Abstract
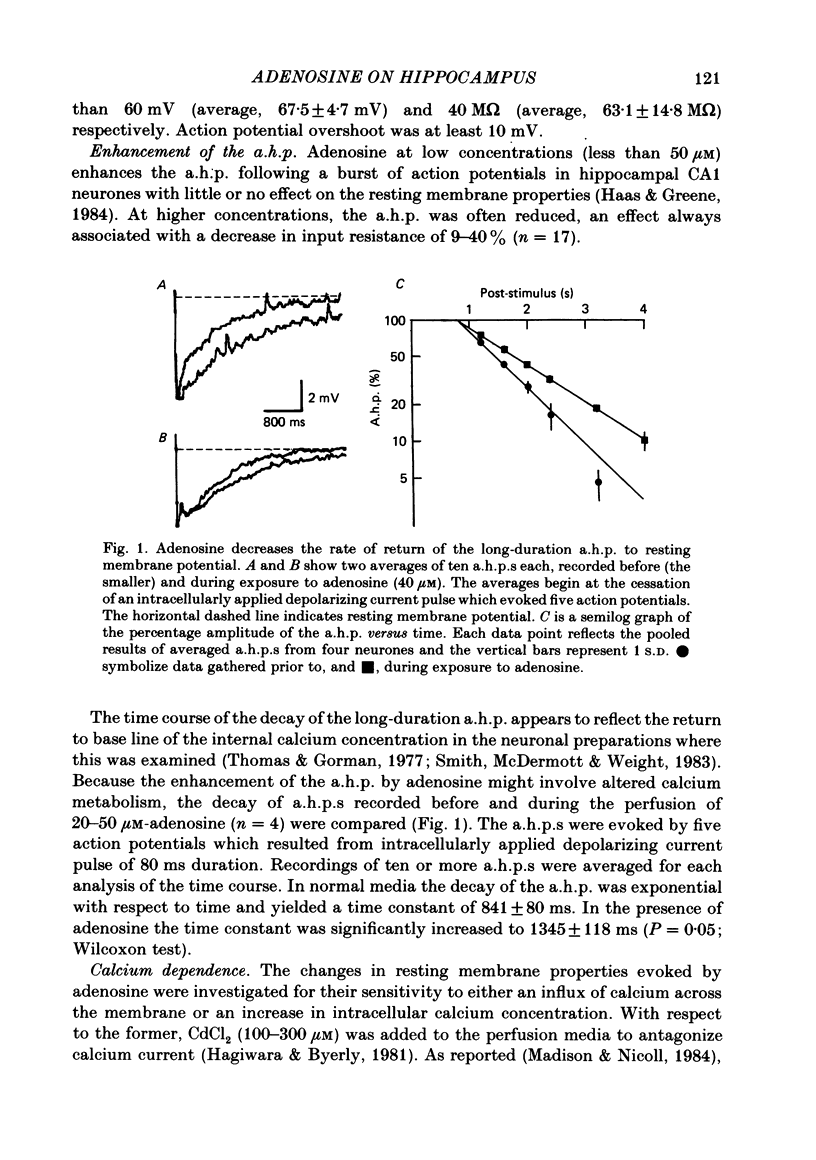
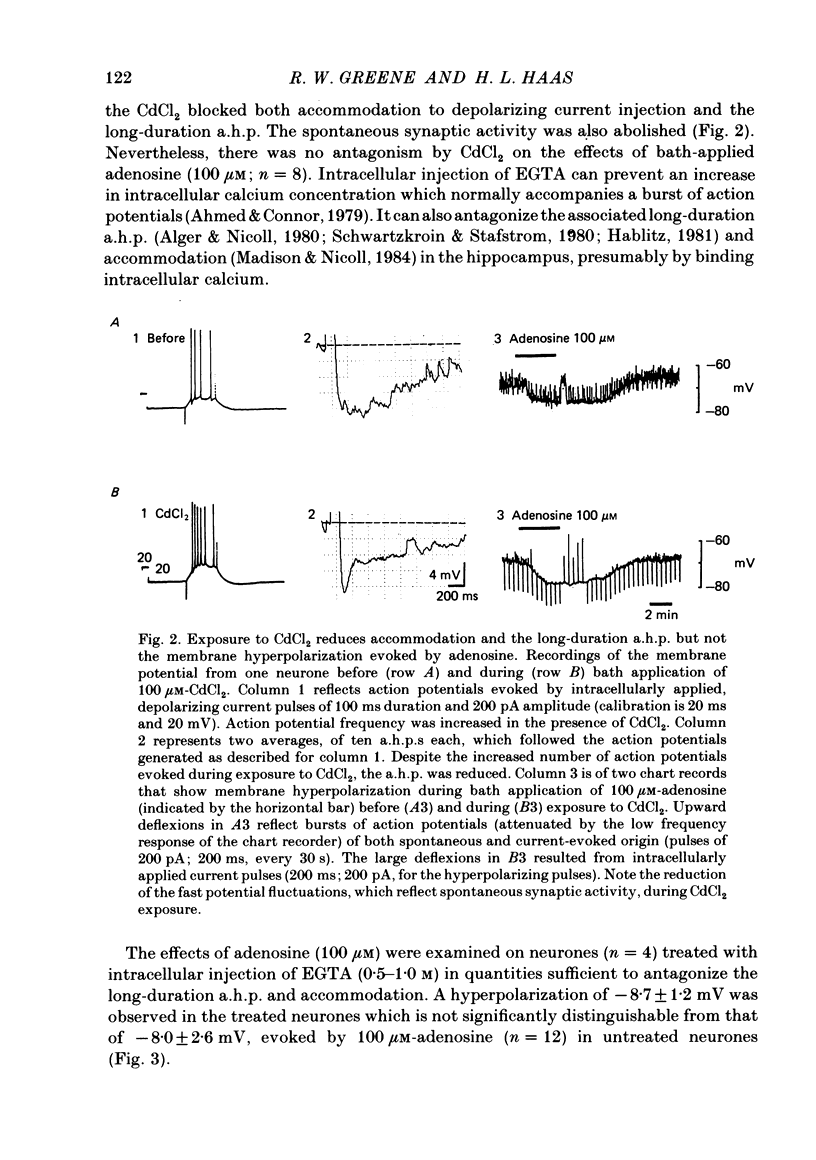
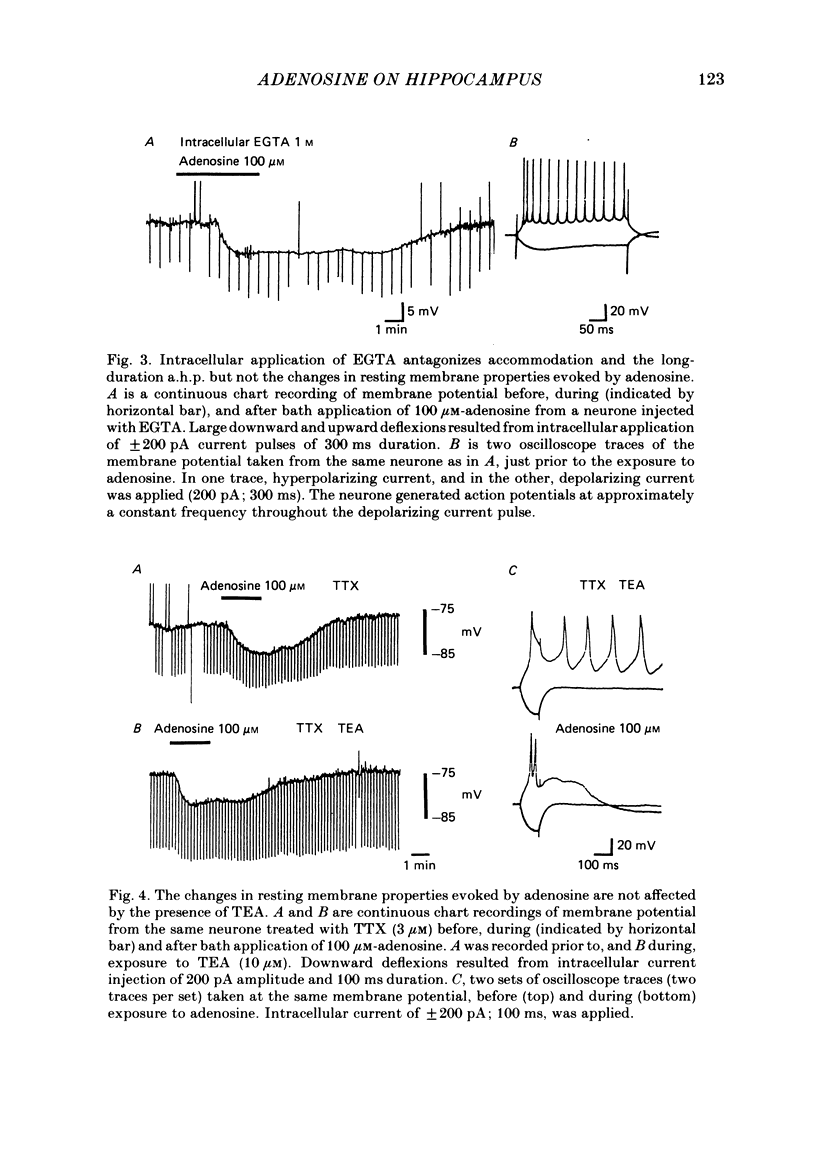
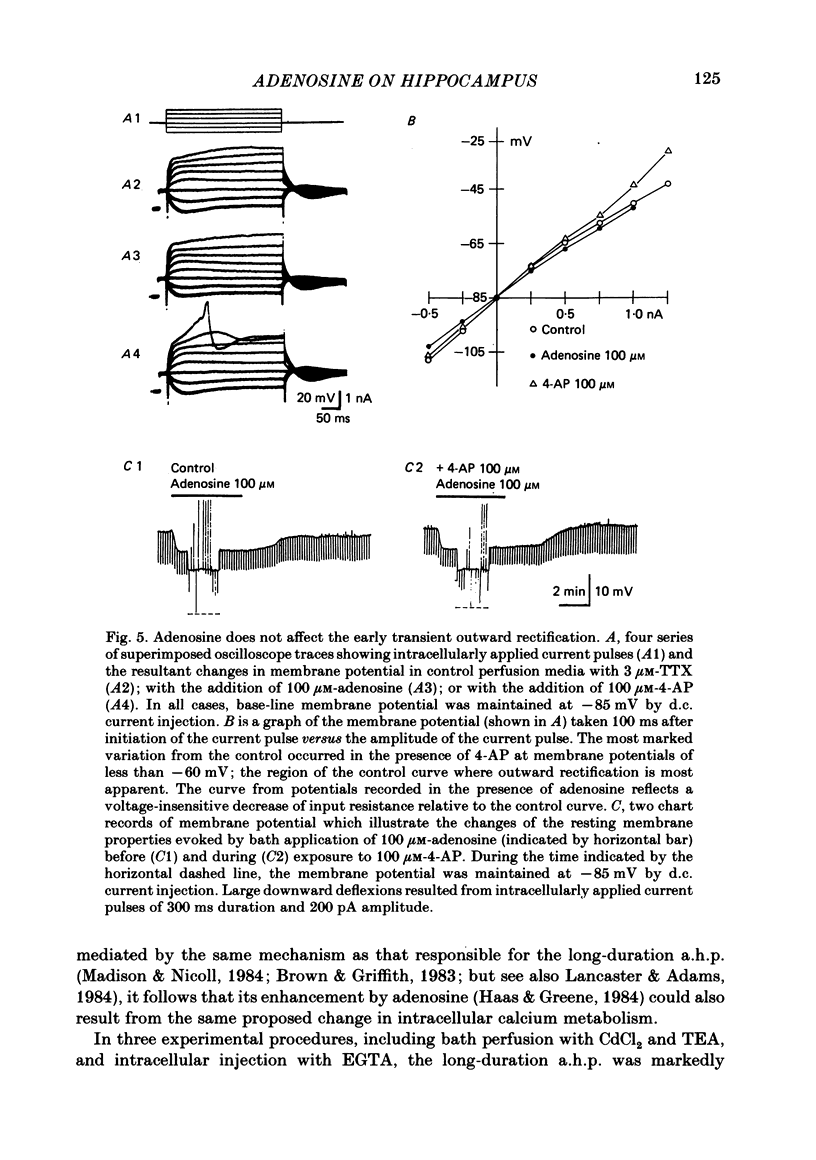
Intracellular recordings with a bridge amplifier of CA1 pyramidal neurones in vitro were employed to study the mechanisms of action of exogenously applied adenosine in the hippocampal slice preparation of the rat. Adenosine enhanced the calcium-dependent, long-duration after-hyperpolarization (a.h.p.) at least in part by a reduction in the rate of decay of the a.h.p. Both the reduced rate of decay and that of the control can be described with a single exponential. Antagonism of the calcium-dependent potassium current (and as a result, the a.h.p.) by bath application of CdCl2 or intracellular injection of EGTA (ethyleneglycolbis-(beta-aminoethyl ether)N,N'-tetraacetic acid) did not reduce the adenosine-evoked hyperpolarization or decrease in input resistance. Similarly, TEA (tetraethylammonium), which antagonizes both the voltage- and calcium-sensitive, delayed, outward rectification, had no effect on the adenosine-evoked changes in resting membrane properties. Adenosine did not affect the early, transient, outward rectification. During exposure to 4-aminopyridine (4-AP) in concentrations sufficient to antagonize this early rectification, the changes in resting membrane properties evoked by adenosine were unaffected. We conclude that the enhancement of the a.h.p. and accommodation by adenosine may be mediated by a change in the regulation of intracellular calcium. However, the mechanism responsible for the hyperpolarization and decrease in input resistance evoked by adenosine is both calcium and voltage insensitive. Thus, it appears distinct from that mediating the enhancement of the a.h.p. and accommodation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980 May;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J., Fredholm B. B. Alkylxanthines elevate hippocampal excitability. Evidence for a role of endogenous adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jul;316(4):326–330. doi: 10.1007/BF00501365. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Greene R. W. Adenosine enhances afterhyperpolarization and accommodation in hippocampal pyramidal cells. Pflugers Arch. 1984 Nov;402(3):244–247. doi: 10.1007/BF00585506. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Jefferys J. G., Slater N. T., Carpenter D. O. Modulation of low calcium induced field bursts in the hippocampus by monoamines and cholinomimetics. Pflugers Arch. 1984 Jan;400(1):28–33. doi: 10.1007/BF00670532. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Schaerer B., Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979 Dec;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Altered burst responses in hippocampal CA3 neurons injected with EGTA. Exp Brain Res. 1981;42(3-4):483–485. doi: 10.1007/BF00237513. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Scholfield C. N. Somatically recorded Ca-currents in guinea-pig hippocampal and olfactory cortex neurones are resistant to adenosine action. Neurosci Lett. 1984 Sep 7;50(1-3):13–18. doi: 10.1016/0304-3940(84)90454-3. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos G. K., Phillis J. W. Purinergic depression of neurons in different areas of the rat brain. Exp Neurol. 1977 Jun;55(3 Pt 1):719–724. doi: 10.1016/0014-4886(77)90296-5. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Saito M., Kobayashi K. Concomitant changes in cyclic AMP level and postsynaptic potentials of olfactory cortex slices induced by adenosine derivatives. Brain Res. 1976 Jun 4;109(1):196–201. doi: 10.1016/0006-8993(76)90393-0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Schubert P., Heinemann U. The anticonvulsive action of adenosine: a postsynaptic, dendritic action by a possible endogenous anticonvulsant. Brain Res. 1984 Oct 29;321(1):160–164. doi: 10.1016/0006-8993(84)90694-2. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Ozawa S. Inhibitory action of adenosine on synaptic transmission in the hippocampus of the guinea pig in vitro. Eur J Pharmacol. 1980 Dec 19;68(4):483–492. doi: 10.1016/0014-2999(80)90424-0. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Connor J. A. A mechanism for minimizing temperature effects on repetitive firing frequency. Am J Physiol. 1978 May;234(5):C155–C161. doi: 10.1152/ajpcell.1978.234.5.C155. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. 4-Aminopyridine blockade of neuronal depressant responses to adenosine triphosphate. Br J Pharmacol. 1980 Nov;70(3):425–428. doi: 10.1111/j.1476-5381.1980.tb08720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Kostopoulos G. K., Limacher J. J. A potent depressant action of adenine derivatives on cerebral cortical neurones. Eur J Pharmacol. 1975 Jan;30(1):125–129. doi: 10.1016/0014-2999(75)90214-9. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972 Dec;130(4):975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Scholfield C. N. Depression of evoked potentials in brain slices by adenosine compounds. Br J Pharmacol. 1978 Jun;63(2):239–244. doi: 10.1111/j.1476-5381.1978.tb09752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P., Mitzdorf U. Analysis and quantitative evaluation of the depressive effect of adenosine on evoked potentials in hippocampal slices. Brain Res. 1979 Aug 17;172(1):186–190. doi: 10.1016/0006-8993(79)90910-7. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Segal M. Intracellular analysis of a postsynaptic action of adenosine in the rat hippocampus. Eur J Pharmacol. 1982 Apr 23;79(3-4):193–199. doi: 10.1016/0014-2999(82)90625-2. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Daly J. Formation of cyclic adenosine 3',5'-monophosphate from adenosine in brain slices. Biochim Biophys Acta. 1970 Nov 24;222(2):465–473. doi: 10.1016/0304-4165(70)90137-6. [DOI] [PubMed] [Google Scholar]
- Smith S. J., MacDermott A. B., Weight F. F. Detection of intracellular Ca2+ transients in sympathetic neurones using arsenazo III. 1983 Jul 28-Aug 3Nature. 304(5924):350–352. doi: 10.1038/304350a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. V., Gorman A. L. Internal calcium changes in a bursting pacemaker neuron measured with arsenazo III. Science. 1977 Apr 29;196(4289):531–533. doi: 10.1126/science.850795. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]


