Abstract
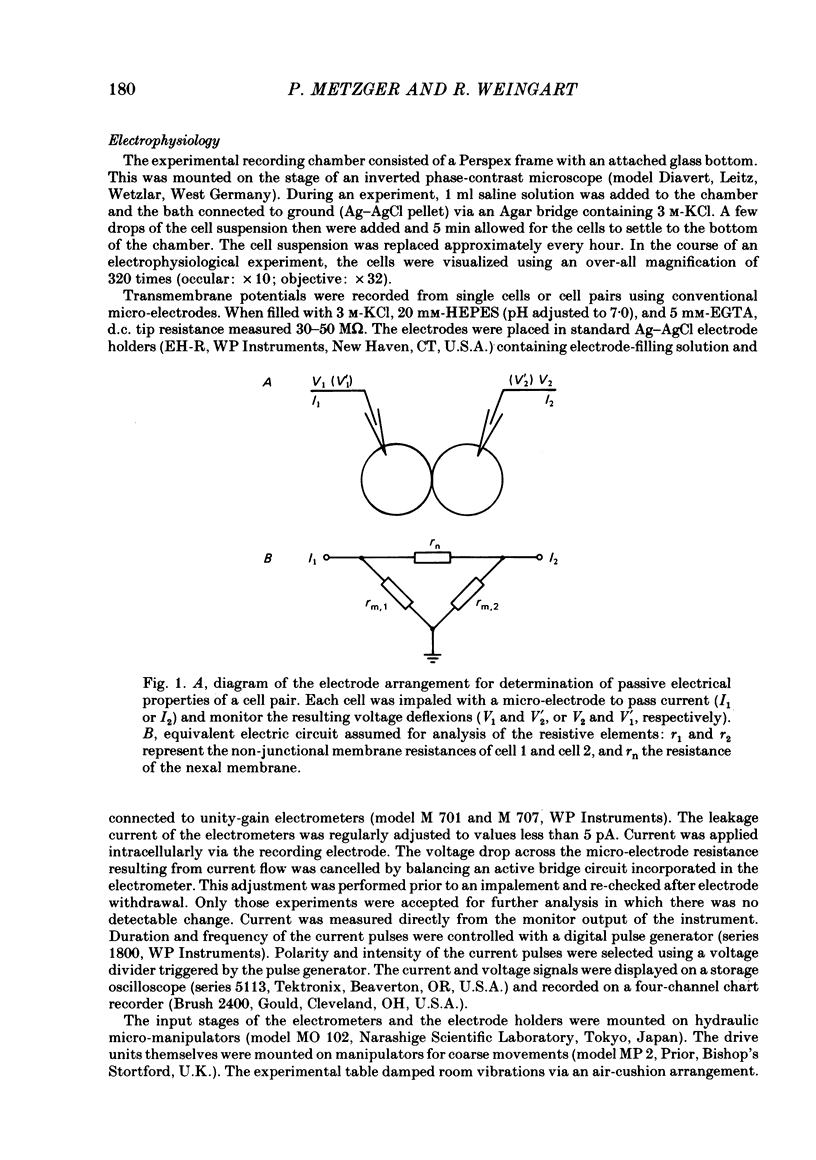
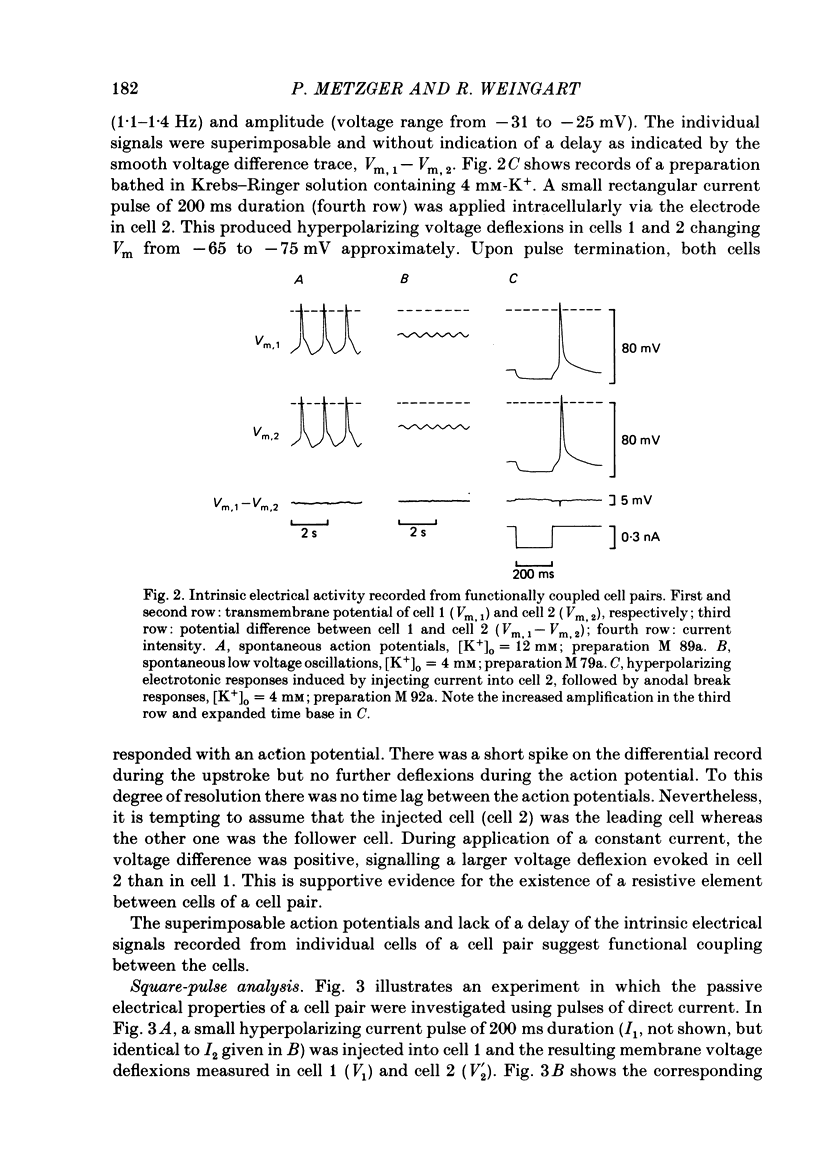
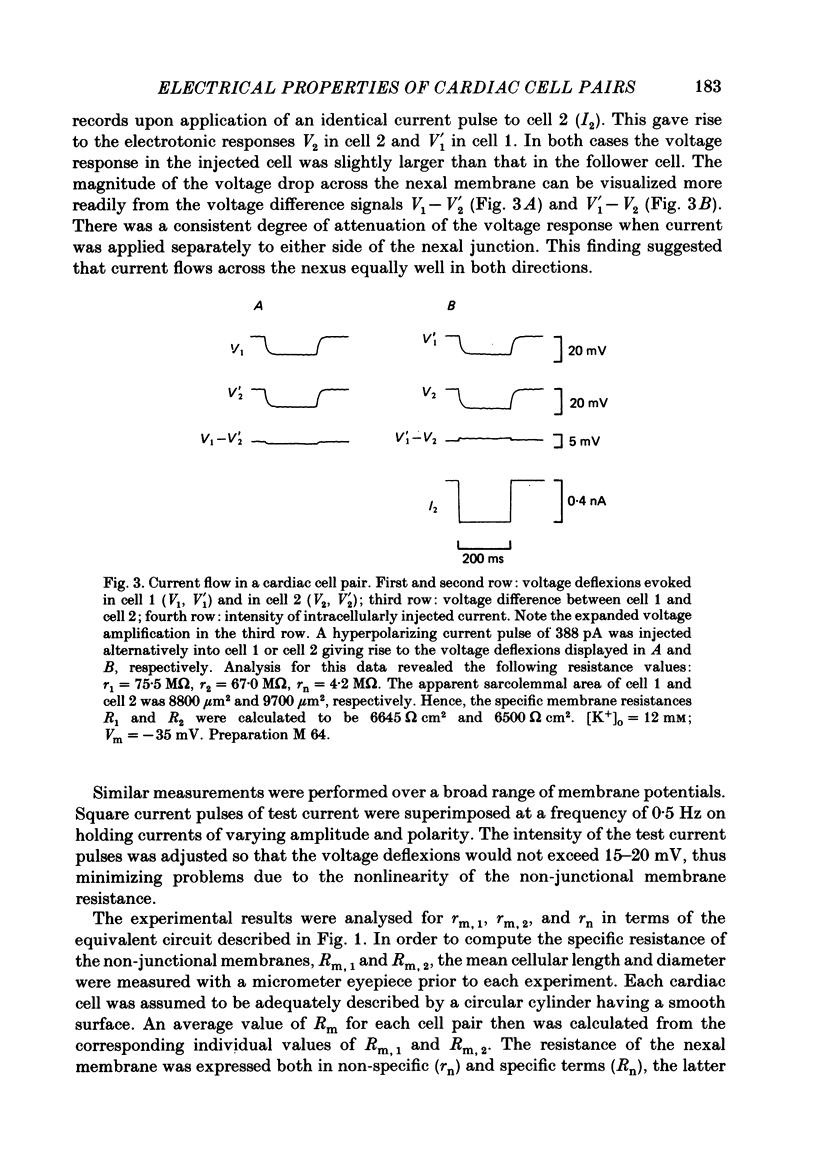
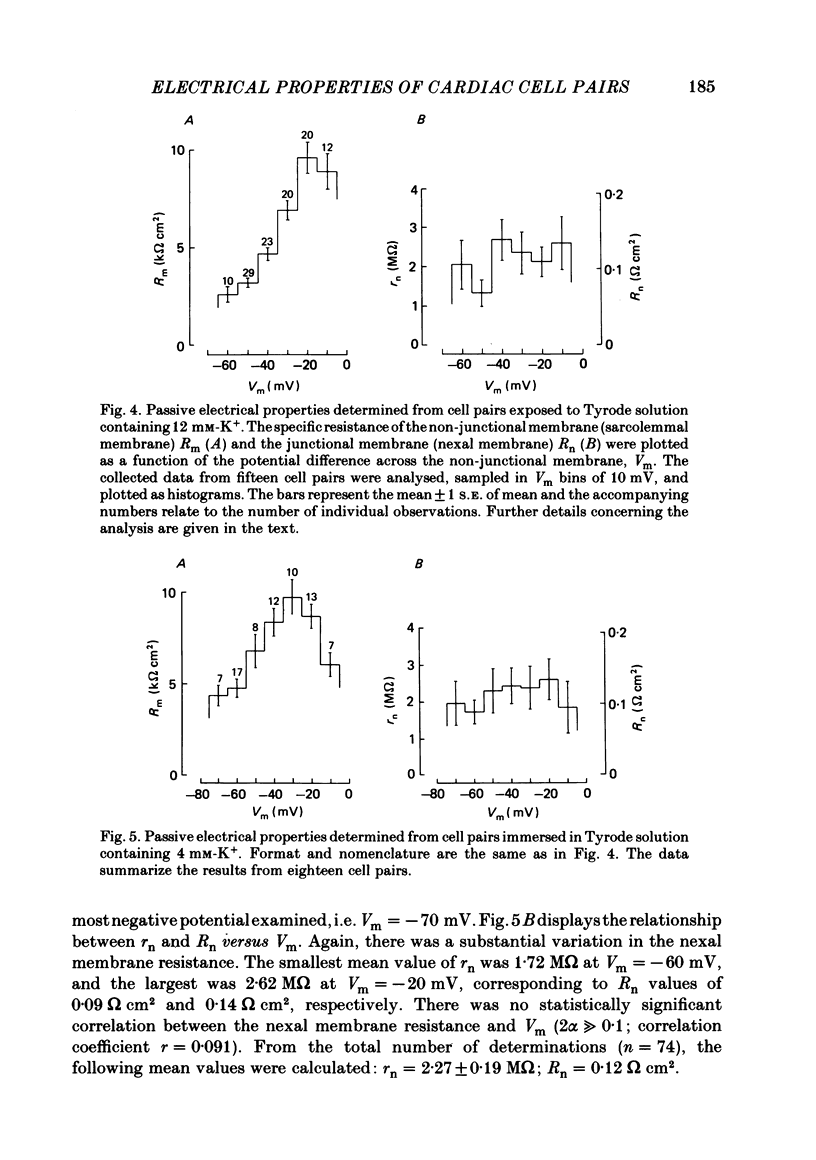
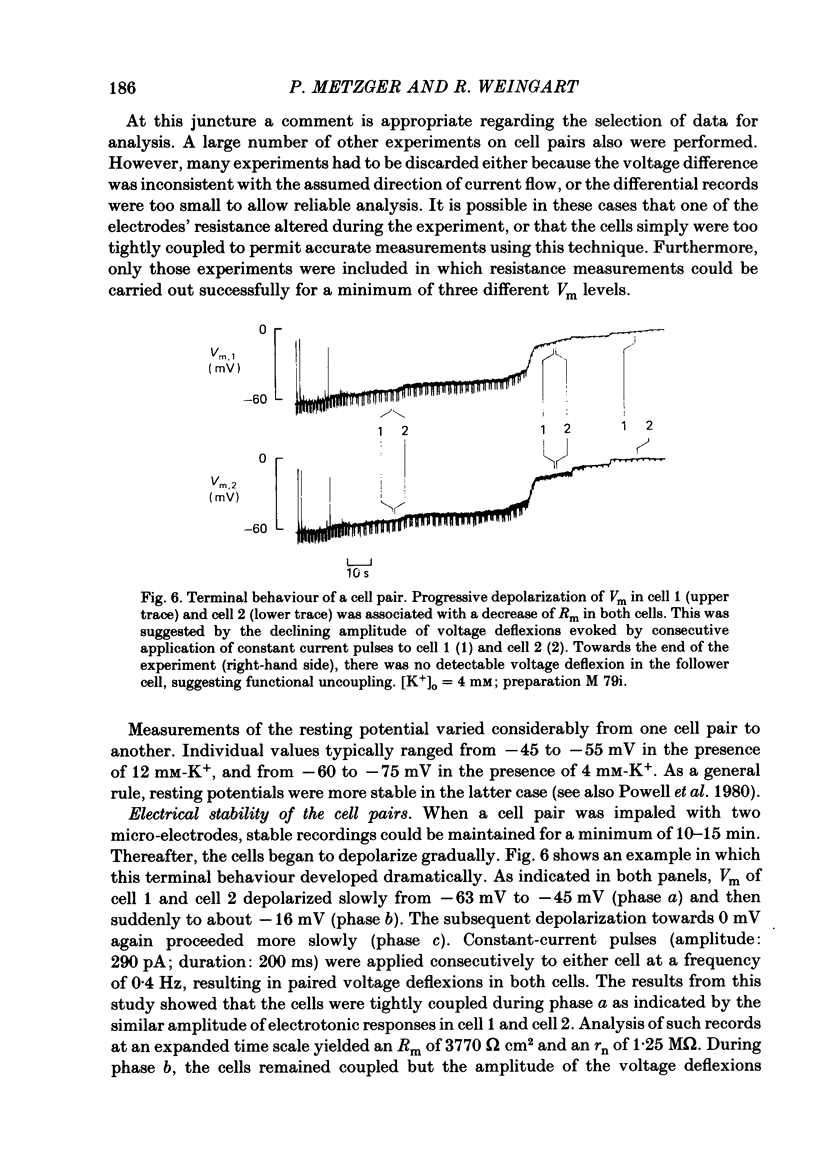
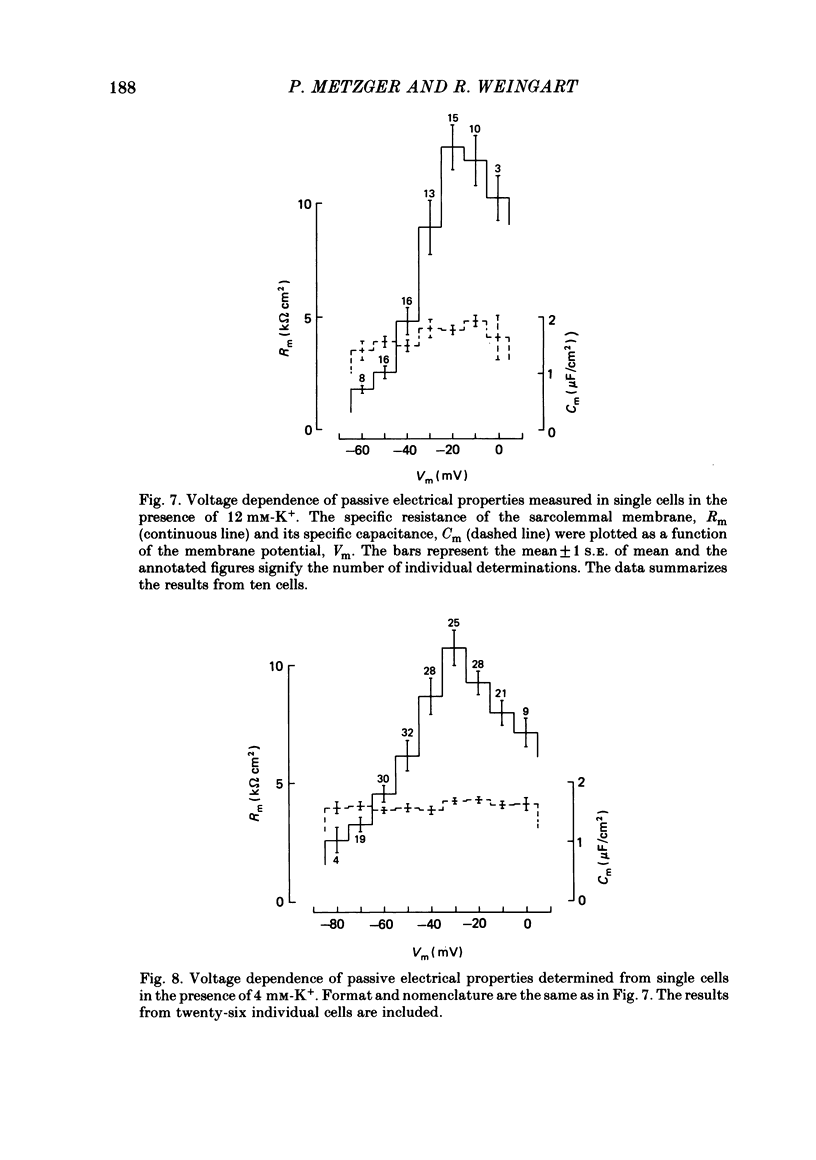
Cell pairs were isolated from ventricles of adult rat hearts so as to study cell-to-cell coupling. Both cells of each pair were impaled with micro-electrodes connected to balanced bridge circuits. Rectangular current pulses were passed and the resulting voltage deflexions monitored. The data were analysed in terms of a delta configuration of three resistive elements, the resistances of the non-junctional membrane of cell 1 and cell 2 (rm, 1 and rm, 2), and the resistance of the nexal membrane (rn). The nexal membrane resistance was found to be insensitive to voltage gradients across the non-junctional membrane (range examined: -70 to -10 mV) and direction of current flow. The mean value of rn was 2.12 M omega ([K+]o = 12 mM). Taking into account morphological parameters, this corresponds to a specific nexal membrane resistance (Rn) of 0.1 omega cm2. Spontaneous uncoupling in which one cell remained polarized while the other one depolarized was never observed. The current-voltage relationship of the non-junctional membrane was found to be bell-shaped. The specific resistance (Rm) at the resting membrane potential (approximately -50 mV) was 3.2 k omega cm2 ([K+]o = 12 mM). Comparative studies performed on single cells revealed a similar relationship Rm versus Vm. Rm at the resting membrane potential (Vm approximately -50 mV) was 2.5 k omega cm2 ([K+]o = 12 mM). The specific capacitance of the non-junctional membrane (Cm) was determined from experiments on single cells. Cm was found to be independent of Vm (voltage range: -80 to 0 mV). The mean value of Cm was 1.66 microF/cm2 ([K+]o = 12 mM). For comparison, experiments on cell pairs and single cells were also carried out with [K+]o = 4 mM. The values obtained for Rn, Rm and Cm did not deviate significantly from those found with [K+]o = 12 mM.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Cell-to-cell communication in heart and other tissues. Prog Biophys Mol Biol. 1982;39(3):147–182. doi: 10.1016/0079-6107(83)90016-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Cohen I. S. The ultrastructure of the cardiac Purkinje strand in the dog: a morphometric analysis. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):191–213. doi: 10.1098/rspb.1983.0006. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Mobley B. A. Size changes in single muscle fibers during fixation and embedding. Tissue Cell. 1975;7(2):383–387. doi: 10.1016/0040-8166(75)90013-0. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Inpocketings of the cell membrane (caveolae) in the rat myocardium. J Ultrastruct Res. 1978 Nov;65(2):135–147. doi: 10.1016/s0022-5320(78)90051-5. [DOI] [PubMed] [Google Scholar]
- Haas H. G., Meyer R., Einwächter H. M., Stockem W. Intercellular coupling in frog heart muscle. Electrophysiological and morphological aspects. Pflugers Arch. 1983 Dec;399(4):321–335. doi: 10.1007/BF00652760. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Isolated bovine ventricular myocytes. Characterization of the action potential. Pflugers Arch. 1982 Oct;395(1):19–29. doi: 10.1007/BF00584964. [DOI] [PubMed] [Google Scholar]
- Jongsma H. J., van Rijn H. E. Electronic spread of current in monolayer cultures of neonatal rat heart cells. J Membr Biol. 1972;9(4):341–360. [PubMed] [Google Scholar]
- Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea-pig heart. J Physiol. 1983 Mar;336:345–357. doi: 10.1113/jphysiol.1983.sp014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecky B., Rakusan K. Normal and hypertrophic growth of the rat heart: changes in cell dimensions and number. Am J Physiol. 1978 Feb;234(2):H123–H128. doi: 10.1152/ajpheart.1978.234.2.H123. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Forletti D., Wittenberg B. A. Uniform sarcomere shortening behavior in isolated cardiac muscle cells. J Gen Physiol. 1980 Nov;76(5):587–607. doi: 10.1085/jgp.76.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R. A., Mathias R. T., Eisenberg R. S. Electrical properties of sheep Purkinje strands. Electrical and chemical potentials in the clefts. Biophys J. 1983 Nov;44(2):225–248. doi: 10.1016/S0006-3495(83)84295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T. Effect of tortuous extracellular pathways on resistance measurements. Biophys J. 1983 Apr;42(1):55–59. doi: 10.1016/S0006-3495(83)84368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez C., Mueller W. J., Urguiaga X. Propagation of impulses across the Prukinje fiber-muscle junctions in the dog heart. Circ Res. 1970 Feb;26(2):135–150. doi: 10.1161/01.res.26.2.135. [DOI] [PubMed] [Google Scholar]
- Metzger P., Weingart R. Electric current flow in a two-cell preparation from Chironomus salivary glands. J Physiol. 1984 Jan;346:599–619. doi: 10.1113/jphysiol.1984.sp015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler M. L. Cable analysis in quiescent and active sheep Purkinje fibres. J Physiol. 1984 Jul;352:739–757. doi: 10.1113/jphysiol.1984.sp015319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs N. J., Slade A. M., Powell T., Twist V. W., Warren R. L. Correlation of ultrastructure and function in calcium-tolerant myocytes isolated from the adult rat heart. J Ultrastruct Res. 1982 Nov;81(2):222–239. doi: 10.1016/s0022-5320(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. M., Page E. Improved stereological techniques for studying myocardial cell growth: application to external sarcolemma, T system, and intercalated disks of rabbit and rat hearts. J Ultrastruct Res. 1978 Nov;65(2):119–134. doi: 10.1016/s0022-5320(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F. Current-voltage relations in ventricular muscle preparations from different species. Pflugers Arch. 1978 Apr 25;374(1):79–89. doi: 10.1007/BF00585700. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Kass R. S., Weingart R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979 Aug;81:205–215. doi: 10.1242/jeb.81.1.205. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Joyner R. W., Rawling D. A. Purkinje and ventricular activation sequences of canine papillary muscle. Effects of quinidine and calcium on the Purkinje-ventricular conduction delay. Circ Res. 1984 May;54(5):500–515. doi: 10.1161/01.res.54.5.500. [DOI] [PubMed] [Google Scholar]
- WATANABE A., GRUNDFEST H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961 Nov;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]



