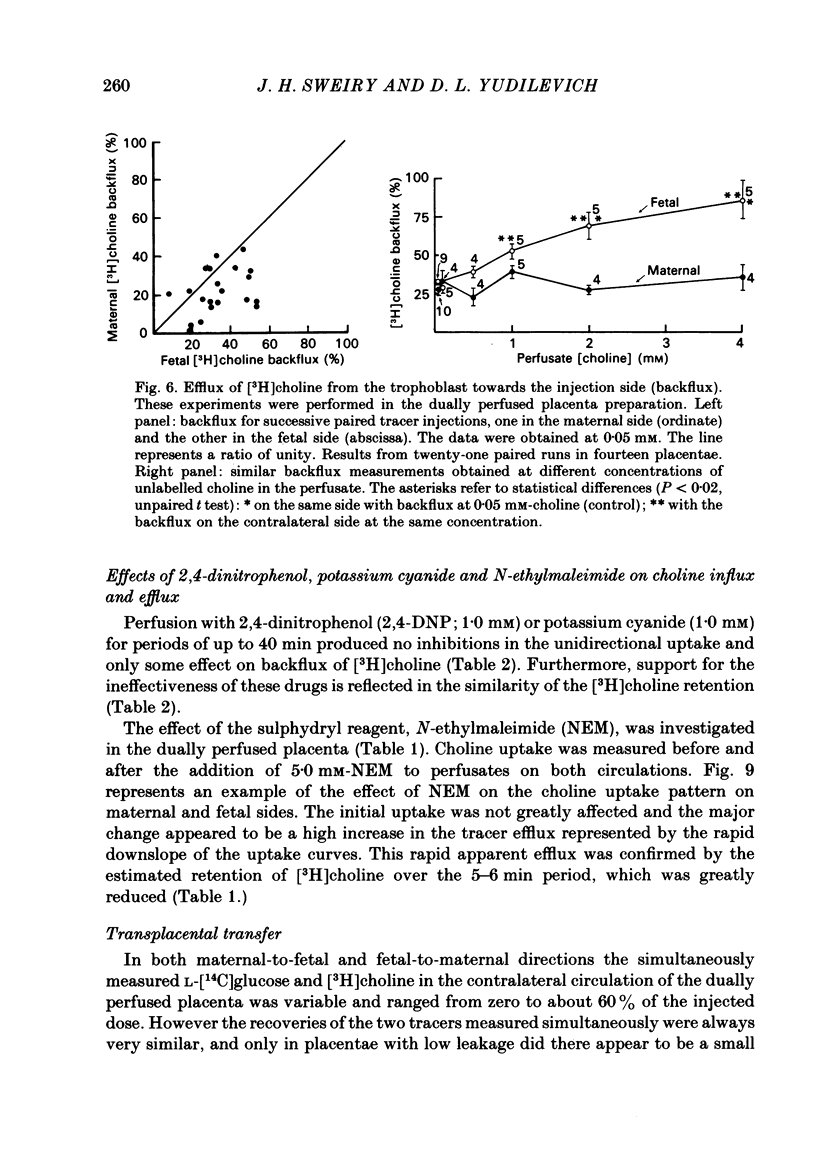
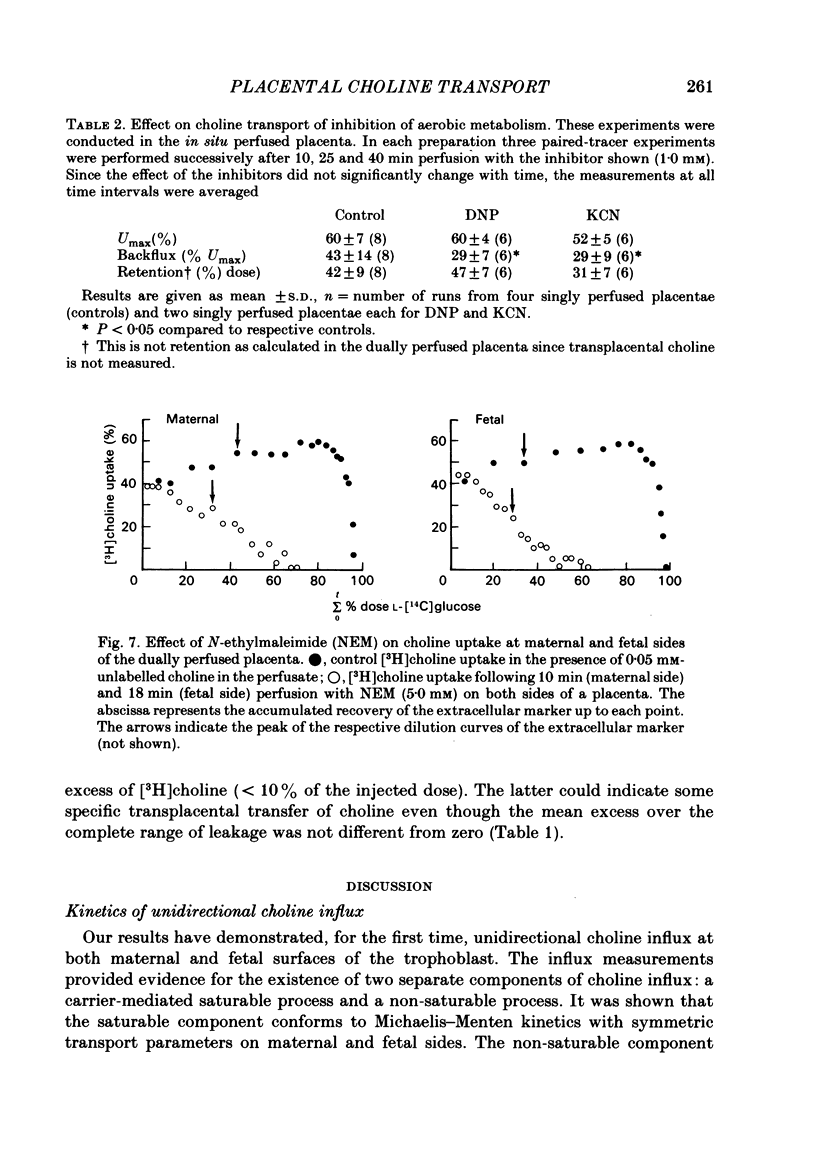
Abstract
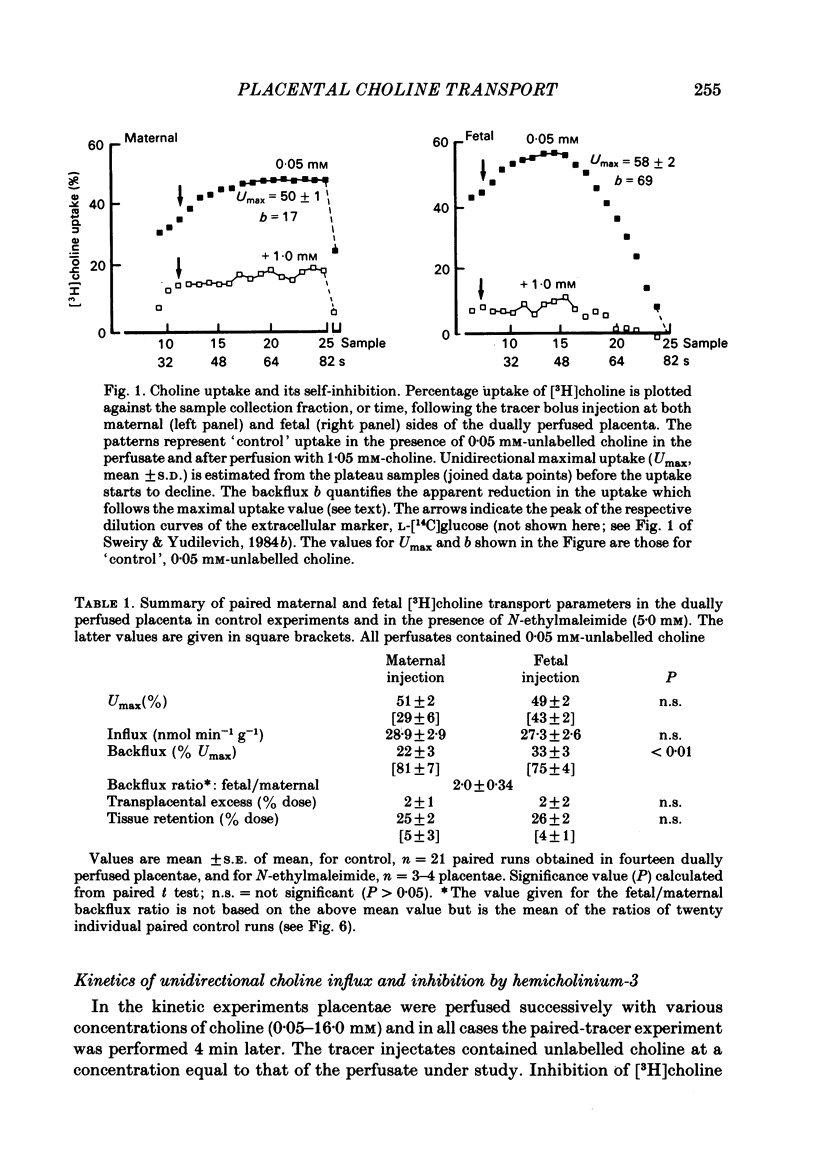
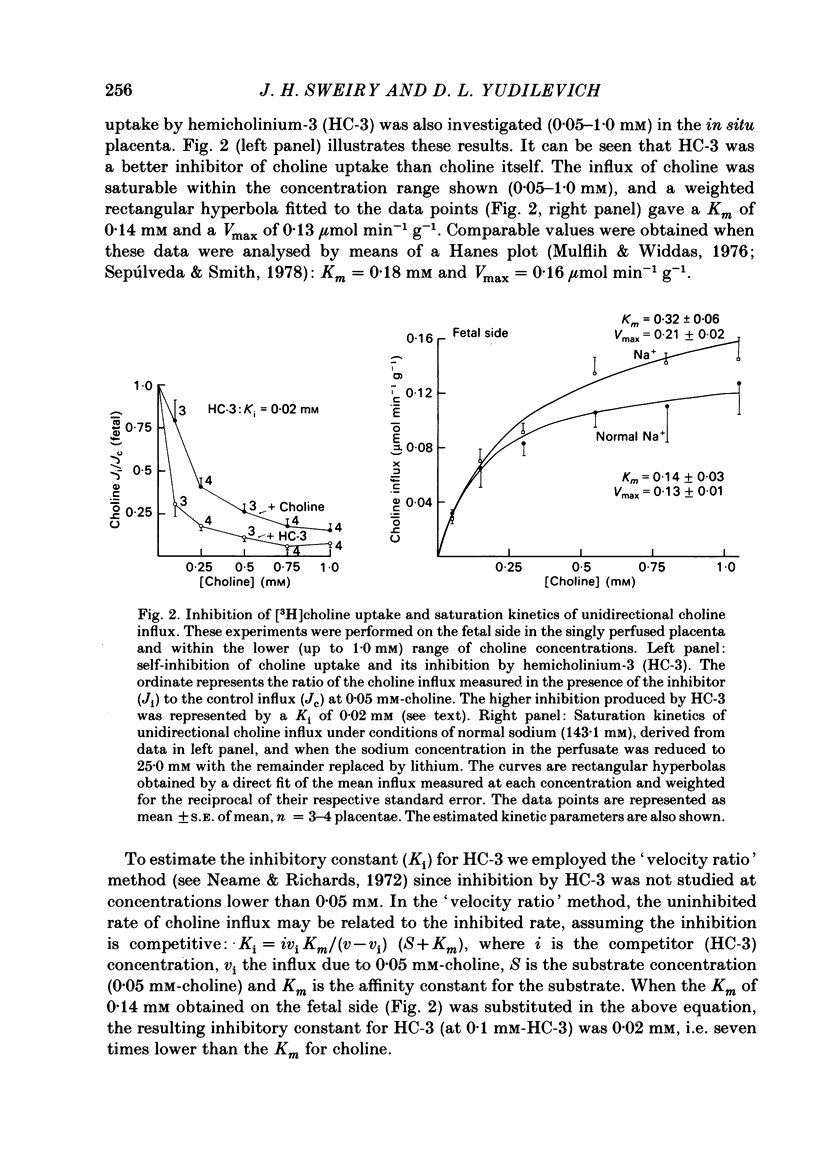
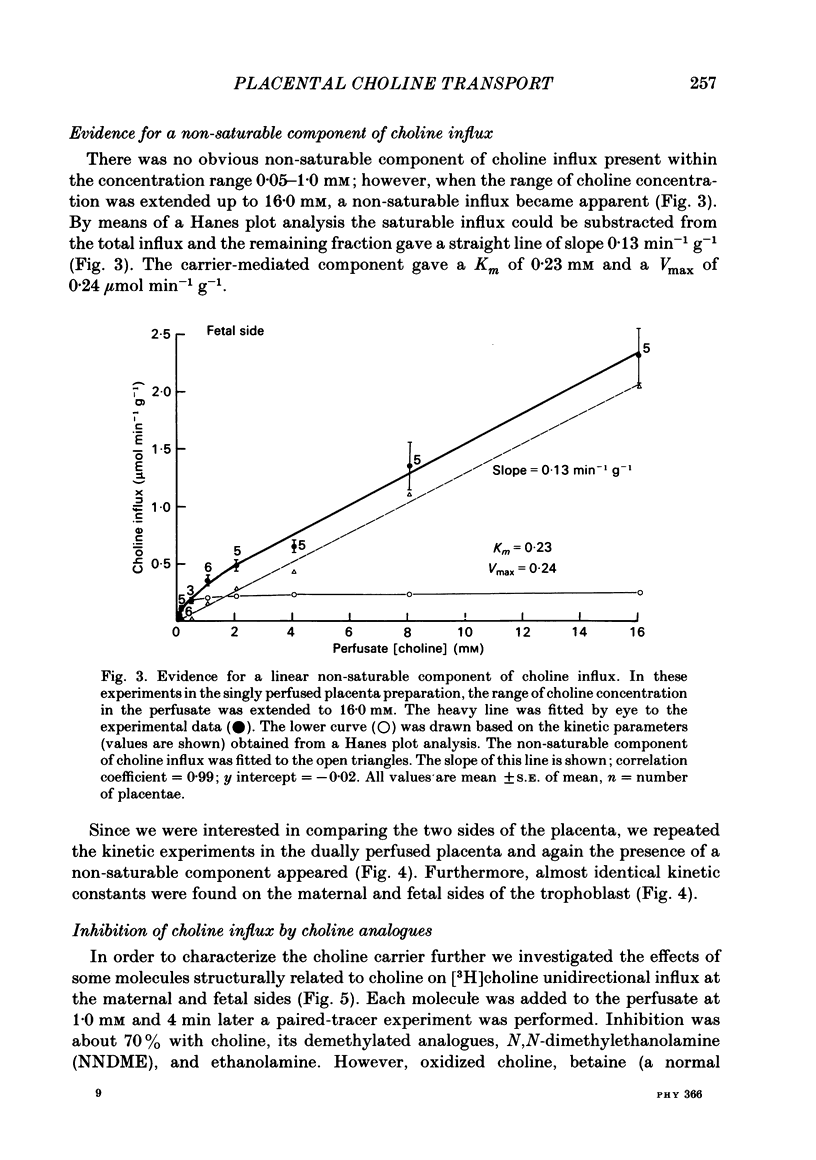
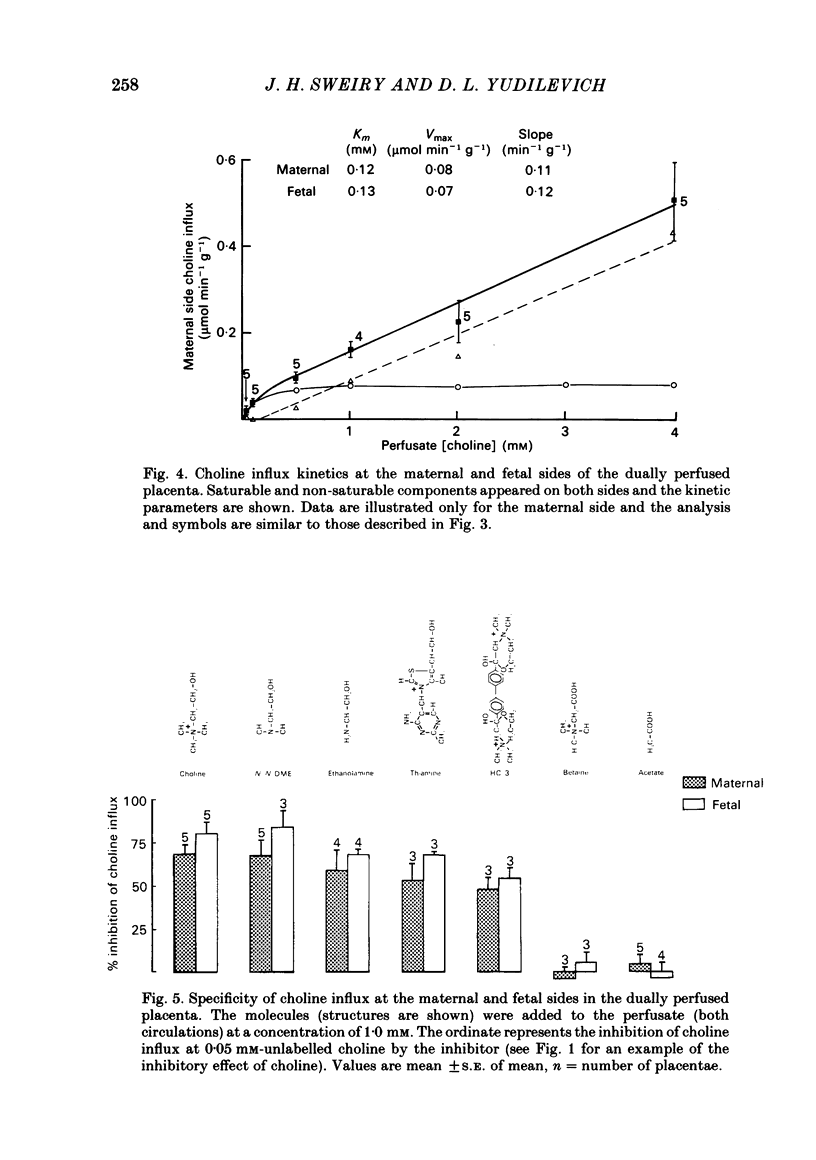
Unidirectional influx and efflux of choline into the syncytiotrophoblast were investigated from both maternal and fetal circulations of the perfused guinea-pig placenta by using a single-circulation paired-tracer (extracellular reference and test substrate) dilution technique. Cellular uptake of [3H]choline at 0.05 mM was (mean percentage +/- S.E. of mean, n = 14 placentae) 51 +/- 2 and 49 +/- 2, on maternal and fetal sides, respectively. Kinetics of unidirectional influx (0.05-4.0 mM-choline) indicated the existence of saturable and non-saturable components on both sides: on maternal and fetal interfaces the Km (mM) values were respectively, 0.12 and 0.13, the Vmax (mumol min-1 g-1) values, 0.08 and 0.07 and the apparent linear transfer constants (min-1 g-1) 0.11 and 0.12. Efflux of [3H]choline from the placenta back into the ipsilateral circulation (backflux) was generally fast (20-60% in 5-6 min) and asymmetric with the fetal: maternal ratio usually above unity. Transplacental specific choline transfer in the dually perfused placenta, when observed, was small (less than 10% of the injected dose) following tracer injections in either direction based on the 5-6 min collection of the contralateral circulation (at 0.05 mM-choline). Placental retention of [3H]choline at the end of the 5-6 min period was about 25% of the injected dose when the tracers were injected from either circulation. Analogues of choline such as hemicholinium-3, thiamine, ethanolamine and N,N-dimethylethanolamine inhibited choline unidirectional influx, whereas betaine and acetate had no effect. The absence of the normal sodium gradient (perfusate sodium was replaced by Tris or by lithium) did not inhibit choline transport. The metabolic inhibitors dinitrophenol (1.0 mM) and potassium cyanide (1.0 mM) were essentially ineffective (up to 40 min perfusion). The sulphydryl reagent N-ethylmaleimide did not appear to inhibit the influx, in comparison with its effect on [3H]choline backflux which was greatly accelerated, resulting in a dramatic reduction in placental net uptake of the label. Our findings show that choline transport into the placenta is a rapid carrier-mediated process occurring at both maternal and fetal sides of the trophoblast, at physiological blood concentrations. This cellular uptake is possibly related to the synthesis of acetylcholine, which is known to occur in human placental tissue. Specific transplacental transfer of choline was a very slow process under the conditions of our experiments and this contrasted with the observed fast and high uptake into the trophoblast.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH J. The level of free choline in plasma. J Physiol. 1952 Jun;117(2):234–240. doi: 10.1113/jphysiol.1952.sp004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I. The mechanism of transfer for L-leucine into the vascular bed of the Anuran small intestine. J Physiol. 1981 Aug;317:91–102. doi: 10.1113/jphysiol.1981.sp013815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Evidence for a two-state mobile carrier mechanism in erythrocyte choline transport: effects of substrate analogs on inactivation of the carrier by N-ethylmaleimide. J Membr Biol. 1981;61(1):21–30. doi: 10.1007/BF01870749. [DOI] [PubMed] [Google Scholar]
- Diamond I., Kennedy E. P. Carrier-mediated transport of choline into synaptic nerve endings. J Biol Chem. 1969 Jun 25;244(12):3258–3263. [PubMed] [Google Scholar]
- Eaton B. M., Mann G. E., Yudilevich D. L. Transport specificity for neutral and basic amino acids at maternal and fetal interfaces of the guinea-pig placenta. J Physiol. 1982 Jul;328:245–258. doi: 10.1113/jphysiol.1982.sp014262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. M., Yudilevich D. L. Uptake and asymmetric efflux of amino acids at maternal and fetal sides of placenta. Am J Physiol. 1981 Sep;241(3):C106–C112. doi: 10.1152/ajpcell.1981.241.3.C106. [DOI] [PubMed] [Google Scholar]
- Herzberg G. R., Lerner J. Intestinal absorption of choline in the chick. Biochim Biophys Acta. 1973 Apr 25;307(1):234–242. doi: 10.1016/0005-2736(73)90040-0. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Kuczler F. J., Nahrwold D. L., Rose R. C. Choline influx across the brush border of guinea pig jejunum. Biochim Biophys Acta. 1977 Feb 14;465(1):131–137. doi: 10.1016/0005-2736(77)90361-3. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Schröder H. Uber den Glucosetransport durch die isolierte, beiderseits künstlich perfundierte Meerschweinchenplacenta. Pflugers Arch. 1971;325(2):139–148. doi: 10.1007/BF00587004. [DOI] [PubMed] [Google Scholar]
- MONEY W. L., DANCIS J. Technique for the in situ study of placental transport in the pregnant guinea pig. Am J Obstet Gynecol. 1960 Aug;80:209–214. doi: 10.1016/0002-9378(60)90114-9. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The activation of presynaptic choline uptake by acetylcholine release. J Physiol (Paris) 1982;78(4):373–378. [PubMed] [Google Scholar]
- Marchbanks R. M. The uptake of [14C] choline into synaptosomes in vitro. Biochem J. 1968 Dec;110(3):533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Extracellular cations and the movement of choline across the erythrocyte membrane. J Physiol. 1972 Jul;224(1):207–230. doi: 10.1113/jphysiol.1972.sp009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Some properties of an SH group essential for choline transport in human erythrocytes. J Physiol. 1971 Mar;213(3):647–664. doi: 10.1113/jphysiol.1971.sp009406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muflih I. W., Widdas W. F. Sugars and sugar derivatives which inhibit the short-circuit current of the everted small intestine of the rat. J Physiol. 1976 Dec;263(2):101–114. doi: 10.1113/jphysiol.1976.sp011623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENNICK B. R. The renal tubular excretion of choline and thiamine in the chicken. J Pharmacol Exp Ther. 1958 Apr;122(4):449–456. [PubMed] [Google Scholar]
- Reynolds M. L., Young M. The transfer of free alpha-amino nitrogen across the placental membrane in the guinea-pig. J Physiol. 1971 May;214(3):583–597. doi: 10.1113/jphysiol.1971.sp009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C. Water-soluble vitamin absorption in intestine. Annu Rev Physiol. 1980;42:157–171. doi: 10.1146/annurev.ph.42.030180.001105. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Choline acetyltransferase. Inhibition by thiol reagents. J Biol Chem. 1974 Apr 10;249(7):2156–2159. [PubMed] [Google Scholar]
- Rowell P. P., Sastry B. V. Human placental cholinergic system: depression of the uptake of alpha-aminoisobutyric acid in isolated human placental villi by choline acetyltransferase inhibitors. J Pharmacol Exp Ther. 1981 Feb;216(2):232–238. [PubMed] [Google Scholar]
- Sanford P. A., Smyth D. H. Intestinal transfer of choline in rat and hamster. J Physiol. 1971 Jul;215(3):769–788. doi: 10.1113/jphysiol.1971.sp009497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry B. V., Sadavongvivad C. Cholinergic systems in non-nervous tissues. Pharmacol Rev. 1978 Mar;30(1):65–132. [PubMed] [Google Scholar]
- Sepúlveda F. V., Smith M. W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 Sep;282:73–90. doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated sounds by auditory neurones of echo-locating bats. J Physiol. 1965 Jul;179(1):26–53. doi: 10.1113/jphysiol.1965.sp007648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiry J. H., Yudilevich D. L. Asymmetric calcium influx and efflux at maternal and fetal sides of the guinea-pig placenta: kinetics and specificity. J Physiol. 1984 Oct;355:295–311. doi: 10.1113/jphysiol.1984.sp015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch F. Choline metabolism in human term placenta--studies on de novo synthesis and the effects of some drugs on the metabolic fate of [N-methyl 3H]choline. Biochem Pharmacol. 1978;27(8):1251–1257. doi: 10.1016/0006-2952(78)90459-8. [DOI] [PubMed] [Google Scholar]
- Welsch F. Effects of drugs on the uptake of acetylcholine by human term placenta fragments. Res Commun Chem Pathol Pharmacol. 1976 Nov;15(3):457–468. [PubMed] [Google Scholar]
- Welsch F. Studies on accumulation and metabolic fate of (N-Me3h)choline in human term placenta fragments. Biochem Pharmacol. 1976 May 1;25(9):1021–1030. doi: 10.1016/0006-2952(76)90490-1. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M. Amino acid carriers at maternal and fetal surfaces of the placenta by single circulation paired-tracer dilution. Kinetics of phenylalanine transport. Biochim Biophys Acta. 1980 Feb 28;596(2):315–319. doi: 10.1016/0005-2736(80)90364-8. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M., Short A. H., Leichtweiss H. P. Glucose carriers at maternal and fetal sides of the trophoblast in guinea pig placenta. Am J Physiol. 1979 Nov;237(5):C205–C212. doi: 10.1152/ajpcell.1979.237.5.C205. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr. 1981;1:95–121. doi: 10.1146/annurev.nu.01.070181.000523. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Epstein M. F., Wurtman R. J. Elevated choline concentration in neonatal plasma. Life Sci. 1980 May 26;26(21):1827–1831. doi: 10.1016/0024-3205(80)90585-8. [DOI] [PubMed] [Google Scholar]


