Abstract
Cognitive impairment resulting from insufficient sleep poses a significant public health concern, particularly in children. The effects and mechanisms of choline on cognitive impairment caused by sleep deprivation are unknown. Chronic sleep deprivation is induced in young mice in this study, followed by feeding diet containing 11.36 g/kg choline bitartrate. Choline supplementation significantly improves spatial learning ability. Functional MRI results reveal the hippocampus as a key region affected by sleep deprivation, where choline supplementation notably preserves hippocampal structural integrity and enhanced connectivity. Additionally, choline ameliorates hippocampal pathological injury, reduces blood-brain barrier permeability and serum brain injury biomarkers. Choline also reduces inflammation and oxidative stress biomarkers, and mitigates microglial activation in the hippocampus, which preserves synaptic plasticity. A key finding is the changes of hippocampal phospholipidomic profile along with cognitive function, and a total of 313 phospholipid molecules are identified. Choline increases the levels of total phospholipid and sub-classes (particularly PC), which are strongly correlated with reduced neuroinflammation and oxidative stress biomarkers, as well as improved cognitive outcomes. Furthermore, there are similar findings in some phospholipid molecules such as PC 36:1, PC O-33:0, PC p-38:3, PE 36:3, PE p-42:4 and PS 44:12. These findings highlight that choline alleviates cognitive impairment in sleep deprivation via reducing neuroinflammation and oxidative stress as well as altering phospholipidomic profile. This study suggests that choline could develop into functional food or medicine ingredient to prevent and treat cognitive impairment by sleep disturbances, particularly children and adolescents.
Keywords: Choline, Sleep deprivation, Cognitive impairment, Neuroinflammation, Magnetic resonance imaging, Phospholipidomics
Graphical abstract
Highlights
-
•
Choline alleviates cognitive impairment caused by sleep deprivation.
-
•
Hippocampus is key brain region affected by sleep deprivation and choline.
-
•
Choline reduces neuroinflammation and oxidative stress.
-
•
Choline inactivates microglia and upregulates α7-nAChR, enhancing neuroprotective.
-
•
Choline restores phospholipid homeostasis disrupted by sleep deprivation.
1. Introduction
Humans spend approximately one-third of their lives asleep, with infants and young children requiring significantly more sleep than adults [1]. Sleep deficiency among children is a widespread concern globally [2], with more than 40 % of Chinese children did not meet the recommended sleep durations [3]. Sleep plays a paramount role in cognition, influencing every level from cellular redox state regulation and neuronal structures to brain morphology and function [4,5]. The developing brain exhibits heightened vulnerability and lacks robust adaptations to sleep deprivation when compared to the adult brain [6]. Sleep disturbances were associated with reduced learning ability and lower academic performance in children and adolescents [7].
Sleep loss has serious pathophysiological consequences, including chronic inflammation [8] and alter lipid metabolism [9]. The studies indicate a correlation between self-reported sleep disturbances and dysregulation of inflammatory markers [10]. Neuroinflammation is characterized by the activation of microglia and astrocytes [11,12], along with the subsequent release of pro-inflammatory molecules [13], contributing to cognitive deficits [4]. Moreover, sleep deprivation can alter lipid metabolism, as lipids particularly susceptible to oxidative stress-induced degradation [9].
Addressing sleep deprivation is very difficult for children. On the other hand, several studies showed that diets rich in antioxidants and anti-inflammatory nutrients were helpful for preventing cognitive impairment and improving brain function [[14], [15], [16]], which hinder that some nutrients could be used to improve cognitive function of children experiencing sleep disturbances. Furthermore, the studies showed that choline supplementation supported neurocognitive development during perinatal period and early life stages [15,17].
Choline is an essential nutrient for various physiological processes, such as cell membrane structure, lipid metabolism, and brain development and function [18]. Choline is converted into the neurotransmitter acetylcholine (ACh) by choline acetyltransferase (ChAT) [19], which is intricately linked to cholinergic neural networks involved in memory and cognitive function [20]. Furthermore, a study indicates that higher dietary choline intake is associated with lower levels of circulating inflammatory markers [21], suggesting that choline possesses anti-inflammatory properties. In addition, choline is an essential component of membrane phospholipids that contributes to the structural integrity of cell membrane [22]. Alterations in membrane phospholipids can lead to the continuous activation of specific receptors [23,24], resulting in signaling dysregulation that undermines homeostatic synaptic plasticity, ultimately leading to cognitive impairment [25]. However, the effects of choline on cognitive deficits resulting from sleep deprivation remain unclear. Thus, we hypothesize that choline could support neurotransmitter synthesis, alleviate sleep deprivation-induced neuroinflammation, modulate phospholipids metabolism and maintain membrane integrity, all of which contribute to optimal brain health and cognitive function.
In this study, we induced cognitive impairment through sleep deprivation in a young mice model. We identified the protective effects of choline on spatial learning ability, brain structure and function. We evaluated microglia and synapse morphology, blood-brain barrier (BBB) integrity, hippocampus (HPC), neuroinflammation and oxidative stress along with associated biomarkers. Furthermore, the change of phospholipidomic profile in HPC was studied. We reported that choline could improve cognitive impairment by modulating the inflammatory response and phospholipids metabolism, thereby mitigating the pathological processes of synaptic damage, and microglial activation. Overall, our results provide valuable insights into utilizing choline to mitigate the detrimental effects of sleep disturbances.
2. Materials and methods
2.1. Experimental animals
Three-week-old male C57BL/6J mice were purchased from Laboratory Animal Center, Sun Yat-sen University. The animals were housed in a specific pathogen-free facility under controlled environmental conditions (22 ± 2 °C, 40–60 % humidity, 12 h light/dark cycle) with unrestricted access to food and water. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
2.2. Experimental design
Mice were adaptively fed for 7 days, and then randomly assigned to three groups (n = 8 per group): Control (CON), Chronic Sleep Deprivation (CSD), and Choline-Supplemented CSD (CHOCSD). Both the CON and CSD groups were maintained on a standard AIN-93G diet containing 2.5 g/kg choline bitartrate. Beginning on day 0, the CSD and CHOCSD groups were subjected to 20 h of sleep deprivation daily for 21 consecutive days, which was carried out using a XR-XS108 feedback-controlled sleep deprivation apparatus (Shanghai XinRuan Information Technology Co., Ltd., Shanghai, China) at a bidirectional rotation speed of 14 rpm with random pauses. The CHOCSD group was provided with a high-choline diet containing 11.36 g/kg choline bitartrate [26]. The experimental timeline is depicted in Fig. 1A.
Fig. 1.
Choline improves spatial learning ability. (A) Schematic outline of the experimental design. (B) Diagram of the NOR test. (C) Diagram of the Y-maze test. (D) Heatmaps from the NOR test. (E) Heatmaps from the Y-maze test. (F) Duration (%) spent in the novel arm of the Y-maze test across groups. (G) Discrimination index analysis in the NOR test across groups. (H) Recognition index in the NOR test across groups. (I) Exploration time of novel versus habituated objects in the NOR test across groups. Data are presented as mean ± SD; n = 8 per group; n defines biological replicates; one-way ANOVA followed by Dunnett's t-test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: NOR, novel object recognition; CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging; BOLD, blood oxygen level dependent.
2.3. Behavioral assessments
Cognitive function was assessed as previously described in our previous research by Y-maze novel arm preference test and the novel object recognition (NOR) test on day 21 [27]. In chief, mice were subjected to a two-phase protocol in the Y-maze test. During the training phase, one of the three arms was blocked, and the mice were placed at the entrance of one of the open arms (start arm) and allowed to explore the maze for 10 min. After 1 h retention interval, the test phase commenced, in which all three arms were accessible. Each mouse was returned to the start arm and permitted to explore all arms for 5 min (Fig. 1B). The percentage of time spent exploring the novel arm was recorded as a measure of spatial memory. For the NOR test, mice were first placed in the center of a chamber and exposed to two identical objects for 10 min (training phase). After 1 h delay, one of the familiar objects was replaced with a novel object (test phase), and the mice were allowed to explore for 5 min (Fig. 1C). The exploration time for both the familiar and novel objects was recorded, and the discrimination index (DI), which measures the ability to distinguish between novel and familiar stimuli by calculating the difference in the time spent exploring the novel object versus the familiar one, normalized by the total exploration time, was calculated along with the recognition index (RI) as the ratio of time spent exploring the novel object to the total exploration time. Exploratory behavior was defined as the orientation of the mouse's nose within a 2 cm radius of the objects. For both tests, the maze and chambers were thoroughly cleaned with 75 % ethanol between trials to prevent olfactory cues. Behavioral data from both tests were captured via an automated video tracking system and analyzed using TopScan 3.0 software (CleverSys, Inc., Reston, VA, USA).
2.4. Magnetic resonance imaging (MRI)
On day 30, all mice underwent MRI to assess structural and functional brain changes. The MRI scans were performed using the Biospec 94/30USR MRI scanner (Bruker, Billerica, MA, USA). Diffusion tensor imaging (DTI) was used to evaluate gray matter integrity, while resting-state functional MRI (rs-fMRI) was used to assess functional connectivity. The DTI data were analyzed to compute fractional anisotropy (FA) and mean diffusivity (MD) in predefined regions of interest (ROIs), including HPC, cingulate cortex (CC), prefrontal cortex (PFC), entorhinal cortex (EC), and hypothalamus (HT). For rs-fMRI, data were processed to calculate the fractional amplitude of low-frequency fluctuations (fALFF), regional homogeneity (ReHo) of both voxel base and ROIs. The seed-based functional connectivity (FC) using HPC as the seed region and z-transformed FC (zFC) of ROIs were conducted. Anesthesia protocols were tailored for imaging modality, and it was induced with 4 % isoflurane, followed by a subcutaneous injection of dexmedetomidine (0.15 mg/kg) [28]. Maintenance was achieved with 0%–0.8 % isoflurane, and the respiratory rate was monitored and adjusted to remain within 50–100 breaths per minute. Imaging parameters for DTI and rs-fMRI are shown in Table 1.
Table 1.
Imaging parameters for DTI and rs-fMRI.
| Imaging Modality | Sequence | TR/TE (ms) | Matrix | FOV (mm2) | Slice Thickness (mm) | Slice Number |
|---|---|---|---|---|---|---|
| DTI | T2 (RARE) | 3200/33 | 256 × 256 | 16 × 16 | 0.5 | 22 |
| DTI (DTIEPI) | 2500/18 | 125 × 125 | 16 × 16 | 0.5 | 22 | |
| Rs-fMRI | T2 (RARE) | 3200/33 | 256 × 256 | 16 × 16 | 0.5 | 29 |
| BOLD (EPI) | 840/15 | 64 × 53 | 16 × 13.25 | 0.5 | 29 |
Abbreviations: DTI, diffusion tensor imaging; EPI, echo planar imaging; FOV, field of view; RARE, rapid acquisition with relaxation enhancement; Rs-fMRI, resting-state functional magnetic resonance imaging; TE, echo time; TR, repetition time.
The DTI images were preprocessed and analyzed in FSL (FMRIB Software Library) software (Oxford University, Oxford, UK) [29]. For preprocess, motion correction and eddy current were first performed using the ‘eddy correct’ tool, and followed by gradient correction. Then, FA and MD images were calculated by fitting a tensor model to the corrected diffusion data. Rs-fMRI image processing and analysis were performed using ParaVision 360.1.1 software (Bruker, Billerica, MA, USA), with additional post-processing performed using MATLAB and SPM12, and all these images were then aligned to the template image of mouse brain in Paxinos & Franklin space [30].
2.5. Measurement of synaptic structural parameters
Following MRI imaging, mice were anesthetized with sodium pentobarbital (50 mg/kg bw, i.p.) and perfused transcranial with 0.9 % saline, followed by 2 % paraformaldehyde and 2.5 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The HPC was carefully dissected, and the CA1 region was isolated for analysis. Samples were post-fixed in 1 % osmium tetroxide for 1.5 h, dehydrated in a graded series of ethanol, and embedded in Epon resin. Coronal sections (70 nm) were prepared and mounted on copper grids. Sections were stained with 4 % uranyl acetate and 0.4 % lead citrate.
JEM-1400 transmission electron microscopy (Japan Electron Optics Laboratory Co., Ltd., Japan) at 30000× magnification was employed to capture images of the synapses in the HPC. Synaptic structural parameters were quantified using Image-Pro Plus 6.1 (Media Cybernetics, Rockville, MD, USA), focusing on Gray's Ⅰ synapses. The width of the synaptic cleft, the curvature of the synaptic interface, the length of the active zone, and the thickness of the postsynaptic density (PSD) were measured. A total of 60 synapses per group were analyzed.
2.6. Immunofluorescence
Frozen brain sections were permeabilized with 0.3 % Triton-X100 for 15 min. After blocking with 5 % goat serum at room temperature for 1 h, the sections were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-iba1 (1:500, Wako, 019–19741), rabbit anti-PSD95 (1:100, Proteintech, 20665-1-AP), and rabbit anti-ChAT (1:100, Proteintech, 20747-1-AP). Following three washes with 1 × phosphate-buffered saline (1 × PBS), the sections were incubated with goat anti-rabbit IgG H&L Alexa Fluor® 488 (1:500, ab150077, Abcam) at 37 °C for 2 h, followed by another three PBS washes. The sections were then counterstained with DAPI (Beyotime Biotechnology, Shanghai, China). Finally, the samples were examined, and fluorescence images were captured using an LSM 900 confocal microscope (Carl Zeiss AG, Oberkochen, Germany). Fluorescence intensity or the percentage of positive areas was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.7. Sholl analysis
Microglial morphology was evaluated using Imaris v10.0.0 software (Bitplane, Zurich, Switzerland) for 3D reconstruction and sholl analysis. Confocal z-stack images were acquired using the LSM980 upright confocal microscope (Carl Zeiss AG, Oberkochen, Germany) equipped with a 100× oil immersion objective. The software traced microglial soma and their branching processes in 3D space, allowing for the quantification of soma volume, the number of endpoints, total process length, and the number of intersections at various radii from the soma.
2.8. Quantification of malondialdehyde (MDA) levels
The levels of MDA in HPC tissues were measured using the AB6500+/EXIONLC ADMP LC-MS/MS system (SCIEX, Framingham, MA, USA). Tissue samples were homogenized in a 1:4 (w/v) ratio with 50 mM 3-nitrophenylhydrazine (N21804, Sigma-Aldrich) as a derivatization reagent, followed by incubation at 50 °C for 30 min. After centrifugation at 15,000 rpm (4 °C) for 10 min, 20 μL of the supernatant was diluted with 180 μL of 50 % methanol-water. The mixture was centrifuged again, and the resulting supernatant was used for LC-MS/MS analysis.
Chromatographic separation was performed on a BEH C18 column (2.1 × 50 mm, 1.7 μm, Waters, USA) with a mobile phase consisting of 0.1 % formic acid containing 2 mM ammonium acetate (solvent A) and acetonitrile (solvent B). The gradient elution program was as follows: 10 % solvent B at 0 min, 90 % solvent B from 2 to 2.5 min, and 10 % solvent B at 2.6 min, with a total runtime of 4 min and a flow rate of 300 μL/min. MDA was detected in positive ion mode using multiple reaction monitoring (MRM), with the ion transition m/z 190.0 → 116.9, corresponding to its derivative formed with 3-nitrophenylhydrazine.
MDA calibration standards were prepared by derivatizing 50 μL of 5 mM MDA (B29980, YuanYe Bio) with 150 μL of 50 mM 3-nitrophenylhydrazine at 50 °C for 30 min to obtain a 1.25 mM stock solution. The stock solution was serially diluted with 50 % methanol-water to prepare working solutions with final concentrations ranging from 0.5 nM to 500 nM. These standards were used to construct a calibration curve. The calibration curve for MDA was generated by plotting the peak area against the concentration of the standard. The linearity of the calibration curve was confirmed with R2 values greater than 0.99.
2.9. Quantification of reduced glutathione (GSH) and oxidized glutathione (GSSG) levels
The concentrations of GSH and GSSG in HPC tissues were determined using the AB6500+/EXIONLC ADMP LC-MS/MS system (SCIEX, Framingham, MA, USA). For GSH, tissue samples were homogenized in a 1:4 (w/v) ratio with 50 mM N-ethylmaleimide (04259, Sigma-Aldrich) for derivatization, followed by incubation at 25 °C for 20 min. GSSG was directly analyzed. The homogenates were centrifuged at 15,000 rpm (4 °C) for 10 min, and 20 μL of the supernatant was diluted with 180 μL of 50 % methanol-water. After a second centrifugation, the supernatant was used for LC-MS/MS analysis.
Chromatographic separation was performed using a BEH HILIC column (4.6 × 250 mm, 5 μm, Waters, USA) with a mobile phase consisting of 0.1 % formic acid containing 2 mM ammonium acetate (solvent A) and acetonitrile (solvent B). The gradient elution program was isocratic, using 60 % solvent B throughout the entire runtime of 5 min, with a flow rate of 800 μL/min. Detection was performed in positive ion mode with MRM monitoring the ion transitions m/z 433.1 → 304.1 for N-ethylmaleimide-derivatized GSH (GSH-NEM) and m/z 613.1 → 484.3 for GSSG.
A mixed calibration solution containing GSH and GSSG was prepared. For GSH, 50 μL of 5 mM GSH (HY-D0844, MedChemExpress) was mixed with 150 μL of 50 mM N-ethylmaleimide and reacted at 25 °C for 20 min to form a derivatized stock solution (1.25 mM GSH-NEM). For GSSG (HY-D0844, MedChemExpress), no derivatization was required, and a 100 μM stock solution was prepared directly. These stock solutions were mixed and serially diluted with 50 % methanol-water to create a series of working solutions with final concentrations targeting 10,000 nM–1 nM for GSH and 100 nM–0.1 nM for GSSG in the same solution. Calibration curves for all analytes were generated by plotting the peak area against the concentration of the standards. The linearity of the calibration curves was confirmed with R2 values greater than 0.99.
2.10. Phospholipidomics
To assess the phospholipid composition in HPC samples, lipid extraction was performed using a modified Folch method in our previous research [31]. Briefly, 15 mg of HPC tissue was placed in 180 μL of PBS containing 2 mg/mL papain (Worthington Biochemical Corporation, USA) and homogenized using a high-throughput tissue grinder until a uniform homogenate was obtained. The homogenate was subjected to lipid extraction by adding chloroform/methanol (2:1, v/v) and vortexed for 30 min. A 0.9 % NaCl solution was added to the homogenate, followed by centrifugation at 8000 rpm for 10 min to separate the organic phase. The organic layer containing lipids was collected and evaporated under nitrogen gas. The dried lipid extract was resuspended in chloroform/methanol (2:1, v/v) for further analysis.
For qualitative analysis, phospholipid molecular species were identified using hydrophilic interaction liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry (HILIC-ESI-IT-TOF-MS), as described in our previous work [31]. Briefly, the extracted lipids were analyzed by collecting scan data in MS, MS2, and MS3 modes. The mass-to-charge ratios (m/z) were compared with calculated exact masses using the LIPID MAPS Structure Database (LMSD), and individual molecular species were further confirmed by fragmentation patterns in the MS2 and MS3 spectra.
For quantitative analysis, hydrophilic interaction liquid chromatography-electrospray ionization tandem mass spectrometry (HILIC-ESI-MS/MS) was employed following established protocols [31]. Chromatographic separation was achieved using a CORTECS HILIC column (2.1 × 150 mm, 1.6 μm, Waters, USA) with a flow rate of 0.4 mL/min. Eluent A consisted of water with 0.1 % formic acid and 10 mM ammonium formate, while eluent B was acetonitrile/water (95:5) with 0.1 % formic acid and 10 mM ammonium formate. The gradient elution program was as follows: 99.5 % B at 0 min, 91 % B at 21 min, 80 % B at 21.1 min, and returning to 99.5 % B at 26.1 min. Mass spectrometric detection was performed in both positive and negative ionization modes depending on the phospholipid class. The MS/MS analysis utilized MRM to detect specific phospholipid species.
Calibration curves were established using standards from Avanti Polar Lipids (Alabaster, AL, USA), with deuterium-labeled internal standards added to each sample for quantification. Quality control samples were injected after every 25 experimental samples to ensure analytical accuracy. Quantification of phospholipid classes, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and others, was performed by comparing the peak areas of samples with those of known standards. Phospholipid concentrations were expressed as nanomoles per milligram of tissue.
2.11. Statistical analysis
Data are presented as mean ± standard deviation (SD). Voxel-based analysis and seed-based functional connectivity analysis of MRI data were performed using one-tailed t-tests, with statistical significance set at p < 0.005 and a voxel size >50. Comparisons between the two groups for ROIs were conducted using two-tailed t-tests. For comparisons across multiple groups, one-way analysis of variance (ANOVA) was used, followed by Dunnett's t-test. The phospholipid data were log-transformed and scaled using center scaling (Ctr). Discriminatory analysis of phospholipidomic profiles across three groups was performed using principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA). MRI data were processed using MATLAB and SPM12, while all other statistical analyses were conducted with SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) and SIMCA software (version 14.1, MKS Umetrics, Sweden). Statistical significance was defined as ∗ p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Further details on other methods are provided in the Supplementary Information.
3. Results
3.1. Effects of choline on cognitive impairment
3.1.1. Choline improves spatial learning ability
Spatial learning ability was assessed by Y-maze and the NOR tests [32]. The CSD group spent a significantly reduced percentage of time exploring the novel arm of the Y-maze compared with CON group. In contrast, the CHOCSD group exhibited a significant increase in exploration time in the novel arm compared with CSD group (Fig. 1F). Heatmaps illustrated differences in exploration preferences among the groups during the Y-maze test (Fig. 1D). Similarly, the CSD group demonstrated significant reductions in exploration time, DI, and RI relative to the CON group in the NOR test (Fig. 1G–H). Conversely, the CHOCSD group demonstrated an evident preference for novel objects (Fig. 1I). Heatmaps also depicted differences in preference for novel versus habituated objects across the groups (Fig. 1E). These findings indicated that choline supplementation could ameliorate cognitive deficits caused by CSD.
3.1.2. Choline preserves brain structure and function
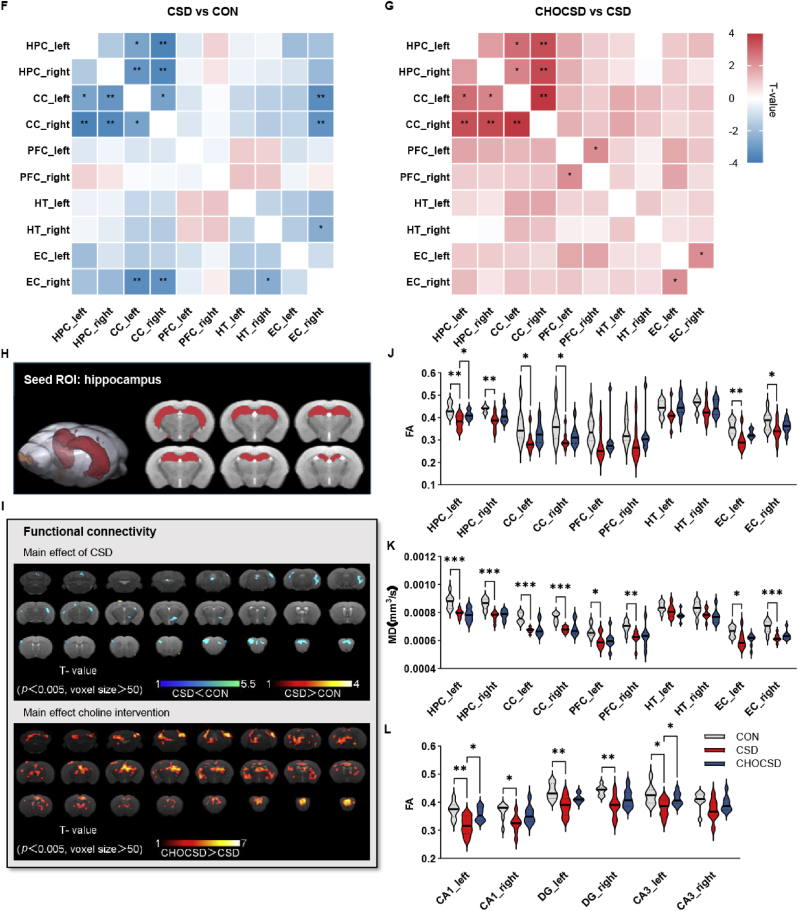
The rs-fMRI was used to examine the impact of choline supplementation on spontaneous neuronal activity and connectivity within brain regions associated with learning and memory [33,34]. Voxel-wise analysis revealed widespread reductions in fALFF across the brain in the CSD group compared with CON group, with significant recovery noted in the CHOCSD group, particularly within the HPC and EC (Fig. 2A, Supplementary Table 1). Additionally, ReHo decreased in several brain regions in the CSD group compared with CON group, with recovery observed in the CHOCSD group, particularly in the HPC, CC, PFC, and HT (Fig. 2B–Supplementary Table 2). Given the pathological changes observed in the HPC, this region was selected for seed-based FC analysis using Paxinos & Franklin space (Fig. 2H). This analysis revealed reduced zFC between the HPC and multiple brain regions in the CSD group, with significant recovery in the CHOCSD group, especially in the HPC, HT, and PFC (Fig. 2I–Supplementary Table 3).
Fig. 2.
Choline preserves brain structure and function. (A) Voxel-wise analysis of fALFF showing the main effects of CSD (upper panel) and the impact of choline supplementation (lower panel); n = 8 per group; one-tailed t-test. (B) Voxel-wise analysis of ReHo changes in the HPC and other brain regions showing the main effects of CSD (upper panel) and the impact of choline supplementation (lower panel); n = 8 per group; one-tailed t-test. (C–E) fALFF value, ReHo value, and zFC analysis of four key DMN-related brain regions and the hypothalamus (HT) across groups; n = 8 per group; one-way ANOVA followed by Dunnett's t-test. (F) Heatmap showing FC correlation differences between the CSD and CON groups in four key DMN-related brain regions and HT; n = 8 per group; Pearson correlation. (G) Heatmap showing FC correlation differences between the CHOCSD and CSD groups in four key DMN-related brain regions and HT; n = 8 per group; Pearson correlation. (H) Anatomical illustration of the seed ROI in the HPC used for FC analysis. (I) Seed-based FC analysis showing the main effects of CSD (upper panel) and the impact of choline supplementation (lower panel); n = 8 per group; one-tailed t-test. (J–L) FA and MD value analysis of four key DMN-related brain regions and the HT across groups; FA values in HPC subregions (CA1, CA3, DG) across groups; n = 8 per group; one-way ANOVA followed by Dunnett's t-test. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; fALFF, fractional amplitude of low-frequency fluctuations; DMN, default mode network; ReHo, regional homogeneity; HPC, hippocampus; zFC, z-transformed functional connectivity; FC, functional connectivity; ROI, region of interest; FA, fractional anisotropy; MD, mean diffusivity; DG, dentate gyrus; CC, cingulate cortex; PFC, prefrontal cortex; HT, hypothalamus; EC; entorhinal cortex.
ROIs were delineated based on Paxinos & Franklin space, focusing on key nodes of the default mode network (DMN) and HT. Compared with CON group, the CSD group exhibited significant reductions in bilateral HPC fALFF, which were substantially reversed by choline. Although a decrease in left EC fALFF was observed in the CSD group, it did not reach the threshold for statistical significance. However, choline supplementation led to a statistically significant increase in fALFF in this region (Fig. 2C). Bilateral PFC and HT ReHo were significantly reduced in the CSD group compared with CON group, whereas choline supplementation led to significant increases in ReHo in both bilateral HT and right PFC. No significant differences were found between the CSD and CON groups in bilateral CC, but choline supplementation resulted in a statistically significant increase in ReHo in these regions (Fig. 2D). Additionally, zFC was significantly diminished in bilateral PFC in CSD group compared with CON group. Moreover, choline supplementation restored zFC levels, with a statistically significant increase in right PFC zFC compared with CSD group. Although bilateral HPC and CC zFC decreased in CSD group, these changes did not reach statistical significance. Nonetheless, choline supplementation led to significant improvements in these parameters (Fig. 2E).
The Pearson correlation analysis matrix for FC, based on ROIs, revealed distinct differences in ROI connectivity among three groups. Overall, CSD group exhibited weaker connectivity compared with CON group, but CHOCSD group reversed this change (Supplementary Figs. 1A–C). Statistical analysis demonstrated decreased connectivity between the bilateral HPC and bilateral CC, as well as between the left and right CC in CSD group compared with CON group. This connectivity was significantly restored in CHOCSD group (Fig. 2F–G). These findings suggest that choline supplementation ameliorates CSD-induced reductions in spontaneous neuronal activity and functional connectivity within ROIs crucial for learning and memory, with the HPC emerging as a key ROI.
DTI was employed to assess the impact of choline supplementation on brain integrity [35]. In the predefined ROIs, significant reductions of FA in CSD group were observed in the bilateral HPC, CC, and EC, compared with CON group. Following choline supplementation, FA levels in these regions demonstrated significant recovery, with a statistically significant increase in the left HPC compared with CSD group (Fig. 2J). Conversely, MD levels were significantly reduced in the bilateral HPC, CC, PFC, and EC of CSD group compared with CON group. Although MD levels were improved with choline supplementation, no statistically significant differences were observed (Fig. 2K). The significant reduction in FA level of HPC following CSD prompted further investigation into microstructural changes within subregions. Three additional ROIs (CA1, CA3, and dentate gyrus (DG)) were extracted from the HPC. Compared with CON group, statistically significant decreases of FA levels were observed in the bilateral CA1, DG, and left CA3 of CSD group, with choline supplementation resulting in significant recovery, primarily in the left CA1 and CA3 (Fig. 2L). These results suggested that choline supplementation could preserve HPC and its subregions structural integrity.
3.1.3. Choline reverses brain injury
Pathological changes in the CA1, CA3, and DG regions were investigated using Nissl and hematoxylin and eosin (H&E) staining to evaluate the impact of choline supplementation on neuronal injury in the HPC. The CON group exhibited normal neuronal morphology characterized by densely and neatly arranged neurons, intact structures, and evident nucleoli (Fig. 3A and B). In contrast, CSD group displayed reduced neuronal density and disorganized cell morphology, with pathological features including cell vacuolation, edema, and deep cytoplasmic staining, as well as ruptured cell membranes, atrophied, and condensed nuclei. Choline supplementation largely preserved neuronal density and structure (Fig. 3B). These findings indicated that choline exerted neuroprotective effects, mitigating HPC damage induced by CSD.
Fig. 3.
Choline reverses brain injury. (A) Representative H&E staining of HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; Scale bar = 100 μm. (B) Representative Nissl staining of HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; Scale bar = 100 μm. (C) Representative images of Evans Blue (EB) staining of HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; Scale bar = 20 μm. (D) Quantification analysis of EB fluorescence intensity in HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; one-way ANOVA followed by Dunnett's t-test. (E) Brain tissue EB concentration across groups; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. (F–G) Serum S100β and NSE levels measured by ELISA across groups; n = 8 per group; one-way ANOVA followed by Dunnett's t-test. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; DG, dentate gyrus; EB, evans blue; NSE, neuron-specific enolase.
The Evans Blue (EB) dye extravasation method was utilized to further assess the protective effects of choline on BBB integrity. The results revealed a statistically significant increase in EB leakage in CSD group compared with CON group, while choline supplementation markedly reduced EB extravasation (Fig. 3E). Similar results were observed in fluorescence images of the HPC, specifically in the CA1, CA3, and DG regions (Fig. 3C–D).
Finally, the severity of brain injury was evaluated by serum levels of S100β and neuron-specific enolase (NSE). Compared with CON group, CSD group exhibited significantly elevated levels of S100β and NSE, whereas choline led to reductions in these biomarkers compared with CSD group (Fig. 3F–G).
3.2. Choline ameliorates cognitive impairment by reducing neuroinflammation and oxidative stress
3.2.1. Choline inhibits inflammation
CSD group exhibited elevated ACh level in the HPC compared with CON group, while CHOCSD group showed the decreased ACh level compared with CSD group (Fig. 4A). ACh synthesis and degradation are regulated by enzymes, including ChAT and Acetylcholinesterase (AChE) [36]. The CSD group displayed upregulation of ChAT and downregulation of AChE compared with CON group, while CHOCSD group reversed these changes (Supplementary Figs. 1D–F). The alpha 7 nicotinic acetylcholine receptor (α7-nAChR) was downregulated in the HPC of CSD group compared with CON group, but it was reversed in CHOCSD group (Fig. 4B–E). These findings suggested that choline could counteract CSD-induced neuroinflammation and abnormal neuronal signaling.
Fig. 4.
Choline inhibits inflammation and oxidative stress. (A) Level of ACh content; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. (B) Representative images of α7-nAChR staining in the HPC across groups; n = 4 per group; Scale bar = 50 μm. (C) Quantitative analysis of α7-nAChR positive area (% of total area); n = 4 per group. (D–E) The protein expression of α7-nAChR protein expression in HPC across groups detected by western blot; n = 3 per group; one-way ANOVA followed by Dunnett's t-test. (F–J) ELISA results showing levels of inflammatory cytokines: TNF-α, IL-1β, IL-6, IL-18, and IL-10 in the HPC across groups; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. (K–Q) Levels of oxidative stress-related markers, including MDA, GSH, GSSG, and the GSH/GSSG ratio measured by LC-MS/MS, as well as GSH-Px, CAT, and SOD activities, were assessed in the HPC across groups; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; ACh, acetylcholine; α7-nAChR, alpha7 nicotinic acetylcholine receptor; HPC, hippocampus; MDA, malondialdehyde; CAT, catalase; GSH, glutathione; GSH-Px, glutathione peroxidase; GSSG, oxidized glutathione; SOD, superoxide dismutase.
The levels of inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-18 (IL-18) were elevated in the HPC of the CSD group compared with CON group. In contrast, choline supplementation reduced these cytokine levels compared with CSD group (Fig. 4F–I). Correspondingly, the anti-inflammatory cytokine interleukin-10 (IL-10) decreased in HPC of CSD group, but increased with choline (Fig. 4J). Similar trends were observed in the serum, where inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-18 were elevated in CSD group, accompanied by a reduction in IL-10, and choline effectively mitigated these inflammatory responses (Supplementary Figs. 1G–K).
3.2.2. Choline reduces oxidative stress and restores antioxidant capacity
MDA level is a reliable marker of lipid peroxidation, and was significantly elevated in the CSD group compared with the CON group, indicating increased oxidative damage. Choline supplementation significantly reduced MDA levels in the CHOCSD group compared with the CSD group (Fig. 4K). For glutathione metabolism, although an insignificant decrease in GSH was observed in the CSD group (Fig. 4L), the levels of GSSG, the oxidized form of glutathione, were significantly higher in the CSD group compared with the CON group. The GSH/GSSG ratio, often used as a measure of oxidative stress, was also reduced in the CSD group. Importantly, choline supplementation decreased GSSG levels in the CHOCSD group and restored the GSH/GSSG ratio compared with the CSD group (Fig. 4M−N). In addition, the activities of antioxidant enzymes, including glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD), were markedly reduced in the CSD group compared with the CON group. Furthermore, choline supplementation significantly improved the activities of GSH-Px, CAT, and SOD in the CHOCSD group (Fig. 4O–Q).
3.2.3. Choline reduces activation of microglia
Immunostaining for IBA1, a marker expressed in all microglial types, revealed significantly increased IBA1-positive cell areas in CA1, CA3, and DG regions of CSD group compared with CON group, indicating substantial microglial proliferation. Choline supplementation contributed to a notable reduction in microglial numbers compared with CSD group (Fig. 5A–B). Furthermore, CSD group exhibited an activated microglial morphology characterized by shorter and sparser processes, enlarged cell soma, and reduced total length compared with CON group, while these characteristics were reversed in CHOCSD group (Fig. 5C–G). These findings suggested that choline could mitigate the CSD-induced pro-inflammatory and oxidative stress environment by inhibiting microglial proliferation and morphological activation.
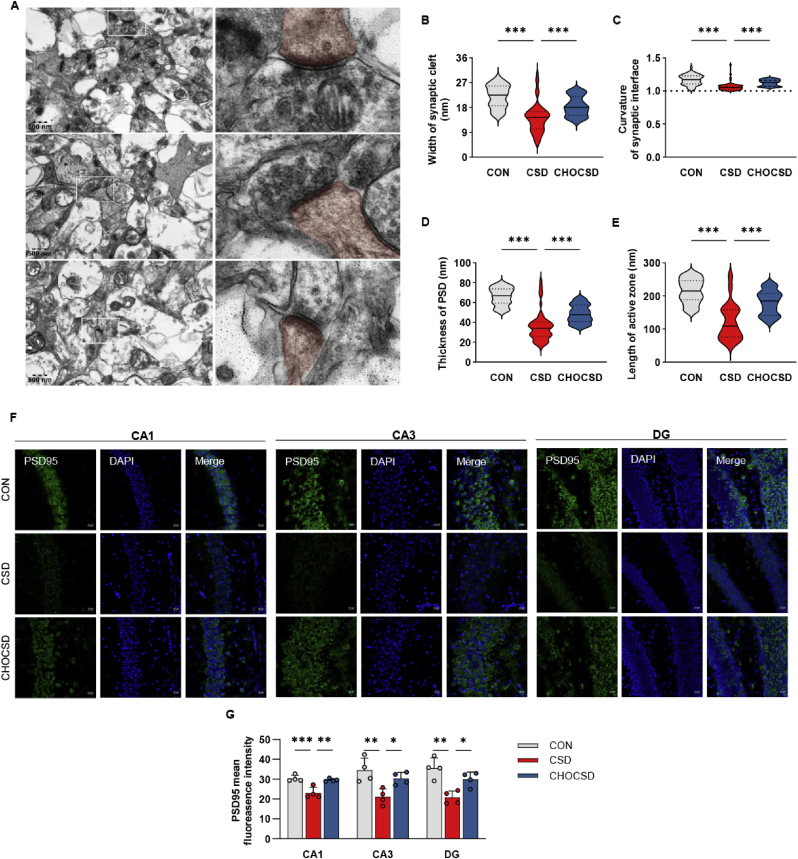
Fig. 5.
Choline reduces activation of microglia. (A) Representative images of Iba1+ microglia staining of HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; Scale bar = 50 μm. (B) Quantitative analysis of IBA1+ microglia as a percentage of the total area in HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; one-way ANOVA followed by Dunnett's t-test. (C) Representative 3D reconstruction of Iba1+ microglia from confocal microscopy images in the CA1 region illustrating morphological differences between groups; n = 4 per group; Scale bar = 10 μm. (D–F) Quantitative analysis of soma volume, total process length and total terminal points of IBA1+ microglia in the CA1 region across groups; n = 4 per group; one-way ANOVA followed by Dunnett's t-test. (G) Sholl analysis of IBA1+ microglial process complexity showing the number of intersections at different distances from the soma; n = 4 per group. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; DG, dentate gyrus.
3.2.4. Choline preserves synaptic plasticity
The CSD group exhibited significantly narrower synaptic clefts, reduced synaptic curvature, shortened active zone lengths, and thinner PSD thickness compared with CON group. Notably, choline supplementation significantly reversed these alterations in CHOSCSD group (Fig. 6A–E). Additionally, immunofluorescence staining revealed reduced PSD95 expression in CSD group compared with CON group, while choline supplementation increased PSD95 expression (Fig. 6F–G). These findings supported that choline not only inhibited the inflammation and oxidative stress within the HPC, but also contributed to the preservation of synaptic structure.
Fig. 6.
Choline preserves synaptic plasticity. (A) Transmission electron microscopy (TEM) images of synaptic structures in the CA1 region across the three groups, with the postsynaptic membrane and its adjacent regions marked in red to highlight the postsynaptic elements; n = 4 per group; Scale bar = 500 nm. (B–E) Quantitative bar graphs depicting the width of the synaptic cleft, curvature of the synaptic interface, thickness of the postsynaptic density (PSD) and length of the active zone across groups, with 60 synapses observed per group; n = 4 per group; one-way ANOVA followed by Dunnett's t-test. (F) Representative images of PSD95 staining of HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; Scale bar = 20 μm. (G) Quantitative analysis of PSD95 mean fluorescence intensity in HPC subregions (CA1, CA3, DG) across groups; n = 4 per group; one-way ANOVA followed by Dunnett's t-test. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; TEM, transmission electron microscopy; PSD, postsynaptic density; HPC, hippocampus; DG, dentate gyrus.
3.3. Choline ameliorates cognitive impairment by modulating phospholipidomic profile
3.3.1. Characteristics of phospholipidomic profile
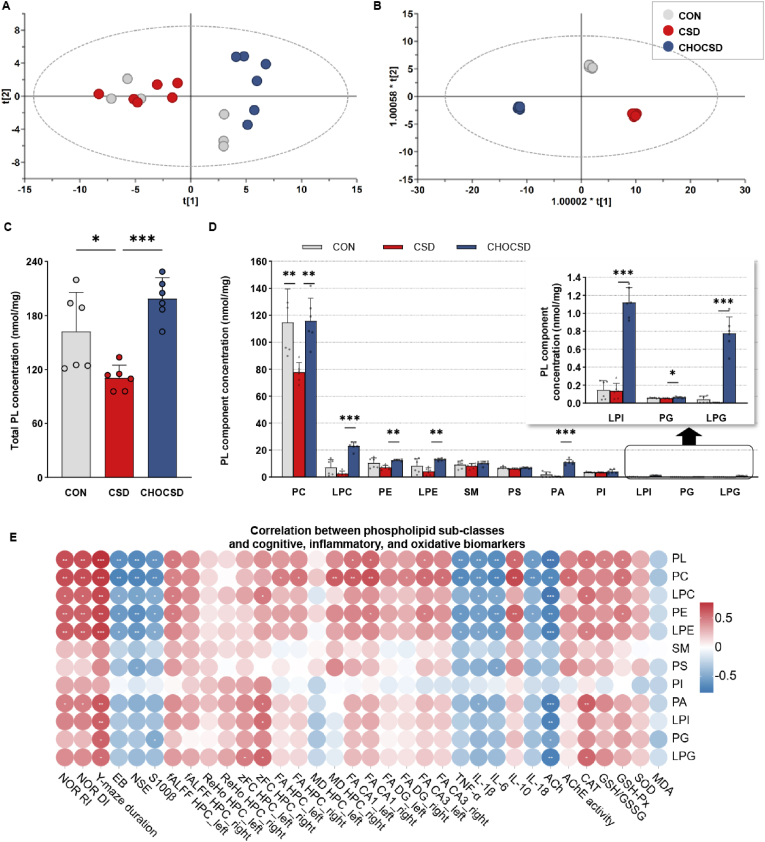
A total of 313 phospholipid molecules were identified from HPC samples (Supplementary Table 4). The phospholipid data were log-transformed and scaled using Ctr scaling. In the PCA model, the concentrations of 313 phospholipid molecules and the 18 HPC samples were designated as the rows and columns of the matrix, respectively. The first two principal components accounted for 60.4 % of the total variance of the data. As depicted in the score plot, for the 18 HPC samples, a distinct separation was observed among the groups although several samples overlapped, indicating that the phospholipidomic profile in the HPC was altered by CSD and choline supplementation (Fig. 7C).
Fig. 7.
Phospholipidomic profile characteristics and the role of phospholipid sub-classes in enhancing cognitive function. (A) Score plots for PL profile in HPC samples from three groups based on PCA model; n = 6 per group. (B) Score plots for PL profile in HPC samples from three groups based on OPLS-DA model; n = 6 per group. (C) Total phospholipid concentration in the HPC across groups; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. (D) PL component analysis showing concentrations of PC, LPC, PE, and other major PL components in the HPC; The inset shows the concentrations of LPI, PG, and LPG across groups; n = 6 per group; one-way ANOVA followed by Dunnett's t-test. (E) Correlation heatmaps showing the relationship between phospholipid sub-classes and cognitive, inflammatory, and oxidative biomarkers; n = 6 per group; Pearson correlation. Data are presented as mean ± SD; n defines biological replicates; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; PL, phospholipid; PC, phosphatidylcholine; LPC, lyso-phosphatidylcholine; PE, phosphatidylethanolamine; LPE, lyso-phosphatidylethanolamine; SM, sphingomyelin; PS, phosphatidylserine; PA, phosphatidic acid; PI, phosphatidylinositol; LPI, lyso-phosphatidylinositol; PG, phosphatidylglycerol; LPG, lyso-phosphatidylglycerol; NOR, novel object recognition; RI, recognition index; DI, discrimination index; EB, evans blue; NSE, neuron-specific enolase; fALFF, fractional amplitude of low-frequency fluctuations; ReHo, regional homogeneity; zFC, z-transformed functional connectivity; FA, fractional anisotropy; MD, mean diffusivity; DG, dentate gyrus; ACh, acetylcholine; α7-nAChR, alpha7 nicotinic acetylcholine receptor; HPC, hippocampus; MDA, malondialdehyde; CAT, catalase; GSH, glutathione; GSSG, oxidized glutathione; SOD, superoxide dismutase; PCA, principal component analysis; OPLS-DA, orthogonal projections to latent structures-discriminant analysis; SD, standard deviation.
To further differentiate phospholipid patterns, OPLS-DA was utilized. The result of the OPLS-DA model is presented in Fig. 7D. The first two components accounted for 99.8 % of the total variance of the data. The score plot demonstrated clear separation among three groups. These findings indicated that CSD induced specific alterations in the HPC phospholipidomic profile. Choline supplementation effectively mitigated these alterations as a part of its neuroprotective effects.
3.3.2. Role of phospholipid sub-classes in cognitive improvement
Based on the phospholipidomic shifts observed, we next analyzed changes within total phospholipid and specific phospholipid sub-classes, focusing on potential associations with cognitive outcomes. The results indicated that total phospholipid level was significantly reduced in CSD group compared with CON group (Fig. 7A). In contrast, choline supplementation resulted in a significant increase in total phospholipid level. PC and lyso-PC (LPC) were the predominant phospholipids in the HPC, followed by PE and lyso-PE (LPE). The CSD group exhibited a significant reduction in PC level compared with CON group. Choline supplementation not only restored PC level, but also significantly increased levels of LPC, PE, LPE, and some trace phospholipids such as phosphatidic acid (PA), lysophosphatidylinositol (LPI), phosphatidylglycerol (PG), and lyso-PG (LPG) (Fig. 7B). These findings suggested that the neuroprotective effects of choline could be partially attributed to its ability to counteract the CSD-induced decline in total phospholipids and PC levels, as well as to elevate the levels of various phospholipid sub-classes.
The relationship between the identified total phospholipid and key phospholipid sub-classes with neurobiological, neuroinflammatory, and neuroprotective biomarkers were further examined (Fig. 7E). Overall, HPC total phospholipid level in this study exhibited a strong negative correlation with ACh content (r = −0.76). Besides, total phospholipids level demonstrated a strong positive correlation with behavioral performance (r = 0.67–0.77). Although total phospholipids showed no significant association with other HPC functional MRI indices, there was a moderate positive correlation with the left HPC fALFF (r = 0.51). At the subregional level, total phospholipids exhibited a positive correlation with fALFF in bilateral CA1 and left CA3 (r = 0.48–0.57). Importantly, total phospholipid level showed a moderate-to-strong negative correlation with brain injury biomarkers NSE, S100β, and the BBB injury marker EB (r = −0.67 ∼ −0.60). Furthermore, total phospholipid level exhibited negative correlations with inflammatory cytokines (r = −0.65 ∼ −0.57) and positive correlations with the anti-inflammatory factor IL-10 (r = 0.57). They also demonstrated a positive correlation with antioxidant enzymes CAT, GSH, and GSH-Px (r = 0.48–0.59).
Among the various phospholipid sub-classes, PC demonstrated particularly noteworthy associations with a wide range of indicators and outcomes (Fig. 7E). For example, PC level exhibited strong positive correlations with behavioral metrics (r = 0.67–0.77). Conversely, PC level demonstrated a negative correlation with brain EB content (r = −0.63). Additionally, PC level was positively correlated with HPC fALFF, HPC and subregional (bilateral CA1, CA3 and right DG) FA, and right HPC MD (r = 0.48–0.66). PC level also displayed negative correlations with inflammatory cytokines (r = −0.69 ∼ −0.62) and positive correlation with the anti-inflammatory factor IL-10 (r = 0.68). PC demonstrated a positive correlation with antioxidant enzymes GSH-Px (r = 0.53), as well as negative correlations with brain injury biomarkers NSE and S100β (r = −0.69, −0.64). Notably, a positive correlation between PC and AChE was also observed (r = 0.56). Other phospholipid sub-classes generally followed similar correlation patterns to those of total phospholipid. These findings suggested that an increase in total phospholipid, particularly PC, could contribute to the maintenance of neural functional integrity by mitigating inflammation and enhancing redox homeostasis.
3.3.3. Role of phospholipid molecules in cognitive improvement
The OPLS-DA models were constructed to compare CON group versus CSD group, and CSD group versus CHOCSD group, in order to further assess the impact of CSD and choline supplementation on phospholipid molecules. The volcano plots integrated fold change, p-values (from t-tests), and variable importance in projection (VIP) values for the phospholipid molecules. Dots farther from the origin indicated greater changes in the represented phospholipids between the two compared groups. The size of each point reflected the VIP value of the corresponding phospholipid molecule derived from the OPLS-DA model, which was positively correlated with its contribution to the distinction between the two groups (Fig. 8A–B). The analysis revealed that choline significantly modulated some phospholipid molecules, particularly in PC, LPC, and PE sub-classes, which were likely integral to maintaining HPC cellular homeostasis and supporting cognitive resilience in the context of CSD.
Fig. 8.
Role of phospholipid molecules in cognitive improvement. (A)–(B) Volcano plots of OPLS-DA models displaying the differential phospholipid species between group comparisons: CON vs. CSD and CSD vs. CHOCSD. (C) The bar plots display the log fold changes in key phospholipid molecules between the CSD vs. CON group and the CHOCSD vs. CSD group; The molecules are arranged along the X-axis in decreasing order of their VIP values derived from the OPLS-DA model comparing the CSD and CON groups. (D) Correlation heatmaps showing the relationship between key phospholipid molecules and cognitive, inflammatory, and oxidative biomarkers; Pearson correlation. n = 6 per group; n defines biological replicates. Abbreviations: CON, control; CSD, chronic sleep deprivation; CHOCSD, choline-supplemented chronic deprivation; PL, phospholipid; PC, phosphatidylcholine; LPC, lyso-phosphatidylcholine; PE, phosphatidylethanolamine; LPE, lyso-phosphatidylethanolamine; SM, sphingomyelin; PS, phosphatidylserine; PA, phosphatidic acid; PI, phosphatidylinositol; LPI, lyso-phosphatidylinositol; PG, phosphatidylglycerol; LPG, lyso-phosphatidylglycerol; NOR, novel object recognition; RI, recognition index; DI, discrimination index; EB, evans blue; NSE, neuron-specific enolase; fALFF, fractional amplitude of low-frequency fluctuations; ReHo, regional homogeneity; zFC, z-transformed functional connectivity; FA, fractional anisotropy; MD, mean diffusivity; DG, dentate gyrus; ACh, acetylcholine; α7-nAChR, alpha7 nicotinic acetylcholine receptor; HPC, hippocampus; MDA, malondialdehyde; CAT, catalase; GSH, glutathione; GSSG, oxidized glutathione; SOD, superoxide dismutase; PCA, principal component analysis; OPLS-DA, orthogonal projections to latent structures-discriminant analysis; VIP, variable importance in projection.
To further understand the changes of key phospholipid molecules in sleep deprivation and choline supplementation, important phospholipids were extracted from the OPLS-DA model comparing CSD group with CON group, specifically those with p-values less than 0.05 and VIP values greater than 1.5. Their variations in CSD and changes following choline supplementation were described (Fig. 8C). Several unique phospholipid molecules were identified, including plasmalogens (PC p-38:3, PC p-35:0, PC p-37:0, PC p-36:3, PC p-36:0, PE p 42:4, PE p-38:5, and PE p-36:3), lysophosphatidic (LPE 19:0, LPC 22:0, and LPG 16:0), and ester-phospholipids (PC O-33:0, and PC O-32:0). Importantly, several molecules containing odd-chain fatty acids (PC 37:4, PC O-33:0, PI 37:4(C17:0), PC p-35:0, PC 34:1, PC 35:1, PC p-37:0, SM 35:1, PC 37:1, LPE 19:0, PC 31:0, SM 41:1, SM 37:1, PC 39:6, PG 34:1 (C18:1), and SM 41:2) were observed as significant for distinction between CSD group and CON group. These results indicate that the influence of sleep deprivation on these phospholipid molecules was significantly reversed by choline supplementation.
Based on the detailed analysis of key phospholipid molecules influenced by sleep deprivation and choline supplementation, specific molecules were further examined to evaluate their associations with cognitive performance and neuroprotective biomarkers (Fig. 8E). Levels of PE 36:3, PE 38:2, PE 40:4, PE p-36:3, PE p-38:5, and PE p-42:4 were observed to be positively correlated with cognitive performance (r = 0.52–0.77), and left HPC fALFF (r = 0.52–0.74), and negatively correlated with brain injury markers (r = −0.75 ∼ −0.53). In addition, these molecules of PE presented negative correlations with pro-inflammation cytokines (r = −0.74 ∼ −0.50), but positive correlations with IL-10 level (r = 0.49–0.64) and antioxidative biomarkers (r = 0.47–0.69). Similar results were observed for a docosahexaenoic acid (DHA)-containing phospholipid, PS 44:12, and its levels were positively correlated with CAT activity (r = 0.71) and the GSH/GSSG ratio (r = 0.65). Additionally, several PC molecules were significantly downregulated by CSD, including PC 36:1, PC 32:0, PC O-33:0, PC p-35:0, PC p-37:0, and PC 34:1. Overall, these findings suggested that choline's neuroprotective effects under CSD conditions could be mediated through its influence on specific phospholipid molecules.
4. Discussion
Choline is an essential nutrient, required for the biosynthesis of the neurotransmitter ACh and various phospholipids [25,37]. In this study, we characterized the structural and functional changes in the brain associated with CSD, highlighting how choline supplementation effectively enhanced neuronal activity and connectivity in specific brain regions. Our results found for the first time that choline could alleviate CSD-induced cognitive impairment by anti-inflammation and antioxidation as well as altering phospholipidomic profile, ultimately exerting neuroprotective effects.
The Rs-fMRI and DTI methods could assess local spontaneous activity, inter-regional communication, and microstructural integrity within brain matter regions. Previous studies have shown that sleep deprivation induces profound molecular alterations in the HPC, including impaired synaptic plasticity, reduced neurogenesis, and alterations in neurotransmitter signaling, all of which contribute to cognitive deficits, which was consistent with our study [38]. Furthermore, choline supplementation has been demonstrated to ameliorate HPC damage across various models of cognitive impairment. For example, in APP/PS1 mice, choline supplementation prevented HPC cholinergic neuron loss and cognitive deficits [39]. Additionally, prenatal choline treatment has been shown to reverse iron deficiency-induced HPC dysfunction in rats [40]. Collectively, these results supported our findings that choline ameliorated sleep deprivation-induced changes in the HPC, establishing it as a key region for further research into cognitive function and potential therapeutic interventions.
A previous study linked a reduced spontaneous neuronal activity measured by fALFF to sleep deprivation-induced cognitive deficits [41], which supported our results. Furthermore, several studies described key nodes of the default mode network (DMN) involving in memory and cognition [[42], [43], [44]], such as HPC [45,46], PFC [47,48], CC [49] and EC [50], as well as HT, which regulates sleep and wakefulness [51]. In this study, we observed decreased ReHo in the HPC, PFC, and CC, reflecting reduced local neuronal synchronization, which was consistent with earlier research demonstrating that sleep deprivation leads to disrupted synchronization, especially in regions associated with higher-order cognitive functions [52,53]. The seed-based FC analysis using the HPC as the seed region demonstrated reduced zFC between the HPC and other regions such as PFC and HT in the CSD group. These disruptions were in line with research highlighting the adverse effects of sleep deprivation on connectivity within cognitive networks, such as the DMN [[54], [55], [56]]. Notably, choline supplementation was able to reverse the disrupted FC and restore structural integrity, which suggested that choline could enhance inter-regional communication, particularly within brain networks that supported memory and executive functions.
Interestingly, our correlation analysis further revealed that choline supplementation improved disrupted connectivity between the HPC and CC, as well as bilateral CC connections. Previous studies indicated the importance of the CC-HPC network in spatial memory, where information about space was relayed via the posterior CC to the HPC, and reward-related information was conveyed from the anterior CC to the HPC formation [57]. Therefore, the reactivity between the CC and HPC after choline supplementation could provide a potential explanation for the observed improvement in spatial learning and memory performance. Furthermore, the DTI results indicated that CSD led to significant reductions in FA in the bilateral HPC, CC, and EC, reflecting compromised microstructural integrity, which aligned with findings that decreased FA was associated with cognitive decline [58]. These findings supported that choline could exert a protective effect on the microstructural integrity of key brain regions involved in cognition.
Sleep restriction has been shown to impair BBB function by reducing the expression of critical endothelial proteins, thereby increasing paracellular permeability and facilitating neuroinflammatory responses [59]. These alterations contributed to cognitive deficits and neuronal injury, as indicated by the leakage of EB dye in various brain regions, which was consistent with our findings [60]. In our study, choline supplementation reduced levels of S100β and NSE, serum markers of brain injury that were elevated by sleep deprivation. This was consistent with previous studies that elevated levels of S100β and NSE were associated with lower levels of circulating choline, indicating that the decline in circulating choline could be linked to postoperative cognitive decline [61].
Inflammation could play an important role in the cognitive impairment [[62], [63], [64]]. In addition, choline could be metabolized into ACh, which is the key molecule in the cholinergic anti-inflammatory pathway [65], and disturbances in the cholinergic pathway have been reported in various cognitive impairment models [19,[66], [67], [68]]. However, the levels of ACh in the HPC of CSD mice did not decrease in this study, while AChE activity was found to decline. AChE hydrolyses and inactivates ACh, terminating the excitatory effect of neurotransmitters on the postsynaptic membrane, thereby ensuring the proper transmission of neural signaling information [69,70]. Additionally, α7-nAChR is integral to the cholinergic anti-inflammatory pathway, whereby ACh binding to α7-nAChR modulates inflammatory responses [69], leading to the suppression of pro-inflammatory cytokine release [50], which was consistent with our observation that choline could significantly reverse the CSD-induced downregulation of α7-nAChR. The α7-nAChR-mediated signaling in macrophages or other subsets of immune cells suppressed inflammatory cytokine secretion and modulates apoptosis, proliferation, and macrophage polarization, eventually reducing the systemic inflammatory response [71]. Previous studies showed that activation of microglia and astrocytes was accompanied by downregulation of α7-nAChR in mice deprived of sleep for seven days, with the use of the α7-nAChR agonist PHA-543613 being able to reverse these changes [72]. It was noteworthy that choline acted as an agonist in our study. Furthermore, we speculated that the elevation of ACh in CSD could be a short-term compensation due to the downregulation of cholinergic receptors. Overall, choline supplementation allows the entire cholinergic anti-inflammatory pathway to function normally, which is disrupted by CSD.
Simultaneously, the activation of the α7-nAChR cholinergic anti-inflammatory pathway not only mitigated inflammatory cytokine release but also reduced oxidative stress [73,74]. In our study, CSD elevated inflammatory cytokines (such as TNF-α, IL-1β, and IL-18), and also caused oxidative damage, as indicated by an increased MDA level and a decreased GSH/GSSG ratio. Moreover, the activation of microglia in response to inflammatory cytokines contributed to the neuroinflammatory environment [12], and their over-activation let to persistent release of inflammatory mediators, which could accumulate to neurotoxic levels, ultimately leading to cognitive impairment [11,26,27,75,76]. This study not only observed an increase in IBA-1 positive cells in CSD mice, but also found that CSD caused microglia to present a highly activated morphology.
In our study, we found that sleep deprivation negatively impacted synaptic plasticity, as evidenced by significant changes in synaptic ultrastructure in the hippocampal CA1 region. These alterations aligned with previous research indicating that oxidative stress and neuroinflammation could lead to similar impairments in synaptic structure and function, ultimately contributing to cognitive deficits [62,75,77]. Moreover, our findings demonstrated that choline supplementation could enhance synaptic plasticity, effectively reversing the structural changes induced by sleep deprivation. These corroborated earlier studies highlighting the neuroprotective effects of choline, particularly in models of neurodegenerative diseases and model of cognitive impairment caused by prenatal low protein, where choline has been shown to restore synaptic function and improve cognitive outcomes [26,39]. In summary, our findings indicated that choline supplementation effectively modulated neuroinflammation and oxidative stress, which helped preserve synaptic plasticity and structural integrity in the HPC.
We observed that phospholipid metabolism was strongly disrupted in CSD mice, which was relevant to cognitive deficits, as alterations in the phospholipid profile played an important role in altered intracellular signaling pathways [23,78,79]. Moreover, in line with choline acting as an important component of phospholipid [80,81], HPC phospholipidomic profile altered in response to choline supplementation. Although phospholipid homeostasis was associated with brain function [82,83], few studies have investigated the phospholipidomic profile in various pathological contexts. A study conducting brain lipidomic analyses has identified a limited number of molecular species and focused solely on calculating their relative amounts [84]. We have identified 313 phospholipid molecules in HPC via HILIC-ESI-IT-TOF-MS, an effective method that combined multistage collision and high mass resolution within the IT-TOF system. This method enabled the characterization of the structure of head groups, backbones, and fatty acyl moieties, as well as the specific positions of fatty acyl substituents.
PLs comprise over 60 % of the total lipid content in neuronal membranes [22]. PC is the most abundant PL in mammalian membranes and, along with other lipids, is essential for synaptic structure and function, influencing membrane fluidity, molecular mobility, and the spatial organization of proteins [25]. Maintaining lipid homeostasis was essential for synaptic plasticity and overall brain function [85]. Altered PC levels was observed in neurodegenerative diseases, with reduced concentrations, particularly of unsaturated PC, being associated with HPC atrophy and cognitive decline [86]. We confirmed that choline supplementation significantly increased the levels of total phospholipids and PC in the HPC of CSD mice, and this increase was associated with improved cognitive function. We also observed an increase in the levels of LPC, PE, and LPE following choline supplementation. PE is the most abundant phospholipid in the mammalian brain [87]. LPC, a metabolite of PC, has been shown to have a positive correlation with corticosterone levels in the blood of rats, suggesting a potential connection between the activity of the hypothalamic-pituitary-adrenal (HPA) axis and phospholipid metabolism [82]. Moreover, LPC is crucial for the synthesis of lyso-pa (LPA) [88], which plays a significant role in modulating presynaptic glutamate release and regulating the excitation/inhibition balance in cortical networks, thereby influencing neuronal excitability and sensory information processing [89]. However, the low concentrations of LPC in the cerebrospinal fluid suggested that LPC availability could limit LPA synthesis in the central nervous system, potentially affecting brain function.
Additionally, plasmalogen phospholipids, which are highly concentrated in the peri-synaptic regions of the postsynaptic membrane, have been shown to benefit cognition [90]. They play a critical role in synaptic plasticity by contributing to vesicle fusion, membrane raft composition, the endoplasmic reticulum stress response, transmembrane protein activity, and cholesterol transport [91,92]. Significant plasmalogen phospholipids were observed in this study, including PC p-35:0, PC p-36:0, PC p-36:3, PC p-37:0, PC p-38:0, PE p-36:3, PE p-38:5, and PE p-42:4. Notably, HPC levels of PE p-36:3, PE p-38:5, and PE p-42:4 were positively correlated with cognitive behavior test performance and negatively correlated with inflammation markers. Similar results were also observed for the HPC levels of PS 44:12, a DHA-containing phospholipid. Its level was positively correlated with the GSH/GSSG ratio and CAT activity, which is consistent with findings from a study that investigated the antioxidant properties of orally administered DHA-PS [93]. Reductions in DHA-containing phospholipids have been reported in several other models of cognitive impairment [83,94].
Interestingly, we found that OCFA-containing phospholipids were distinct discriminants between the CSD group and controls. Although research on the function of OCFAs remains scarce, some studies suggest that OCFAs could offer cognitive benefits [95,96]. Additionally, protective associations between OCFA-containing lipids and metabolic health have been demonstrated in several studies, including the PREDIMED trial [97], and the EPIC study [98,99]. We are the first to report that OCFA-containing phospholipids serve as effective predictors of CSD-induced cognitive impairment, offering a novel target for future research aimed at improving cognitive function. The extent of choline-driven modifications in the HPC phospholipid profile, especially regarding pathways of key phospholipids involving PC, lysophospholipids, plasmalogen phospholipids and OCFA-containing phospholipids, could explain the wide range of cognitive and neuroprotective benefits observed in the context of CSD. Further investigations are needed to clarify the roles of these phospholipids in regulating the balance between neuroinflammatory and neuroprotective responses.
As discussed above, we have established that CSD induces cognitive impairment by disrupting phospholipid homeostasis. There was also a close relationship between phospholipid homeostasis and neuroinflammation [78,100]. Importantly, membrane phospholipids served as substrates for the biosynthesis of bioactive lipid mediators [22], and the resolution of inflammation was partially governed by a shift in lipid mediator production from pro-inflammatory to pro-resolving classes [101]. We reported a negative correlation between HPC phospholipid levels and inflammatory markers. Interestingly, we also observed that choline supplementation markedly reduced EB extravasation, and further analysis revealed that higher phospholipids levels were associated with lower EB concentrations, indicating improved BBB integrity. This suggested that choline supplementation could drive potential changes in several interconnected pathways, including neural plasticity, BBB permeability, phospholipid dynamics, as well as neuroglial functions and inflammation, which collectively restored systems that are disrupted by CSD.
4.1. Strengths and limitations
Choline, a key source of methyl groups, is naturally present in various foods and also available as a dietary supplement [18]. Epidemiological studies have shown positive associations between dietary choline intake and cognitive function in older adults [21,[102], [103], [104], [105]]. In this study, we are the first to demonstrate choline's neuroprotective effects in the context of sleep deprivation. This could have significance for individuals experiencing sleep disturbances, as the effects of sleep restriction on brain function was reported [4,5] and led to neurocognitive dysfunction [106]. Whereas extending sleep duration or drug-based regulating sleep homeostasis could help some patients with severe sleep problems, choline supplementation could potentially benefit a larger population, representing an effective approach to mitigate cognitive deficits caused by sleep deprivation. Our results using CSD mice, demonstrating cognitive improvements with choline supplementation, are highly promising and further support the potential for translating these benefits into broader applications.
One important question with practical relevance is whether this intervention remains effective in milder cases of sleep deprivation, such as those commonly experienced by students, where insufficient sleep leads to poorer academic performance and cognitive decline [107,108]. In this experimental study, some factors could introduce certain positive or negative errors, which can be minimized through researcher training in the pre-experimental phase and adherence to standard operating procedures during the experiment, and the SD values were shown in the related figures. Additionally, the study relied on a specific animal model, which is well-established for the investigation of cognitive impairment, it is still important to note whether the observed improvements are clinically significant or merely statistically significant.
5. Conclusions
In conclusion, our study proved that choline supplementation effectively mitigated sleep deprivation-induced cognitive impairment using neurocognitive behavioral test, functional MRI, and so on. Underlying mechanisms included anti-inflammatory effects by modulating microglial activation and reducing inflammatory cytokine levels, and antioxidant action, as well as restoring phospholipidomic homeostasis. Notably, choline enhanced key phospholipid sub-classes, particularly PC, which correlated strongly with cognitive performance and hippocampal integrity, while specific PC, PE molecules and PS 44:12 were linked to reduced neuroinflammation and oxidative stress. Choline supplementation offers a promising approach to improve cognitive impairment caused by sleep deprivation, likely benefiting a broader population compared with drug-based interventions, considering potential side-effects of drugs. These findings supported that choline has potential to be developed into functional food or medicine ingredient to prevent and manage cognitive impairment induced by sleep disturbances after further human studies.
CRediT authorship contribution statement
Si-Yu Huang: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Zhi-Jun Yang: Methodology, Investigation. Jin Cheng: Writing – review & editing, Investigation. Hang-Yu Li: Methodology, Investigation. Si Chen: Methodology, Investigation, Funding acquisition. Zi-Hui Huang: Investigation. Jie-Dong Chen: Investigation. Ruo-Gu Xiong: Investigation. Meng-Tao Yang: Methodology, Investigation. Chen Wang: Methodology, Investigation. Meng-Chu Li: Methodology, Investigation. Shuang Song: Methodology, Investigation. Wen-Ge Huang: Methodology, Investigation. Dong-Liang Wang: Writing – review & editing, Methodology. Hua-Bin Li: Writing – review & editing, Supervision, Conceptualization. Qiu-Ye Lan: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Funding
This study was supported by the National Natural Science Youth Foundation of China (No. 82103829), and National Natural Science Foundation of China (No. 82473619).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors declare no conflict of interest.
Abbreviations
- Ach
acetylcholine
- AChE
acetylcholinesterase
- ANOVA
one-way analysis of variance
- BBB
blood-brain barrier
- CAT
catalase
- CC
cingulate cortex
- ChAT
choline acetyltransferase
- CHOCSD
choline-supplemented chronic sleep deprivation
- CON
control
- CSD
chronic sleep deprivation
- Ctr
center scaling
- DG
dentate gyrus
- DHA
docosahexaenoic acid
- DI
discrimination index
- DMN
default mode network
- DTI
diffusion tensor imaging
- EB
evans blue
- EC
entorhinal cortex
- FA
fractional anisotropy
- fALFF
fractional amplitude of low-frequency fluctuations
- FC
functional connectivity
- FOV
field of view
- FSL
FMRIB Software Library
- GSH
glutathione
- GSH-NEM
N-ethylmaleimide-derivatized glutathione
- GSH-Px
glutathione peroxidase
- GSSG
oxidized glutathione
- H&E
hematoxylin and eosin
- HILIC-ESI-IT-TOF-MS
hydrophilic interaction liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry
- HILIC-ESI-MS/MS
hydrophilic interaction liquid chromatography-electrospray ionization tandem mass spectrometry
- HPA
hypothalamic-pituitary-adrenal
- HPC
hippocampus
- HT
hypothalamus
- IL-10
interleukin-10
- IL-18
interleukin-18
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LMSD
LIPID MAPS structure database
- LPC
lyso-phosphatidylcholine
- LPE
lyso-phosphatidylethanolamine
- LPG
lyso-phosphatidylglycerol
- LPI
lyso-phosphatidylinositol
- MD
mean diffusivity
- MDA
malondialdehyde
- MRI
magnetic resonance imaging
- MRM
multiple reaction monitoring
- NOR
novel object recognition
- NSE
neuron-specific enolase
- OPLS-DA
orthogonal projections to latent structures-discriminant analysis
- PA
phosphatidic acid
- PBS
phosphate-buffered saline
- PC
phosphatidylcholine
- PCA
principal component analysis
- PE
phosphatidylethanolamine
- PFC
prefrontal cortex
- PG
phosphatidylglycerol
- PS
phosphatidylserine
- PSD
postsynaptic density
- RARE
rapid acquisition with relaxation enhancement
- ReHo
regional homogeneity
- RI
recognition index
- ROIs
regions of interest
- rs-fMRI
resting-state functional magnetic resonance imaging
- SD
standard deviation
- SM
sphingomyelin
- SOD
superoxide dismutase
- TE
echo time
- TNF-α
tumor necrosis factor-alpha
- TR
repetition time
- VIP
variable importance in projection
- zFC
z-transformed functional connectivity
- α7-nAChR
alpha7 nicotinic acetylcholine receptor
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2025.103578.
Contributor Information
Hua-Bin Li, Email: lihuabin@mail.sysu.edu.cn.
Qiu-Ye Lan, Email: lanqy@link.cuhk.edu.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O'Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Adams Hillard P.J. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Matricciani L., Olds T., Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med. Rev. 2012;16:203–211. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Lan Q.-Y., Chan K.C., Yu K.N., Chan N.Y., Wing Y.K., Li A.M., Au C.T. Sleep duration in preschool children and impact of screen time. Sleep Med. 2020;76:48–54. doi: 10.1016/j.sleep.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Lowe C.J., Safati A., Hall P.A. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci. Biobehav. Rev. 2017;80:586–604. doi: 10.1016/j.neubiorev.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Giri B., Kinsky N., Kaya U., Maboudi K., Abel T., Diba K. Sleep loss diminishes hippocampal reactivation and replay. Nature. 2024;630:935–942. doi: 10.1038/s41586-024-07538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gay S.M., Chartampila E., Lord J.S., Grizzard S., Maisashvili T., Ye M., Barker N.K., Mordant A.L., Mills C.A., Herring L.E., Diering G.H. Developing forebrain synapses are uniquely vulnerable to sleep loss. Proc. Natl. Acad. Sci. U. S. A. 2024;121 doi: 10.1073/pnas.2407533121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quach J.L., Nguyen C.D., Williams K.E., Sciberras E. Bidirectional associations between child sleep problems and internalizing and externalizing difficulties from preschool to early adolescence. JAMA Pediatr. 2018;172 doi: 10.1001/jamapediatrics.2017.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua E.C., Shui G., Cazenave-Gassiot A., Wenk M.R., Gooley J.J. Changes in plasma lipids during exposure to total sleep deprivation. Sleep. 2015;38:1683–1691. doi: 10.5665/sleep.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin M.R., Vitiello M.V. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol. 2019;18:296–306. doi: 10.1016/s1474-4422(18)30450-2. [DOI] [PubMed] [Google Scholar]
- 11.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 12.Madore C., Yin Z., Leibowitz J., Butovsky O. Microglia, lifestyle stress, and neurodegeneration. Immunity. 2020;52:222–240. doi: 10.1016/j.immuni.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velazquez R., Ferreira E., Knowles S., Fux C., Rodin A., Winslow W., Oddo S. Lifelong choline supplementation ameliorates Alzheimer's disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019;18 doi: 10.1111/acel.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X., West A.A., Caudill M.A. Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol. Metabol. 2014;25:263–273. doi: 10.1016/j.tem.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Poly C., Massaro J.M., Seshadri S., Wolf P.A., Cho E., Krall E., Jacques P.F., Au R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort1234. Am. J. Clin. Nutr. 2011;94:1584–1591. doi: 10.3945/ajcn.110.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velazquez R., Ferreira E., Winslow W., Dave N., Piras I.S., Naymik M., Huentelman M.J., Tran A., Caccamo A., Oddo S. Maternal choline supplementation ameliorates Alzheimer's disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatr. 2020;25:2620–2629. doi: 10.1038/s41380-018-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blusztajn J.K., Wurtman R.J. Choline and cholinergic neurons. Science (New York, N.Y.) 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 19.Sarter M., Parikh V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 20.Ananth M.R., Rajebhosale P., Kim R., Talmage D.A., Role L.W. Basal forebrain cholinergic signalling: development, connectivity and roles in cognition. Nat. Rev. Neurosci. 2023;24:233–251. doi: 10.1038/s41583-023-00677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave N., Judd J.M., Decker A., Winslow W., Sarette P., Villarreal Espinosa O., Tallino S., Bartholomew S.K., Bilal A., Sandler J., McDonough I., Winstone J.K., Blackwood E.A., Glembotski C., Karr T., Velazquez R. Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer's disease hallmarks. Aging Cell. 2023;22 doi: 10.1111/acel.13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shyu P., Jr., Ng B.S.H., Ho N., Chaw R., Seah Y.L., Marvalim C., Thibault G. Membrane phospholipid alteration causes chronic ER stress through early degradation of homeostatic ER-resident proteins. Sci. Rep. 2019;9:8637. doi: 10.1038/s41598-019-45020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalinichenko L.S., Gulbins E., Kornhuber J., Müller C.P. Sphingolipid control of cognitive functions in health and disease. Prog. Lipid Res. 2022;86 doi: 10.1016/j.plipres.2022.101162. [DOI] [PubMed] [Google Scholar]
- 25.Shea T.B. Choline and phosphatidylcholine may maintain cognitive performance by multiple mechanisms. Am. J. Clin. Nutr. 2019;110:1268–1269. doi: 10.1093/ajcn/nqz244. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C.H., Wu T., Jin Y., Huang B.X., Zhou R.F., Wang Y.Q., Luo X.L., Zhu H.L. Prenatal choline supplementation attenuates spatial learning deficits of offspring rats exposed to low-protein diet during fetal period. J. Nutr. Biochem. 2016;32:163–170. doi: 10.1016/j.jnutbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z.J., Huang S.Y., Zhong K.Y., Huang W.G., Huang Z.H., He T.T., Yang M.T., Wusiman M., Zhou D.D., Chen S., Huang B.X., Luo X.L., Li H.B., Zhu H.L. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m(6)A-YTHDF2-dependent manner. Redox Biol. 2024;69 doi: 10.1016/j.redox.2024.103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandjean J., Schroeter A., Batata I., Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;102:838–847. doi: 10.1016/j.neuroimage.2014.08.043. Pt 2. [DOI] [PubMed] [Google Scholar]
- 29.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Nie B., Wu D., Liang S., Liu H., Sun X., Li P., Huang Q., Zhang T., Feng T., Ye S., Zhang Z., Shan B. A stereotaxic MRI template set of mouse brain with fine sub-anatomical delineations: application to MEMRI studies of 5XFAD mice. Magn. Reson. Imaging. 2019;57:83–94. doi: 10.1016/j.mri.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Song S., Liu T.T., Liang X., Liu Z.Y., Yishake D., Lu X.T., Yang M.T., Man Q.Q., Zhang J., Zhu H.L. Profiling of phospholipid molecular species in human breast milk of Chinese mothers and comprehensive analysis of phospholipidomic characteristics at different lactation stages. Food Chem. 2021;348:10. doi: 10.1016/j.foodchem.2021.129091. [DOI] [PubMed] [Google Scholar]
- 32.Liu S., Fan M., Xu J.X., Yang L.J., Qi C.C., Xia Q.R., Ge J.F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflammation. 2022;19:35. doi: 10.1186/s12974-022-02393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorenzato E., Moaveninejad S., Weis L., Biundo R., Antonini A., Porcaro C. Brain dynamics complexity as a signature of cognitive decline in Parkinson's disease. Mov. Disord. 2024;39:305–317. doi: 10.1002/mds.29678. [DOI] [PubMed] [Google Scholar]
- 34.Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Silva-Rudberg J.A., Salardini E., O'Dell R.S., Chen M.K., Ra J., Georgelos J.K., Morehouse M.R., Melino K.P., Varma P., Toyonaga T., Nabulsi N.B., Huang Y., Carson R.E., van Dyck C.H., Mecca A.P. Assessment of gray matter microstructure and synaptic density in alzheimer's disease: a multimodal imaging study with DTI and SV2A pet. Am. J. Geriatr. Psychiatr. 2024;32:17–28. doi: 10.1016/j.jagp.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S., Kim J., Seo S.G., Choi B.R., Han J.S., Lee K.W., Kim J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol. Res. 2014;85:23–32. doi: 10.1016/j.phrs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Kansakar U., Trimarco V., Mone P., Varzideh F., Lombardi A., Santulli G. Choline supplements: an update. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1148166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havekes R., Abel T. The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr. Opin. Neurobiol. 2017;44:13–19. doi: 10.1016/j.conb.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Guan X., Chen X., Cai Y., Ma Y., Ma J., Zhang Q., Dai L., Fan X., Bai Y. Choline supplementation ameliorates behavioral deficits and alzheimer's disease-like pathology in transgenic APP/PS1 mice. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201801407. [DOI] [PubMed] [Google Scholar]
- 40.Liu S.X., Fredrickson T.K., Calixto Mancipe N., Georgieff M.K., Tran P.V. Sex-specific effects of early-life iron deficiency and prenatal choline treatment on adult rat hippocampal transcriptome. Nutrients. 2023;15 doi: 10.3390/nu15061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao L., Wang Y., Gao Y., Gao H., Guo X. The role of the fronto-parietal network in modulating sustained attention under sleep deprivation: an functional magnetic resonance imaging study. Front. Psychiatr. 2023;14 doi: 10.3389/fpsyt.2023.1289300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu H., Zou Q., Gu H., Raichle M.E., Stein E.A., Yang Y. Rat brains also have a default mode network. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu L.M., Liang X., Gu H., Brynildsen J.K., Stark J.A., Ash J.A., Lin C.P., Lu H., Rapp P.R., Stein E.A., Yang Y. Constituents and functional implications of the rat default mode network. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4541–E4547. doi: 10.1073/pnas.1601485113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckner R.L., DiNicola L.M. The brain's default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019;20:593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- 45.Ferbinteanu J., Shapiro M.L. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- 46.Wood E.R., Dudchenko P.A., Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 47.Le Merre P., Ährlund-Richter S., Carlén M. The mouse prefrontal cortex: unity in diversity. Neuron. 2021;109:1925–1944. doi: 10.1016/j.neuron.2021.03.035. [DOI] [PubMed] [Google Scholar]
- 48.Miller E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 49.Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grienberger C., Magee J.C. Entorhinal cortex directs learning-related changes in CA1 representations. Nature. 2022;611:554–562. doi: 10.1038/s41586-022-05378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krause A.J., Simon E.B., Mander B.A., Greer S.M., Saletin J.M., Goldstein-Piekarski A.N., Walker M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai X.J., Gong H.H., Wang Y.X., Zhou F.Q., Min Y.J., Zhao F., Wang S.Y., Liu B.X., Xiao X.Z. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13:720–727. doi: 10.1016/j.sleep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Wang T., Li S., Jiang G., Lin C., Li M., Ma X., Zhan W., Fang J., Li L., Li C., Tian J. Regional homogeneity changes in patients with primary insomnia. Eur. Radiol. 2016;26:1292–1300. doi: 10.1007/s00330-015-3960-4. [DOI] [PubMed] [Google Scholar]
- 54.Verweij I.M., Romeijn N., Smit D.J., Piantoni G., Van Someren E.J., van der Werf Y.D. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;15:88. doi: 10.1186/1471-2202-15-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z., Chang Y., Guo F., Wang C., Chai N., Zheng M., Fang P., Zhu Y. The restoration ability of a short nap after sleep deprivation on the brain cognitive function: a dynamic functional connectivity analysis. CNS Neurosci. Ther. 2024;30 doi: 10.1111/cns.14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Dai C., Shao Y., Peng J., Yang Y., Hou Y. Decreased functional connectivity in the reward network and its relationship with negative emotional experience after total sleep deprivation. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.641810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyashita Y. Cognitive memory: cellular and network machineries and their top-down control. Science. 2004;306:435–440. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- 58.Obenaus A., Rodriguez-Grande B., Lee J.B., Dubois C.J., Fournier M.L., Cador M., Caille S., Badaut J. A single mild juvenile TBI in male mice leads to regional brain tissue abnormalities at 12 months of age that correlate with cognitive impairment at the middle age. Acta Neuropathol. Commun. 2023;11:32. doi: 10.1186/s40478-023-01515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He J., Hsuchou H., He Y., Kastin A.J., Wang Y., Pan W. Sleep restriction impairs blood-brain barrier function. J. Neurosci. 2014;34:14697–14706. doi: 10.1523/jneurosci.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A., Muresanu D.F., Lafuente J.V., Patnaik R., Tian Z.R., Buzoianu A.D., Sharma H.S. Sleep deprivation-induced blood-brain barrier breakdown and brain dysfunction are exacerbated by size-related exposure to Ag and Cu nanoparticles. Neuroprotective effects of a 5-HT3 receptor antagonist ondansetron. Mol. Neurobiol. 2015;52:867–881. doi: 10.1007/s12035-015-9236-9. [DOI] [PubMed] [Google Scholar]
- 61.Ilcol Y.O., Basagan-Mogol E., Cengiz M., Ulus I.H. Elevation of serum cerebral injury markers correlates with serum choline decline after coronary artery bypass grafting surgery. Clin. Chem. Lab. Med. 2006;44:471–478. doi: 10.1515/cclm.2006.074. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M., Chen H., Zhang W., Liu Y., Ding L., Gong J., Ma R., Zheng S., Zhang Y. Biomimetic remodeling of microglial riboflavin metabolism ameliorates cognitive impairment by modulating neuroinflammation. Adv. Sci. 2023;10 doi: 10.1002/advs.202300180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nantachai G., Vasupanrajit A., Tunvirachaisakul C., Solmi M., Maes M. Oxidative stress and antioxidant defenses in mild cognitive impairment: a systematic review and meta-analysis. Ageing Res. Rev. 2022;79 doi: 10.1016/j.arr.2022.101639. [DOI] [PubMed] [Google Scholar]
- 64.Xu Y., Yang Y., Shi Y., Li B., Xie Y., Le G. Dietary methionine supplementation improves cognitive dysfunction associated with transsulfuration pathway upregulation in subacute aging mice. NPJ Sci. Food. 2024;8:104. doi: 10.1038/s41538-024-00348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox M.A., Bassi C., Saunders M.E., Nechanitzky R., Morgado-Palacin I., Zheng C., Mak T.W. Beyond neurotransmission: acetylcholine in immunity and inflammation. J. Intern. Med. 2020;287:120–133. doi: 10.1111/joim.13006. [DOI] [PubMed] [Google Scholar]
- 66.Lu X., Xiong W., Chen Z., Li Y., Xu F., Yang X., Long M., Guo W., Wu S., Sun L., Wang G. Exercise-conditioned plasma ameliorates postoperative cognitive dysfunction by activating hippocampal cholinergic circuit and enhancing BDNF/TrkB signaling. Cell Commun. Signal. 2024;22:551. doi: 10.1186/s12964-024-01938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno-Rodríguez M., Martínez-Gardeazabal J., Bengoetxea de Tena I., Llorente-Ovejero A., Lombardero L., González de San Román E., Giménez-Llort L., Manuel I., Rodríguez-Puertas R. Cognitive improvement via cortical cannabinoid receptors and choline-containing lipids. Br. J. Pharmacol. 2025;182:1038–1058. doi: 10.1111/bph.17381. [DOI] [PubMed] [Google Scholar]
- 68.Botelho R., Kirstein C.L., Philpot R.M. Cyclophosphamide- and doxorubicin-induced impairment of high affinity choline uptake and spatial memory can be prevented by dietary choline supplementation in breast tumor bearing mice. PLoS One. 2024;19 doi: 10.1371/journal.pone.0305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu E.Y.L., Xia Y., Kong X., Guo M.S.S., Yu A.X.D., Zheng B.Z.Y., Mak S., Xu M.L., Tsim K.W.K. Interacting with α 7 nAChR is a new mechanism for AChE to enhance the inflammatory response in macrophages. Acta Pharm. Sin. B. 2020;10:1926–1942. doi: 10.1016/j.apsb.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soreq H., Seidman S. Acetylcholinesterase--new roles for an old actor. Nat. Rev. Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 71.Keever K.R., Yakubenko V.P., Hoover D.B. Neuroimmune nexus in the pathophysiology and therapy of inflammatory disorders: role of α7 nicotinic acetylcholine receptors. Pharmacol. Res. 2023;191 doi: 10.1016/j.phrs.2023.106758. [DOI] [PubMed] [Google Scholar]
- 72.Xue R., Wan Y., Sun X., Zhang X., Gao W., Wu W. Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front. Immunol. 2019;10:2546. doi: 10.3389/fimmu.2019.02546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 74.Moon J.H., Kim S.Y., Lee H.G., Kim S.U., Lee Y.B. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1-42)-stimulated microglia. Exp. Mol. Med. 2008;40:11–18. doi: 10.3858/emm.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hidalgo C., Arias-Cavieres A. Calcium, reactive oxygen species, and synaptic plasticity. Physiology. 2016;31:201–215. doi: 10.1152/physiol.00038.2015. [DOI] [PubMed] [Google Scholar]
- 76.Pei W., Meng F., Deng Q., Zhang B., Gu Y., Jiao B., Xu H., Tan J., Zhou X., Li Z., He G., Ruan J., Ding Y. Electroacupuncture promotes the survival and synaptic plasticity of hippocampal neurons and improvement of sleep deprivation-induced spatial memory impairment. CNS Neurosci. Ther. 2021;27:1472–1482. doi: 10.1111/cns.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Donnell V.B., Rossjohn J., Wakelam M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018;128:2670–2679. doi: 10.1172/jci97944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fitz N.F., Nam K.N., Wolfe C.M., Letronne F., Playso B.E., Iordanova B.E., Kozai T.D.Y., Biedrzycki R.J., Kagan V.E., Tyurina Y.Y., Han X., Lefterov I., Koldamova R. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer's disease. Nat. Commun. 2021;12:3416. doi: 10.1038/s41467-021-23762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blusztajn J.K., Slack B.E., Mellott T.J. Neuroprotective actions of dietary choline. Nutrients. 2017;9 doi: 10.3390/nu9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibellini F., Smith T.K. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 82.Donoso F., Schverer M., Rea K., Pusceddu M.M., Roy B.L., Dinan T.G., Cryan J.F., Schellekens H. Neurobiological effects of phospholipids in vitro: relevance to stress-related disorders. Neurobiol Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Do Carmo S., Kautzmann M.I., Bhattacharjee S., Jun B., Steinberg C., Emmerson J.T., Malcolm J.C., Bonomo Q., Bazan N.G., Cuello A.C. Differential effect of an evolving amyloid and tau pathology on brain phospholipids and bioactive lipid mediators in rat models of Alzheimer-like pathology. J. Neuroinflammation. 2024;21:185. doi: 10.1186/s12974-024-03184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdullah L., Evans J.E., Ferguson S., Mouzon B., Montague H., Reed J., Crynen G., Emmerich T., Crocker M., Pelot R., Mullan M., Crawford F. Lipidomic analyses identify injury-specific phospholipid changes 3 mo after traumatic brain injury. FASEB J. 2014;28:5311–5321. doi: 10.1096/fj.14-258228. [DOI] [PubMed] [Google Scholar]
- 85.Yang L., Liang J., Lam S.M., Yavuz A., Shui G., Ding M., Huang X. Neuronal lipolysis participates in PUFA-mediated neural function and neurodegeneration. EMBO Rep. 2020;21 doi: 10.15252/embr.202050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin F. Lipid metabolism and Alzheimer's disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023;290:1420–1453. doi: 10.1111/febs.16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morita S.Y., Ikeda Y. Regulation of membrane phospholipid biosynthesis in mammalian cells. Biochem. Pharmacol. 2022;206 doi: 10.1016/j.bcp.2022.115296. [DOI] [PubMed] [Google Scholar]
- 88.Law S.H., Chan M.L., Marathe G.K., Parveen F., Chen C.H., Ke L.Y. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geraldo L.H.M., Spohr T., Amaral R.F.D., Fonseca A., Garcia C., Mendes F.A., Freitas C., dosSantos M.F., Lima F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: novel therapeutic strategies. Signal Transduct. Targeted Ther. 2021;6:45. doi: 10.1038/s41392-020-00367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.da Silva T.F., Eira J., Lopes A.T., Malheiro A.R., Sousa V., Luoma A., Avila R.L., Wanders R.J., Just W.W., Kirschner D.A., Sousa M.M., Brites P. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Investig. 2014;124:2560–2570. doi: 10.1172/jci72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferreira da Silva T., Granadeiro L.S., Bessa-Neto D., Luz L.L., Safronov B.V., Brites P. Plasmalogens regulate the AKT-ULK1 signaling pathway to control the position of the axon initial segment. Prog. Neurobiol. 2021;205 doi: 10.1016/j.pneurobio.2021.102123. [DOI] [PubMed] [Google Scholar]
- 92.Fujino T., Yamada T., Asada T., Tsuboi Y., Wakana C., Mawatari S., Kono S. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild alzheimer's disease and mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled trial. EBioMedicine. 2017;17:199–205. doi: 10.1016/j.ebiom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang Y., Zhao R., Pu Q., Jiang S., Yu F., Yang Z., Han T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023;892 doi: 10.1016/j.scitotenv.2023.164808. [DOI] [PubMed] [Google Scholar]
- 94.Emre C., Do K.V., Jun B., Hjorth E., Alcalde S.G., Kautzmann M.I., Gordon W.C., Nilsson P., Bazan N.G., Schultzberg M. Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer's disease. Acta Neuropathol. Commun. 2021;9:116. doi: 10.1186/s40478-021-01216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parets S., Irigoyen Á., Ordinas M., Cabot J., Miralles M., Arbona L., Péter M., Balogh G., Fernández-García P., Busquets X., Lladó V., Escribá P.V., Torres M. 2-Hydroxy-Docosahexaenoic acid is converted into heneicosapentaenoic acid via α-oxidation: implications for alzheimer's disease therapy. Front. Cell Dev. Biol. 2020;8:164. doi: 10.3389/fcell.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gozzo S., Oliverio A., Salvati S., Serlupi-Crescenzi G., Tagliamonte B., Tomassi G. Effects of dietary phospholipids and odd-chain fatty acids on the behavioural maturation of mice. Food Chem. Toxicol. 1982;20:153–157. doi: 10.1016/s0278-6915(82)80240-8. [DOI] [PubMed] [Google Scholar]
- 97.Razquin C., Toledo E., Clish C.B., Ruiz-Canela M., Dennis C., Corella D., Papandreou C., Ros E., Estruch R., Guasch-Ferré M., Gómez-Gracia E., Fitó M., Yu E., Lapetra J., Wang D., Romaguera D., Liang L., Alonso-Gómez A., Deik A., Bullo M., Serra-Majem L., Salas-Salvadó J., Hu F.B., Martínez-González M.A. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care. 2018;41:2617–2624. doi: 10.2337/dc18-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prada M., Wittenbecher C., Eichelmann F., Wernitz A., Drouin-Chartier J.P., Schulze M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: a targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin. Nutr. 2021;40:4988–4999. doi: 10.1016/j.clnu.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Zheng J.S., Sharp S.J., Imamura F., Koulman A., Schulze M.B., Ye Z., Griffin J., Guevara M., Huerta J.M., Kröger J., Sluijs I., Agudo A., Barricarte A., Boeing H., Colorado-Yohar S., Dow C., Dorronsoro M., Dinesen P.T., Fagherazzi G., Franks P.W., Feskens E.J.M., Kühn T., Katzke V.A., Key T.J., Khaw K.T., de Magistris M.S., Mancini F.R., Molina-Portillo E., Nilsson P.M., Olsen A., Overvad K., Palli D., Quirós J.R., Rolandsson O., Ricceri F., Spijkerman A.M.W., Slimani N., Tagliabue G., Tjonneland A., Tumino R., van der Schouw Y.T., Langenberg C., Riboli E., Forouhi N.G., Wareham N.J. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017;15:203. doi: 10.1186/s12916-017-0968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhivaki D., Kagan J.C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 2022;22:322–330. doi: 10.1038/s41577-021-00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiurchiù V., Leuti A., Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia X., Su C., Zhang J., Huang F., Bai J., Guan F., Wei Y., Li L., Liu Y., Ji J., Du W., Ouyang Y., Zhang X., Zhang B., Wang H. Age and gender disparities in the association of long-term dietary choline and choline compound intakes with incident cognitive decline in middle-aged and older Chinese adults: a prospective cohort study. Nutrients. 2024;16 doi: 10.3390/nu16234121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guan F., Jia X., Huang F., Zhang J., Wei Y., Li L., Bai J., Wang H. Vertical association between dietary total choline and L-alpha-glycerylphosphorylcholine and the cognitive function in Chinese adults aged over 55, result from China health and nutrition survey 1997-2018. Nutrients. 2024;16 doi: 10.3390/nu16213713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mone P., Kansakar U., Lucariello A., Marro A., Pansini A., Varzideh F., Nittolo G., De Angelis L., Trimarco V., Martinelli G., De Luca A., Santulli G. Choline supplementation improves cognitive performance in frail hypertensive patients: novel insights on endothelial function from the INTERVENTIONIST study. Eur. J. Prev. Cardiol. 2023 doi: 10.1093/eurjpc/zwad120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mone P., Trimarco V., Kansakar U., Izzo R., Santulli G., Trimarco B. Combining choline bitartrate and vitamin B12 ameliorates cognitive impairment in hypertensive elders with cognitive frailty. Pharmacol. Res. 2024;201 doi: 10.1016/j.phrs.2024.107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goel N., Rao H., Durmer J.S., Dinges D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Curcio G., Ferrara M., De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med. Rev. 2006;10:323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 108.Dewald J.F., Meijer A.M., Oort F.J., Kerkhof G.A., Bögels S.M. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med. Rev. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.