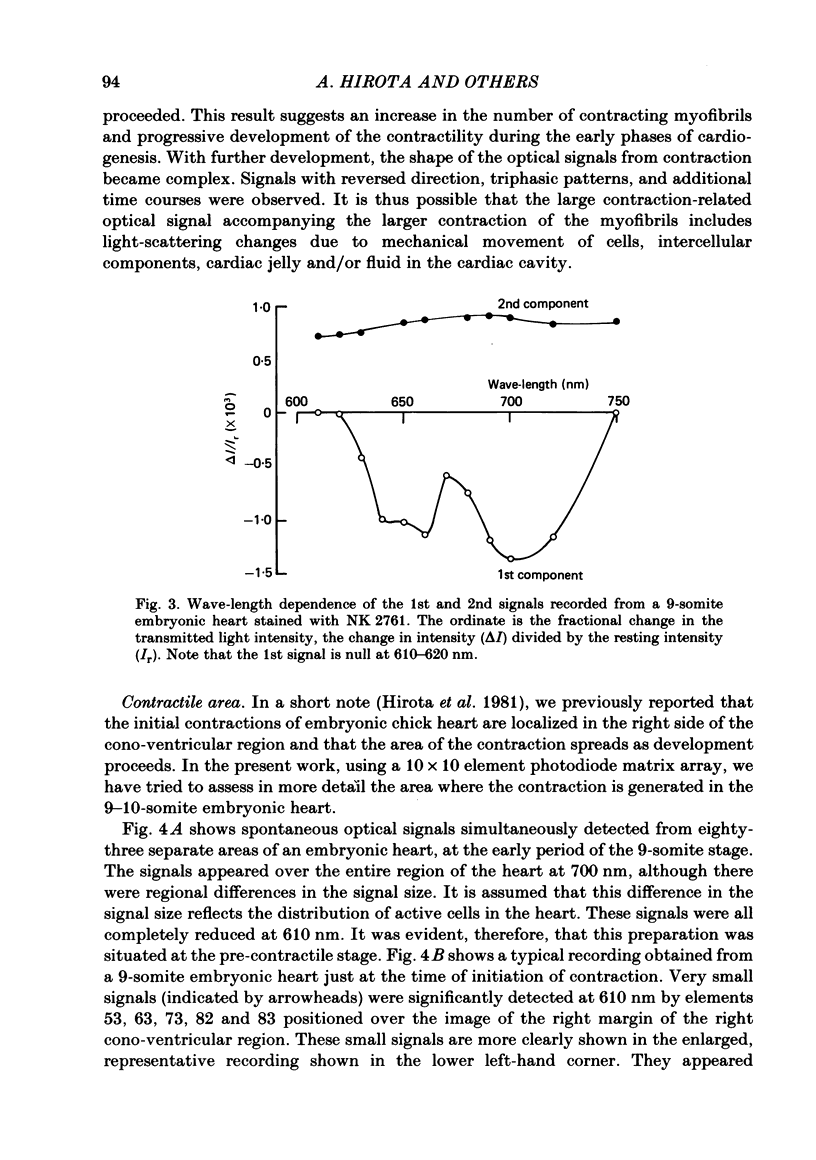
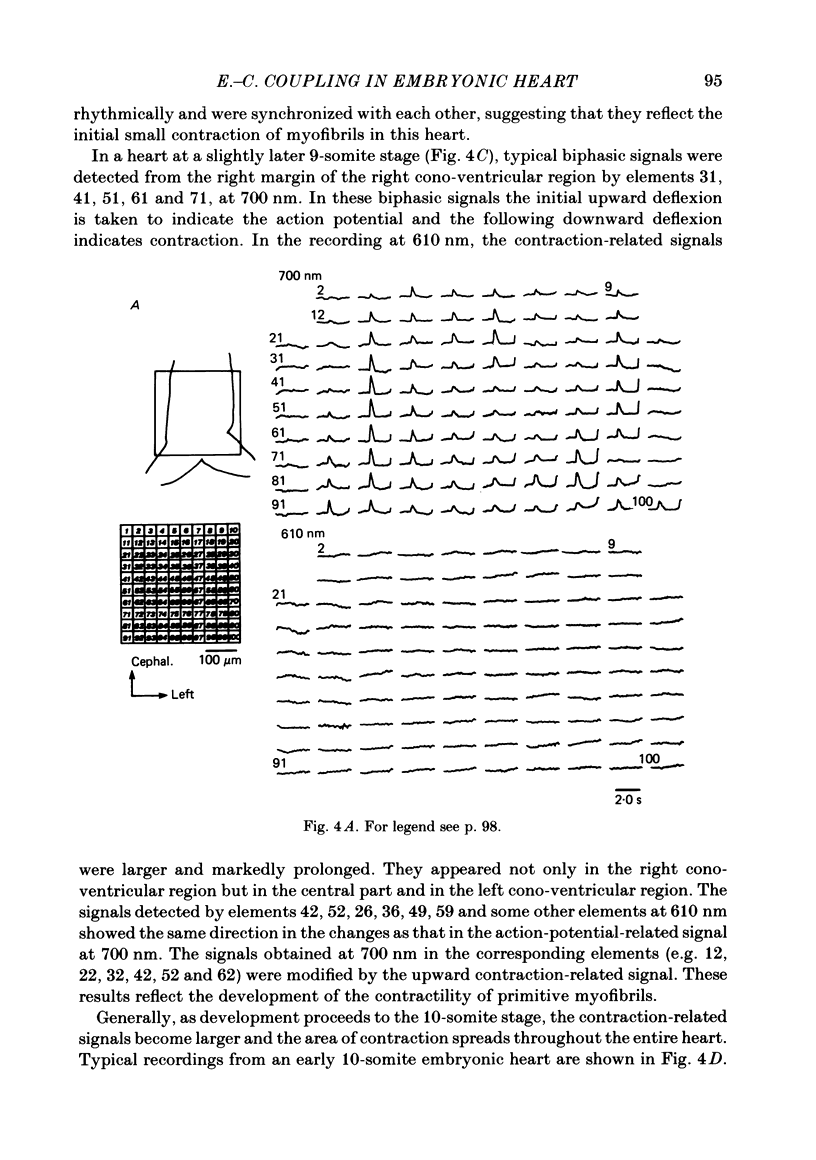
Abstract
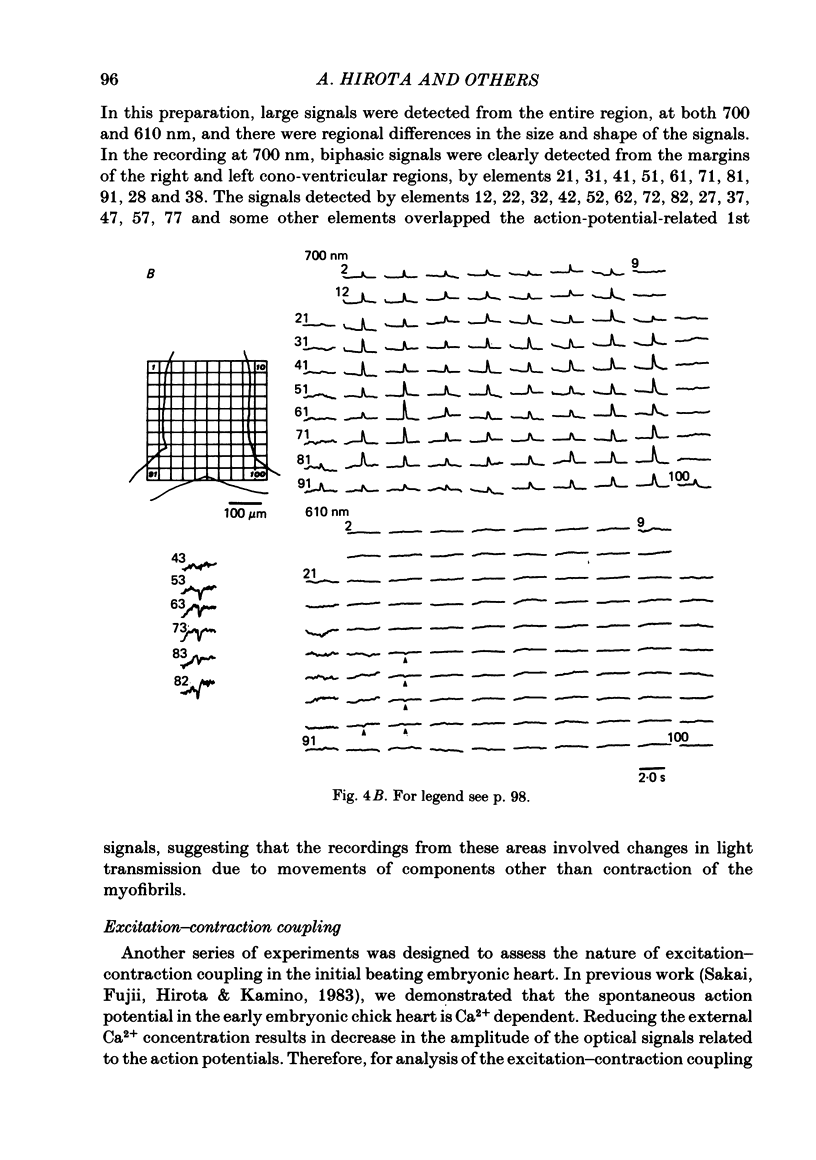
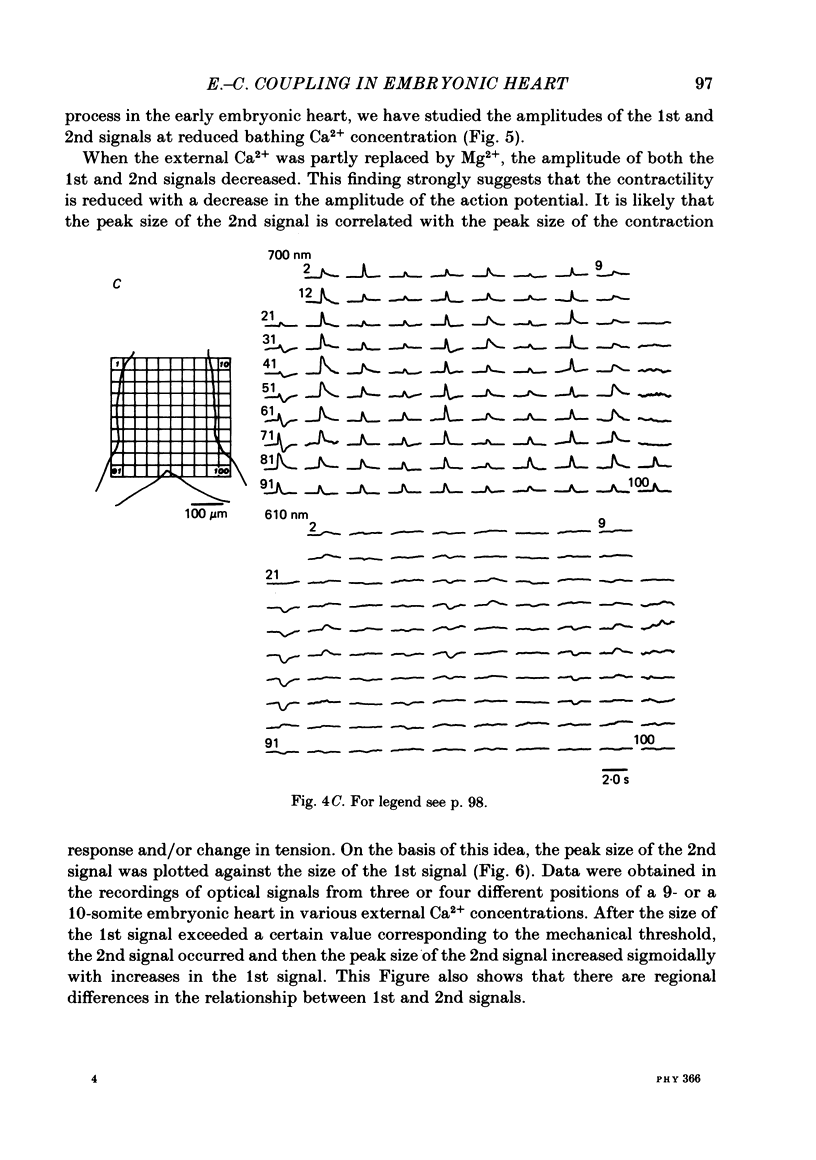
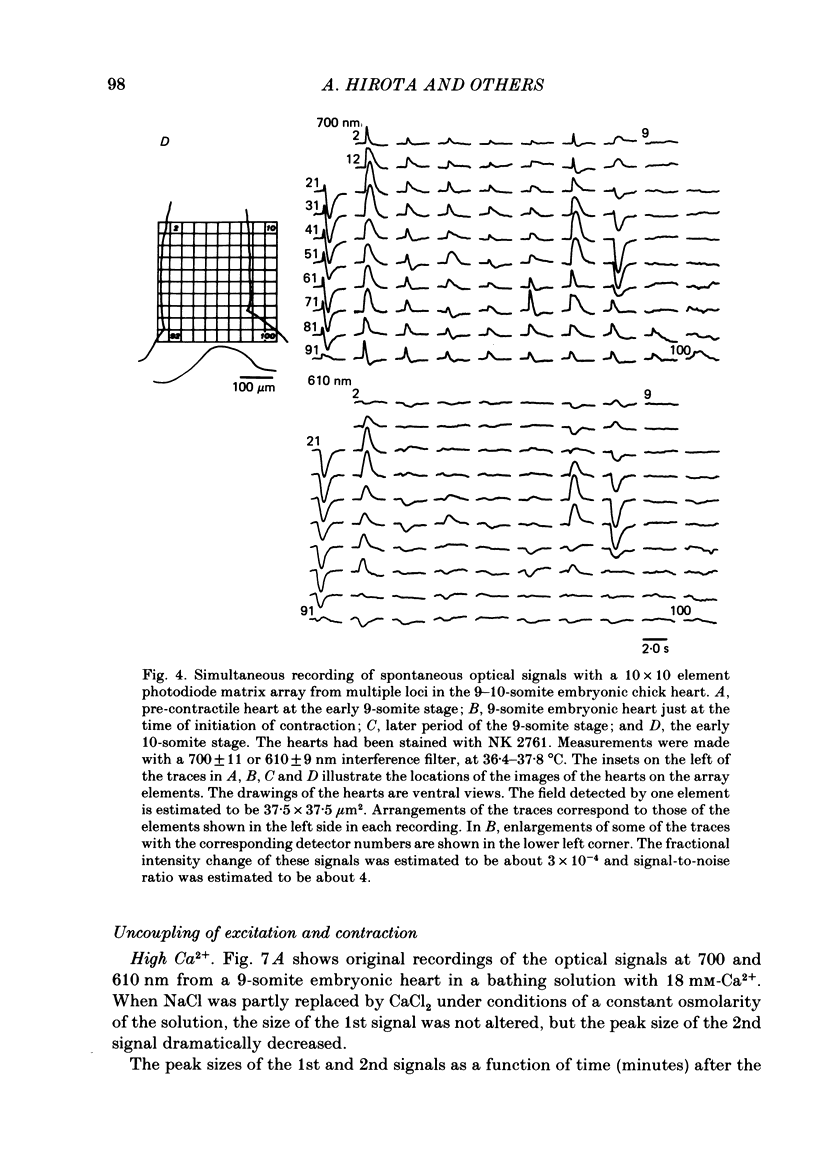
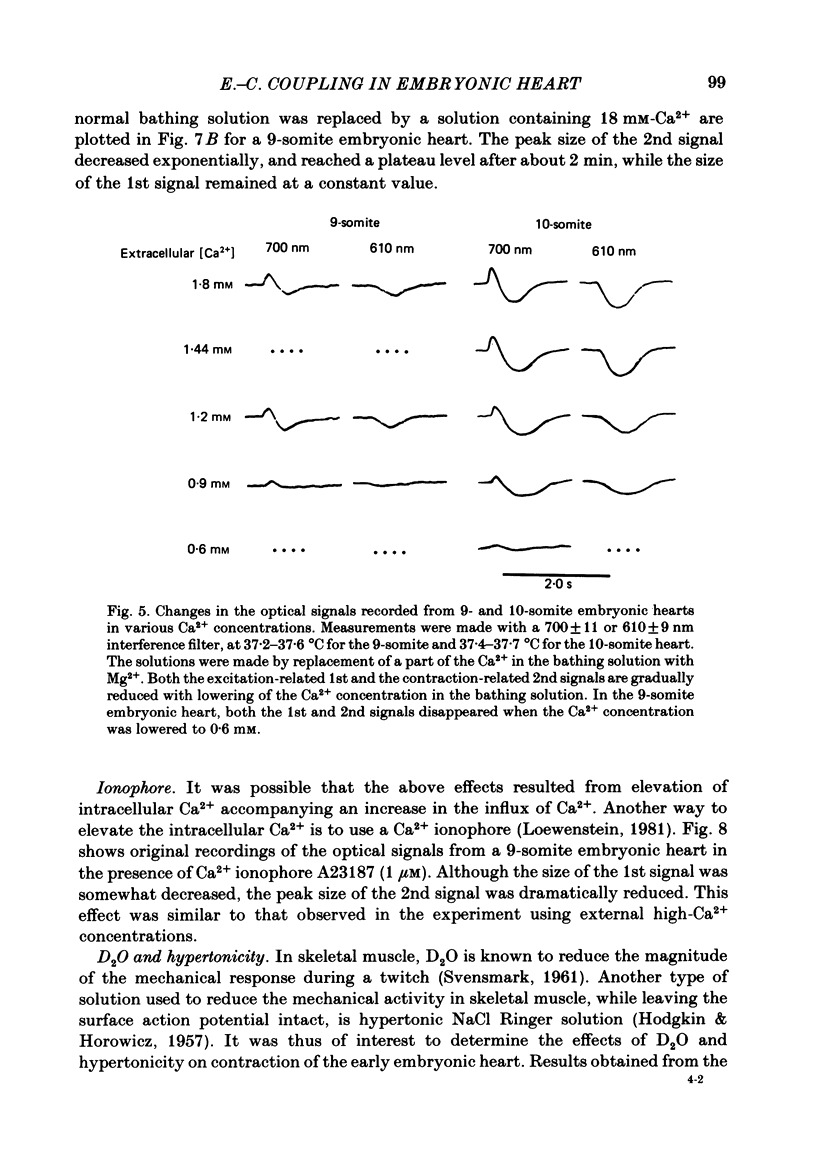
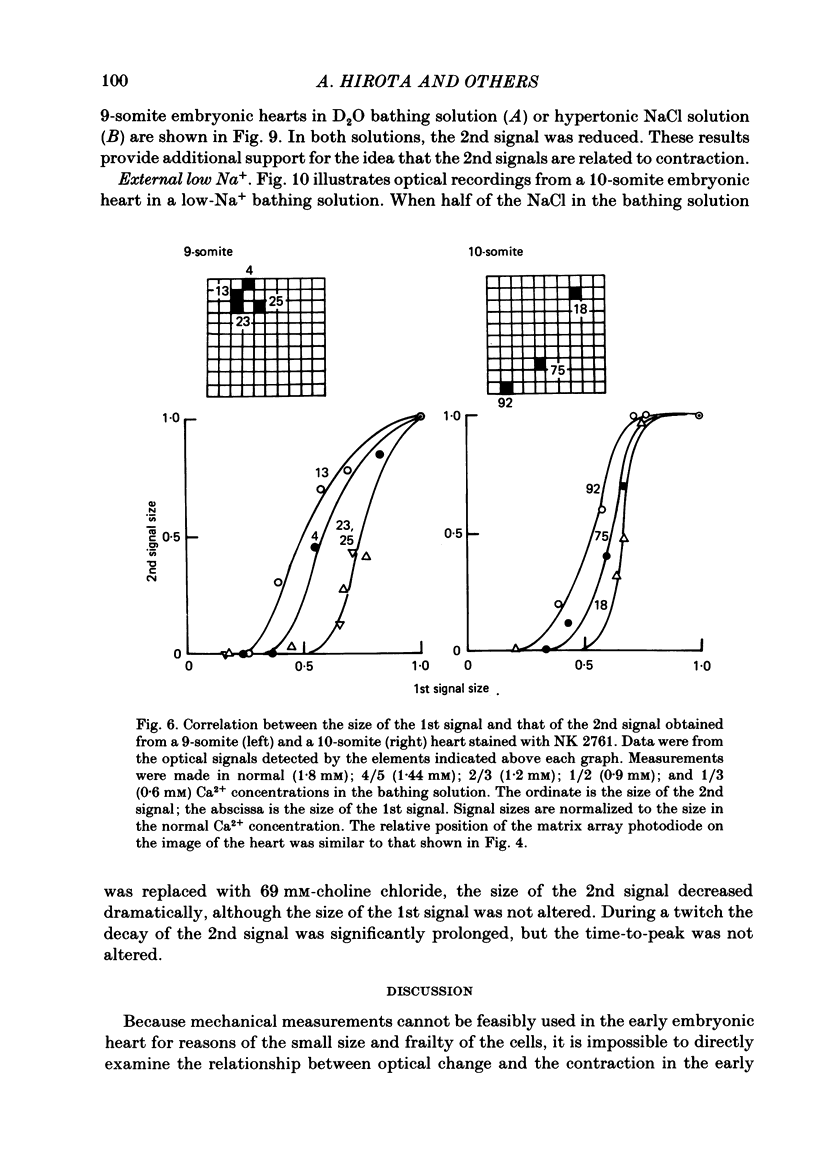
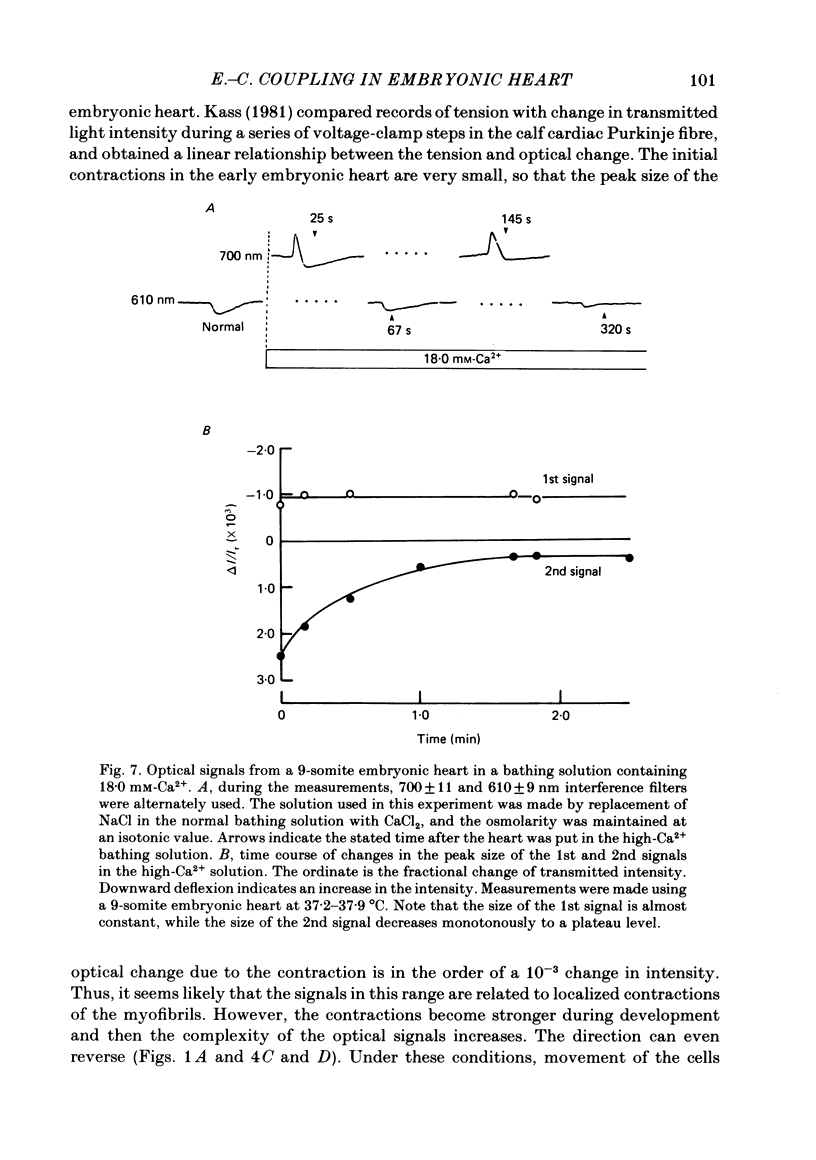
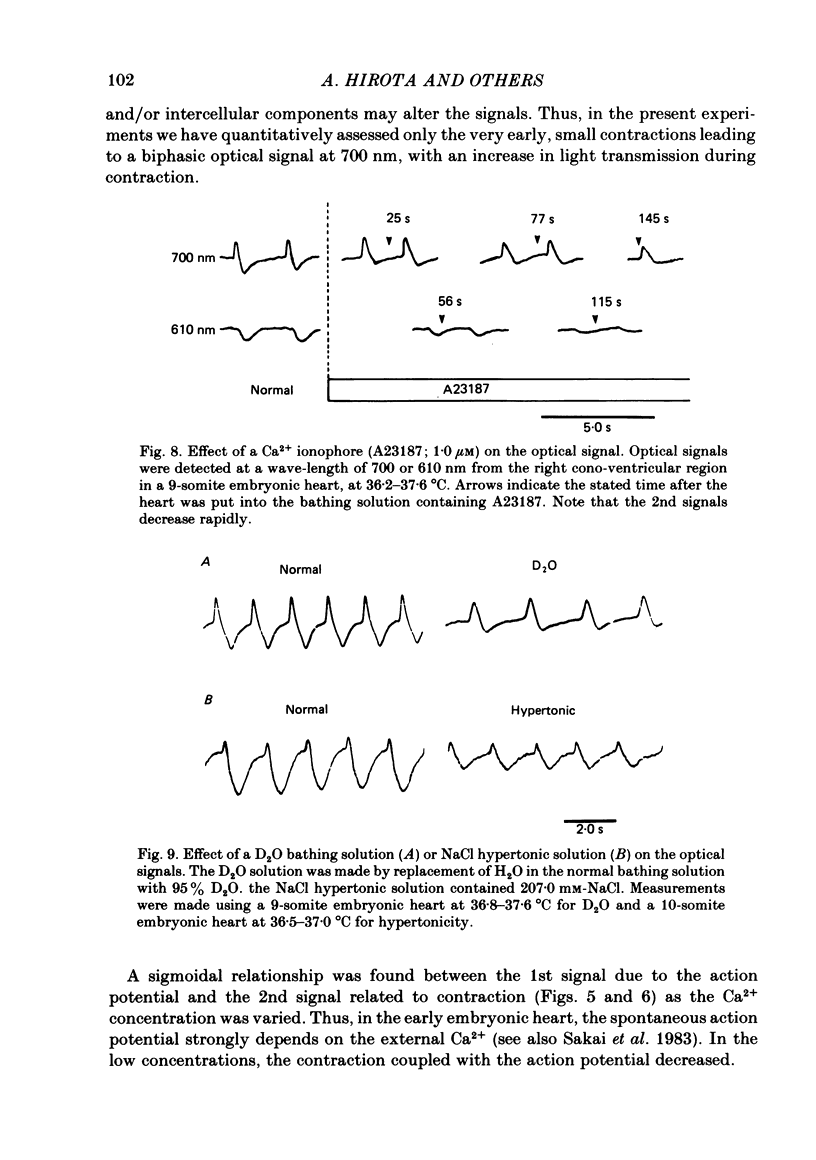
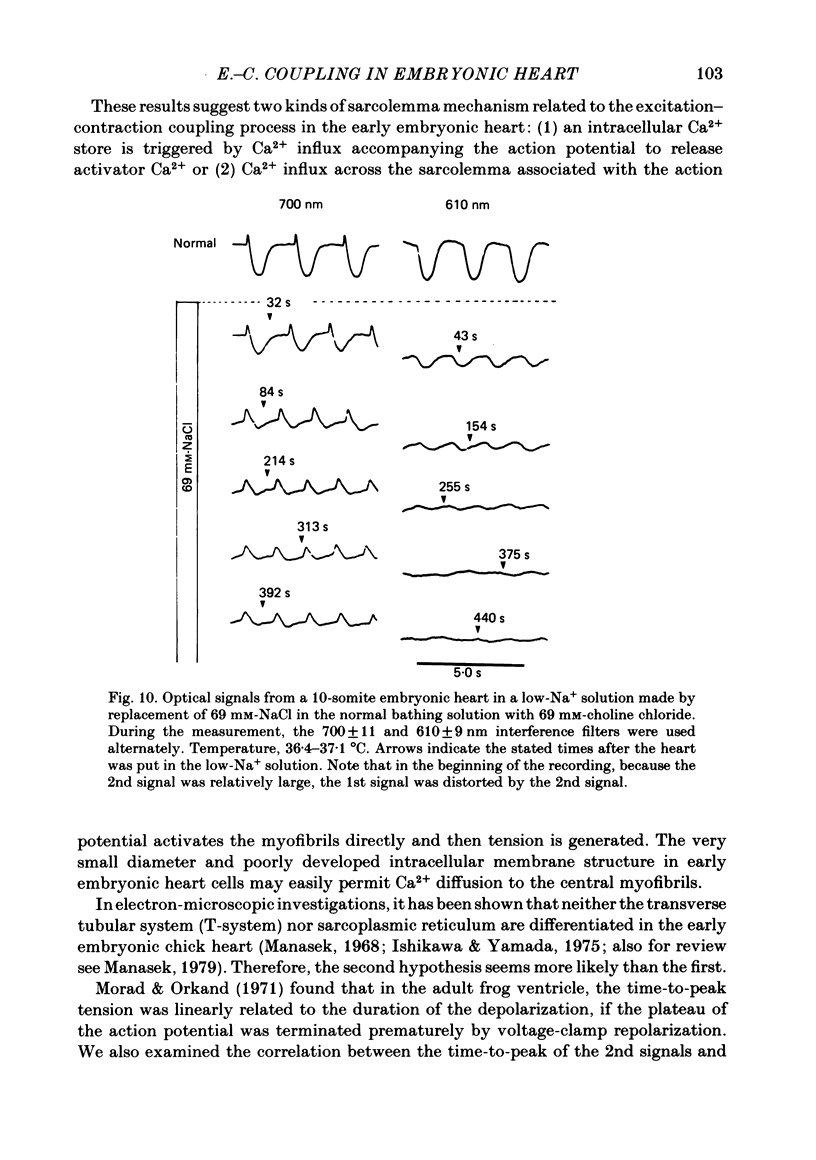
Excitation-contraction coupling at the onset of beating in the 9-10-somite embryonic chick heart was studied by means of an optical method together with a voltage-sensitive merocyanine-rhodanine dye. Spontaneous optical signals were recorded simultaneously from many areas of the embryonic heart, using a square photodiode matrix array. At time of initiation of the heartbeat, spontaneous optical signals consisting of two components were often detected. The first component (1st signal) is a dye-related absorption change due to the action potential, and the second component (2nd signal) is a light scattering change due to contraction. When Ca2+ in the bathing solution was partly replaced by Mg2+, the peak size of both signals was reduced. The correlation between the 1st and 2nd signals corresponded to the relationship between excitation and contraction. The formation of excitation-contraction coupling exhibited a regional non-uniformity in the developing 9-10-somite embryonic hearts: contraction was first generated in the right ventricular region, and then the contractile area spread widely over the whole of the heart. The curves of the excitation-related 1st signal vs. the contraction-related 2nd signal obtained from different areas were not superimposable. Decoupling of excitation from contraction was produced by raising the Ca2+ concentration in the bathing solution, by lowering the Na2+ concentration or by inclusion of a Ca2+ ionophore (A23187). Replacement of the bathing solution with D2O or hypertonic solution also suppressed excitation-contraction coupling. The results suggest that in the early embryonic initial beating chick heart, the contractile system is activated by Ca2+ influx across the sarcolemma accompanying the action potential, and that a Na+-Ca2+ exchange mechanism participates in the relaxation phase of the heartbeat.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. A study of the contractures induced in frog atrial trabeculae by a reduction of the bathing sodium concentration. J Physiol. 1974 Mar;237(2):295–313. doi: 10.1113/jphysiol.1974.sp010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol. 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J Physiol. 1981 Mar;312:253–263. doi: 10.1113/jphysiol.1981.sp013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981 Feb;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: evidence for electrical activity. J Physiol. 1980 Jul;304:503–518. doi: 10.1113/jphysiol.1980.sp013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E. Some effects of hypertonic solutions on contraction and excitation-contraction coupling in frog skeletal muscles. J Gen Physiol. 1970 Feb;55(2):254–275. doi: 10.1085/jgp.55.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Courtney K. R. Voltage-sensitive dyes. Discerning contraction and electrical signals in myocardium. Biophys J. 1982 Dec;40(3):255–257. doi: 10.1016/S0006-3495(82)84481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Fujii S., Sakai T., Kamino K. Temperature dependence of spontaneous electrical activity in early embryonic heart monitored optically with a potential-sensitive dye. Jpn J Physiol. 1983;33(1):85–100. doi: 10.2170/jjphysiol.33.85. [DOI] [PubMed] [Google Scholar]
- Kamino K., Hirota A., Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981 Apr 16;290(5807):595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S. An optical monitor of tension for small cardiac preparations. Biophys J. 1981 Apr;34(1):165–170. doi: 10.1016/S0006-3495(81)84843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol. 1968 Jul;125(3):329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENSMARK O. The effect of deuterium oxide on the mechanical properties of muscle. Acta Physiol Scand. 1961 Sep;53:75–84. doi: 10.1111/j.1748-1716.1961.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Sakai T., Fujii S., Hirota A., Kamino K. Optical evidence for calcium-action potentials in early embryonic precontractile chick heart using a potential-sensitive dye. J Membr Biol. 1983;72(3):205–212. doi: 10.1007/BF01870587. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Sawanobori T., Hirota A., Fujii S., Kamino K. Optical recording of conducted action potential in heart muscle using a voltage-sensitive dye. Jpn J Physiol. 1981;31(3):369–380. doi: 10.2170/jjphysiol.31.369. [DOI] [PubMed] [Google Scholar]
- Vassort G. Influence of sodium ions on the regulation of frog myocardial contractility. Pflugers Arch. 1973 Mar 30;339(3):224–240. doi: 10.1007/BF00587374. [DOI] [PubMed] [Google Scholar]


