Abstract
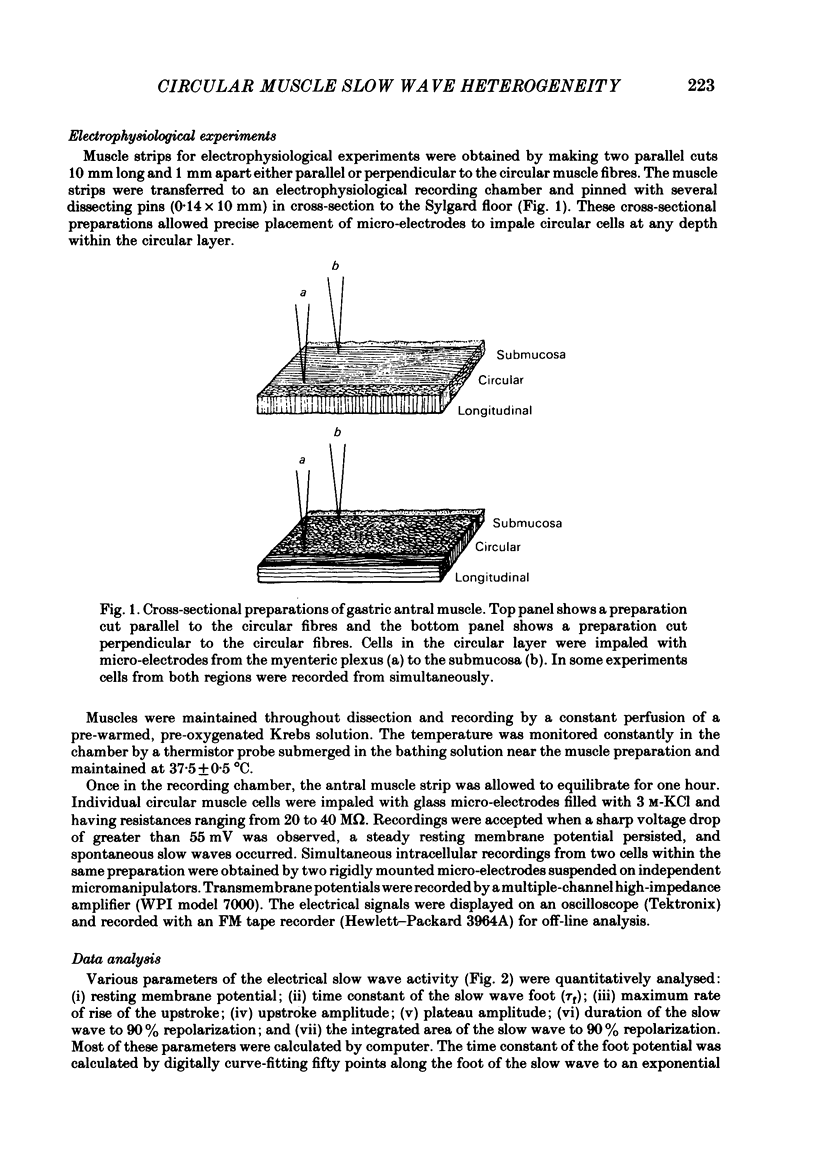
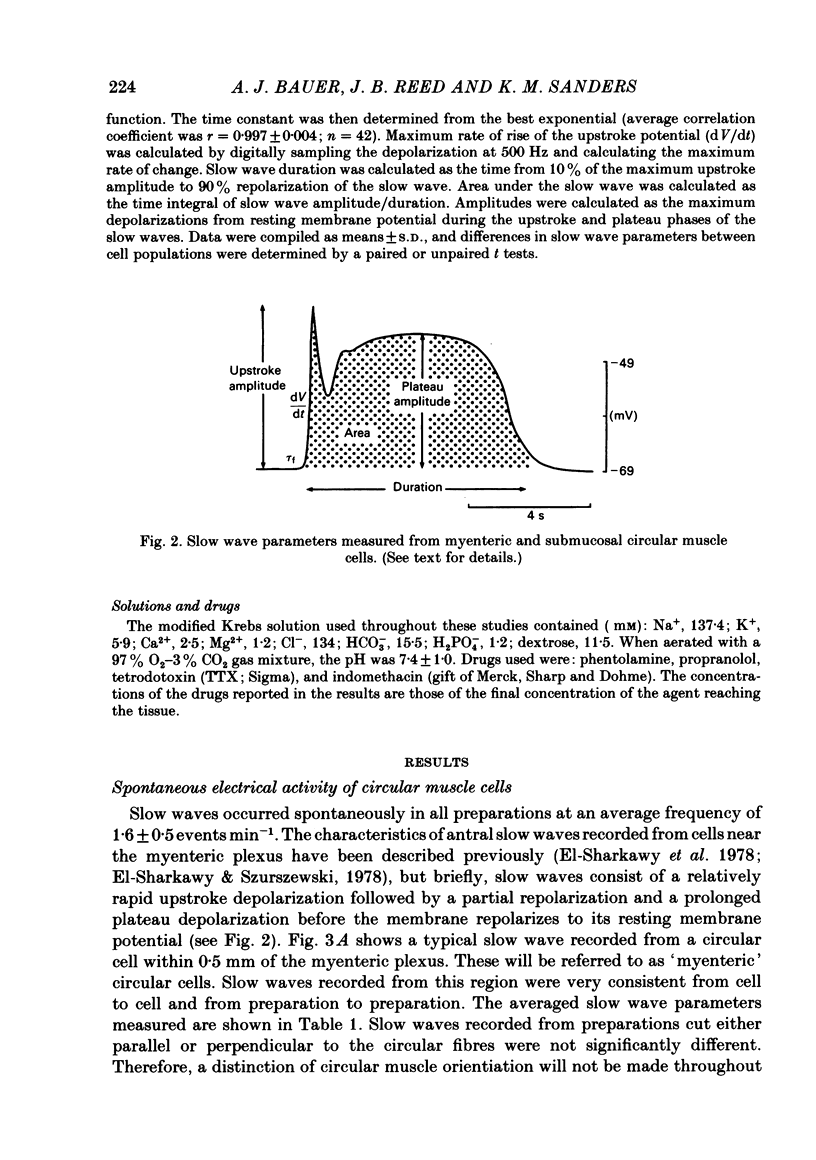
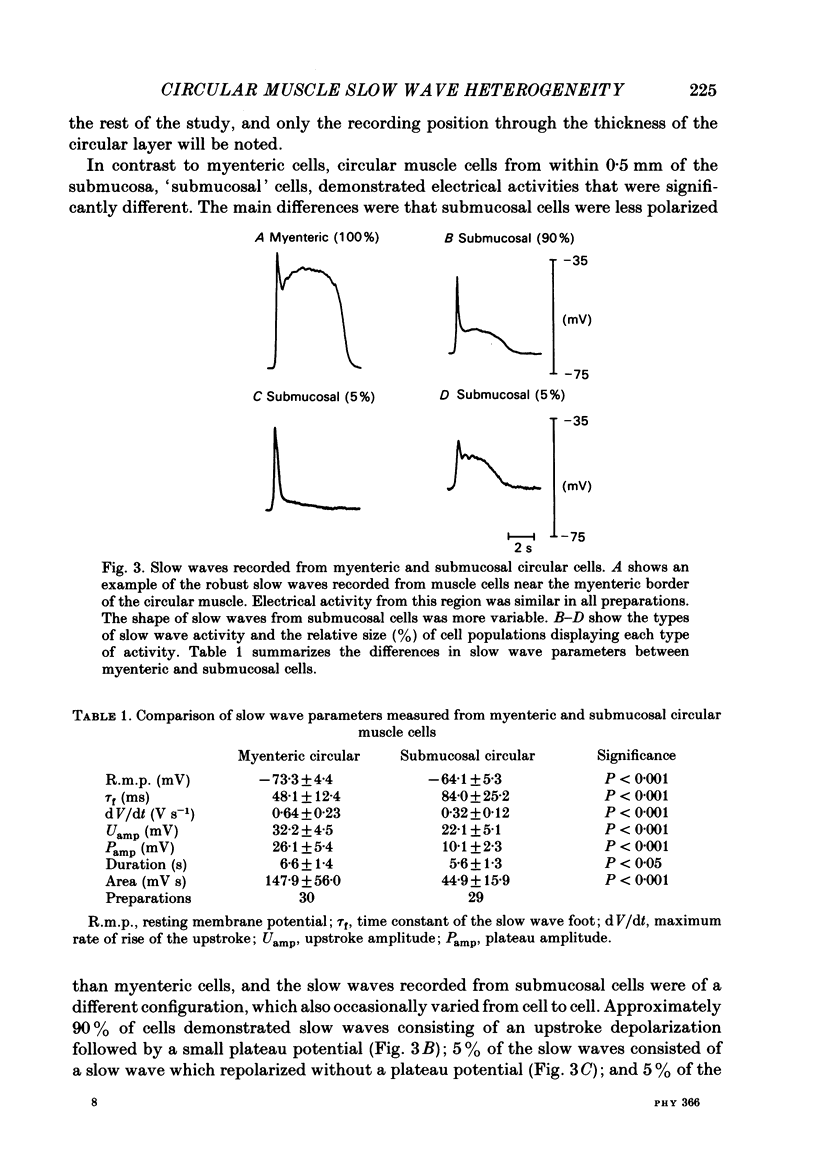
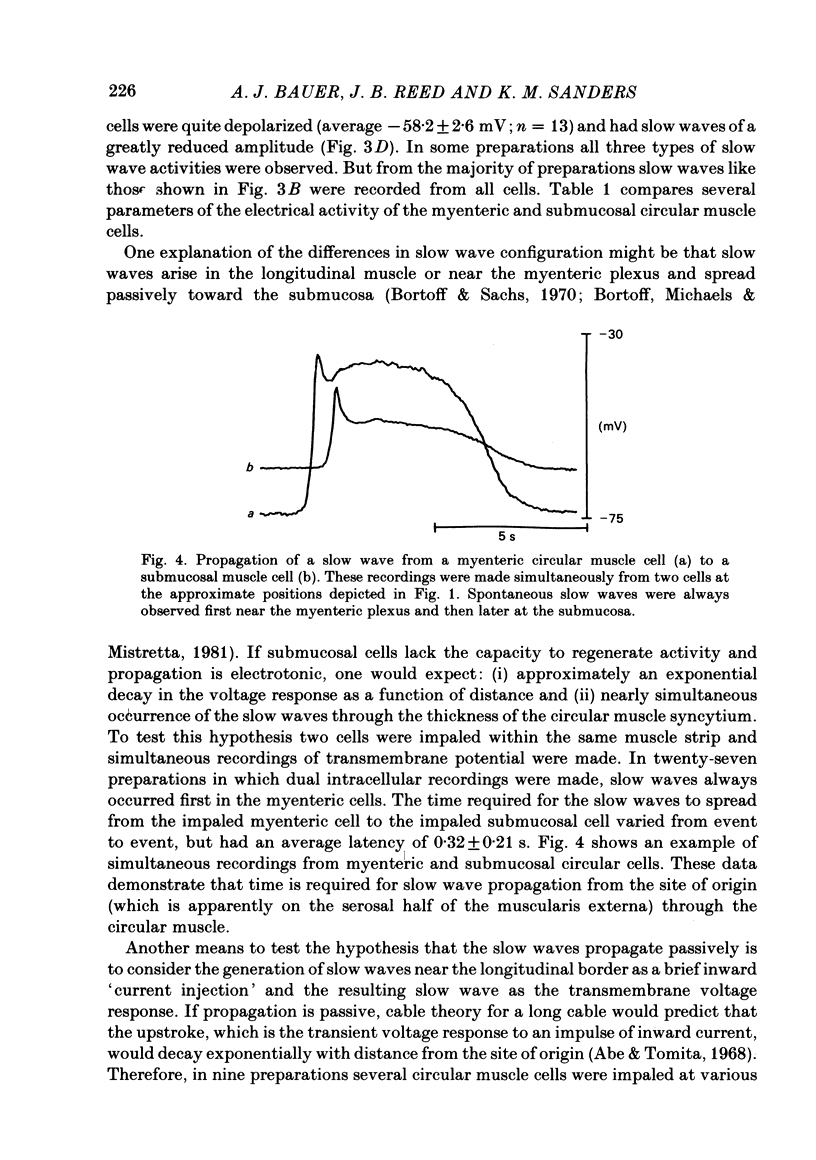
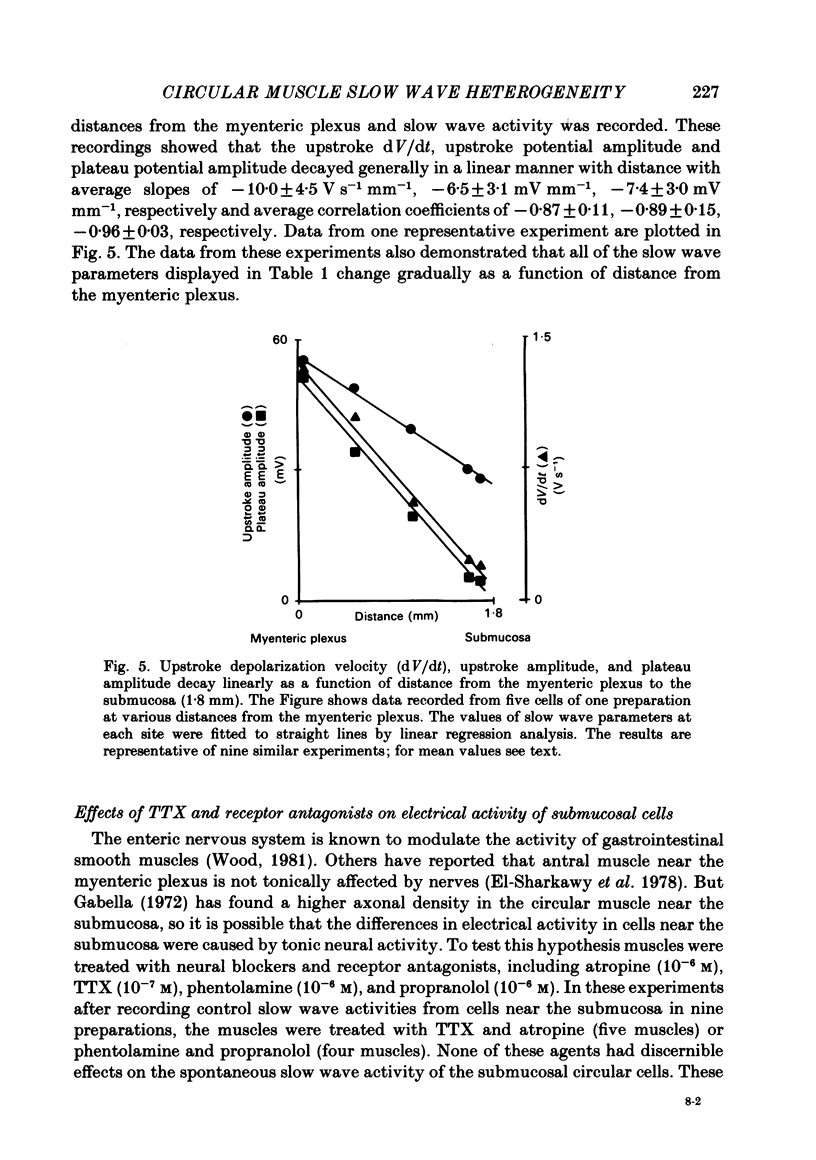
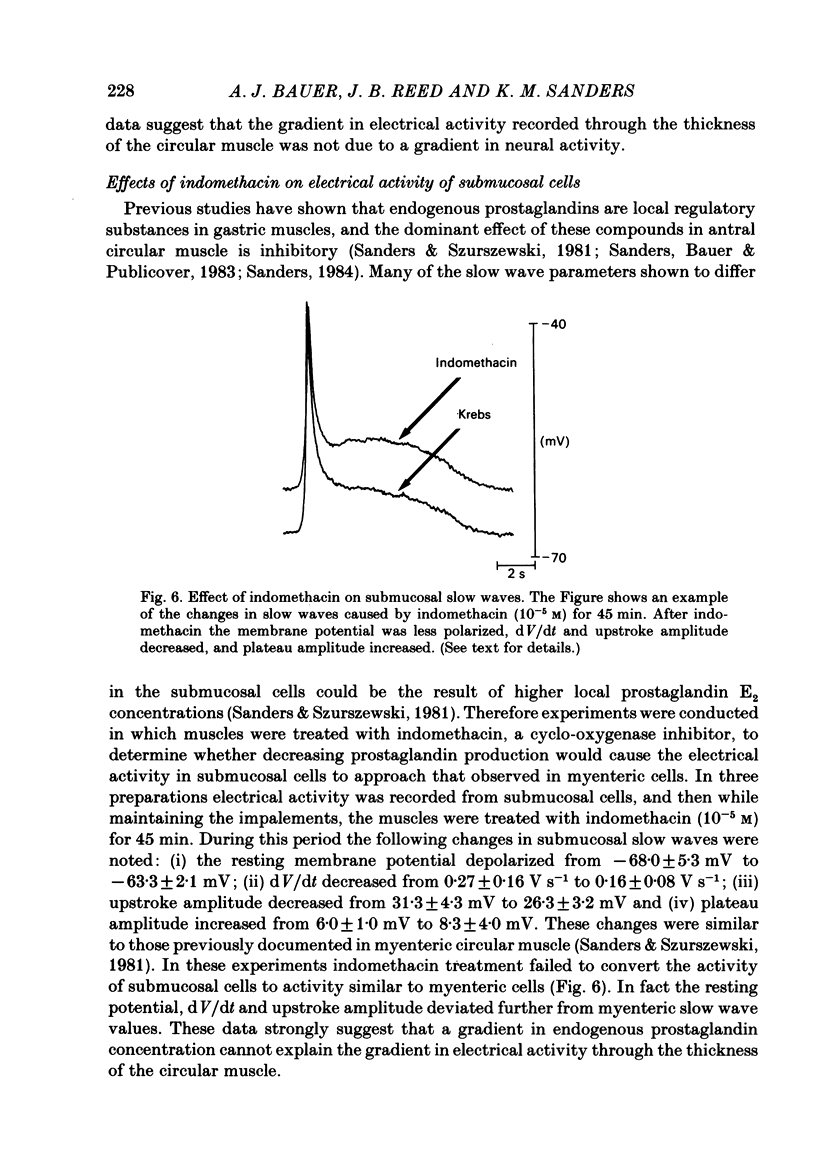
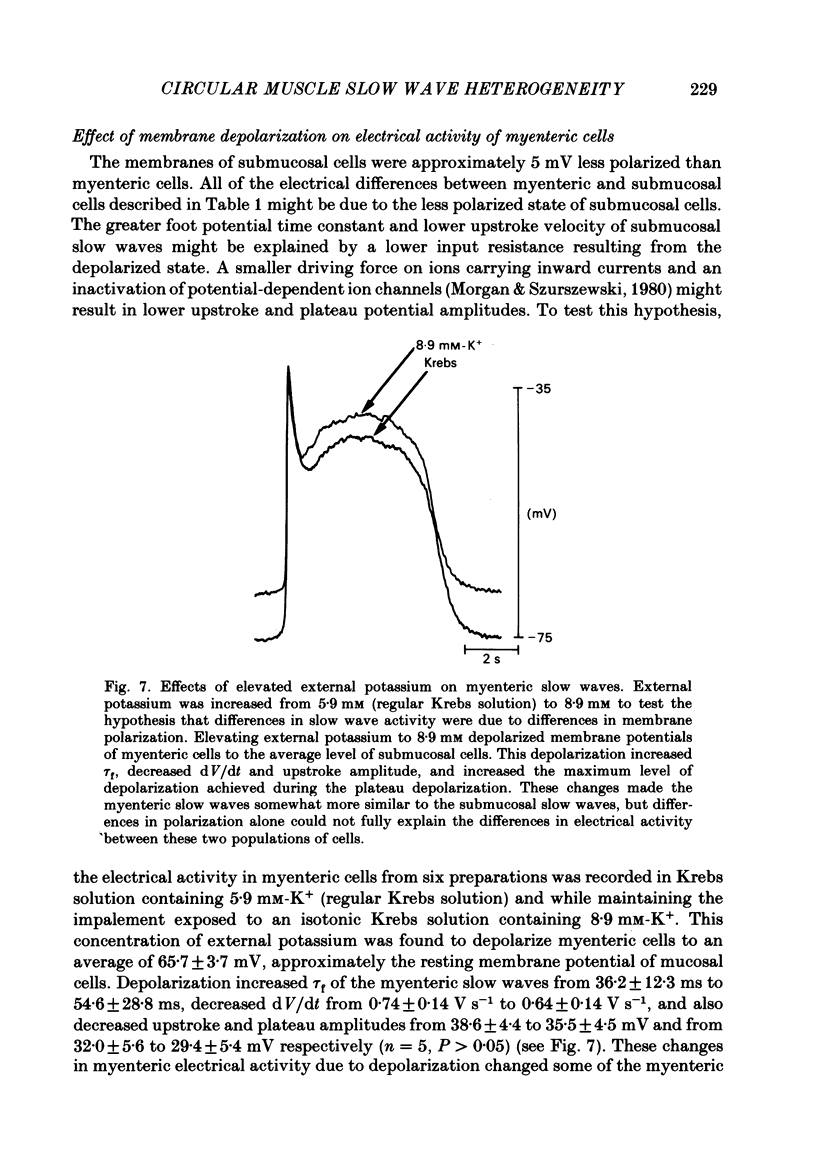
A cross-sectional preparation was designed in which multiple micro-electrodes can be precisely positioned to impale smooth muscle cells anywhere from the serosa to the submucosa. Intracellular electrical recordings were obtained from gastric antral circular muscle cells from the myenteric plexus to the submucosa. The resting membrane potential changed linearly as a function of distance from the myenteric plexus to the submucosa. Slow wave upstroke dV/dt, upstroke potential amplitude, and plateau potential amplitude changed linearly as a function of distance from the myenteric plexus to the submucosa. When slow waves were recorded simultaneously from a circular cell near the myenteric plexus and from a cell near the submucosa, the event always occurred first in the cell near the myenteric plexus. Electrical differences did not appear to be caused by electrotonic decay of slow waves as they propagated through the circular muscle. Electrical differences could not be explained on the basis of differences in intrinsic neural activity or prostaglandin synthesis. Membrane polarization could not explain the differences in slow waves between myenteric and submucosal circular muscle cells. The conclusion of this paper is that fundamental differences exist between the excitability mechanisms and/or passive membrane properties of cells near the myenteric plexus and the submucosa. These differences might be manifest in different motor performance of these two muscle cell populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A., Michaels D., Mistretta P. Dominance of longitudinal muscle in propagation of intestinal slow waves. Am J Physiol. 1981 Mar;240(3):C135–C147. doi: 10.1152/ajpcell.1981.240.3.C135. [DOI] [PubMed] [Google Scholar]
- Bortoff A., Sachs F. Electrotonic spread of slow waves in circular muscle of small intestine. Am J Physiol. 1970 Feb;218(2):576–581. doi: 10.1152/ajplegacy.1970.218.2.576. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Kreulen D., Prosser C. L., Weigel R. Interaction between longitudinal and circular muscle in intestine of cat. J Physiol. 1977 Dec;273(3):665–689. doi: 10.1113/jphysiol.1977.sp012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Mangel A. W., Nelson B. Propagation and entrainment of slow waves in cat small intestine. Am J Physiol. 1979 Nov;237(5):C237–C246. doi: 10.1152/ajpcell.1979.237.5.C237. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Daniel V. P., Duchon G., Garfield R. E., Nichols M., Malhotra S. K., Oki M. Is the nexus necessary for cell-to-cell coupling of smooth muscle? J Membr Biol. 1976 Aug 26;28(2-3):207–239. doi: 10.1007/BF01869698. [DOI] [PubMed] [Google Scholar]
- Elden L., Bortoff A. Electrical coupling of longitudinal and circular intestinal muscle. Am J Physiol. 1984 May;246(5 Pt 1):G618–G626. doi: 10.1152/ajpgi.1984.246.5.G618. [DOI] [PubMed] [Google Scholar]
- Gabella G. Innervation of the intestinal muscular coat. J Neurocytol. 1972 Dec;1(4):341–362. doi: 10.1007/BF01102939. [DOI] [PubMed] [Google Scholar]
- Gabella G. Proceedings: A special muscle layer in the intestinal muscular coat. J Physiol. 1974 Jul;240(2):1P–3P. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Nagai T., Prosser C. L. Electrical interaction between muscle layers of cat intestine. Am J Physiol. 1966 Dec;211(6):1281–1291. doi: 10.1152/ajplegacy.1966.211.6.1281. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Szurszewski J. H. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 Apr;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Bauer A. J. Ethyl alcohol interferes with excitation-contraction mechanisms of canine antral muscle. Am J Physiol. 1982 Mar;242(3):G222–G230. doi: 10.1152/ajpgi.1982.242.3.G222. [DOI] [PubMed] [Google Scholar]
- Sanders K. M. Role of prostaglandins in regulating gastric motility. Am J Physiol. 1984 Aug;247(2 Pt 1):G117–G126. doi: 10.1152/ajpgi.1984.247.2.G117. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Intrinsic neural control of intestinal motility. Annu Rev Physiol. 1981;43:33–51. doi: 10.1146/annurev.ph.43.030181.000341. [DOI] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Szurszewski J. H. Modulation of canine antral circular smooth muscle by acetylcholine, noradrenaline and pentagastrin. J Physiol. 1978 Jun;279:309–320. doi: 10.1113/jphysiol.1978.sp012346. [DOI] [PMC free article] [PubMed] [Google Scholar]


