Abstract
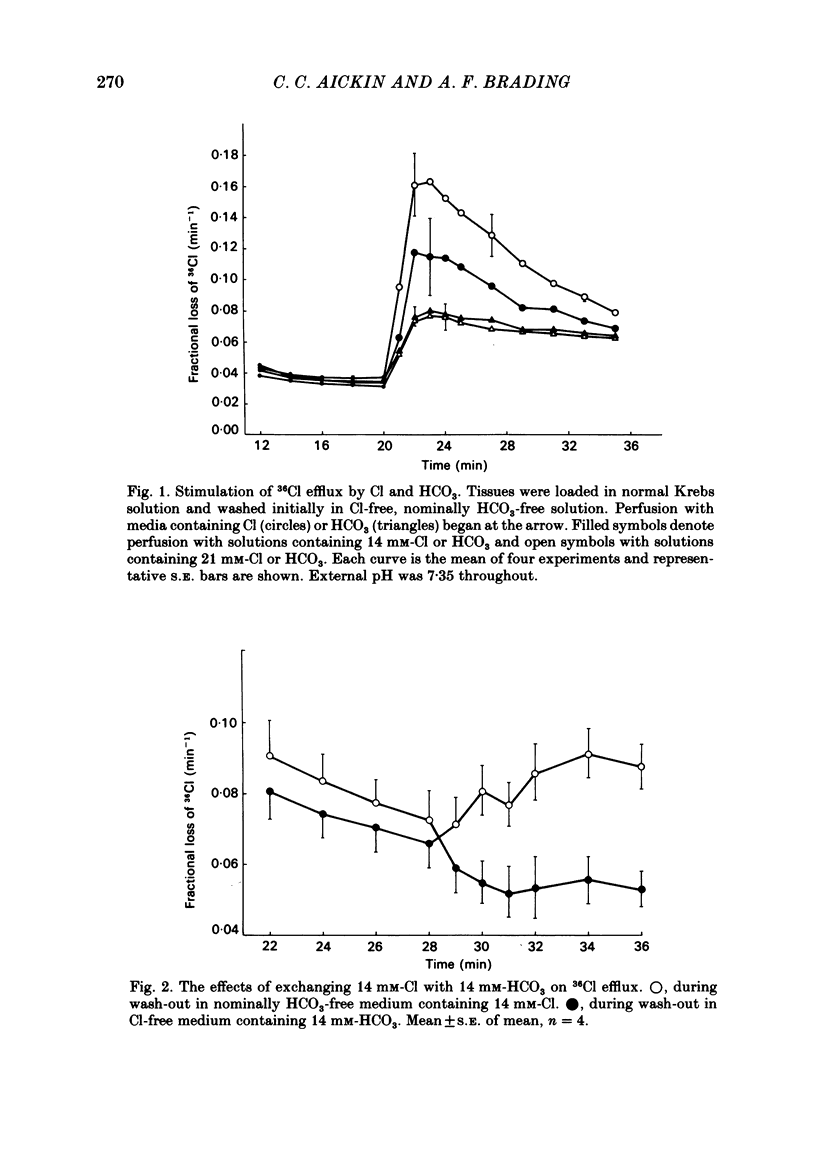
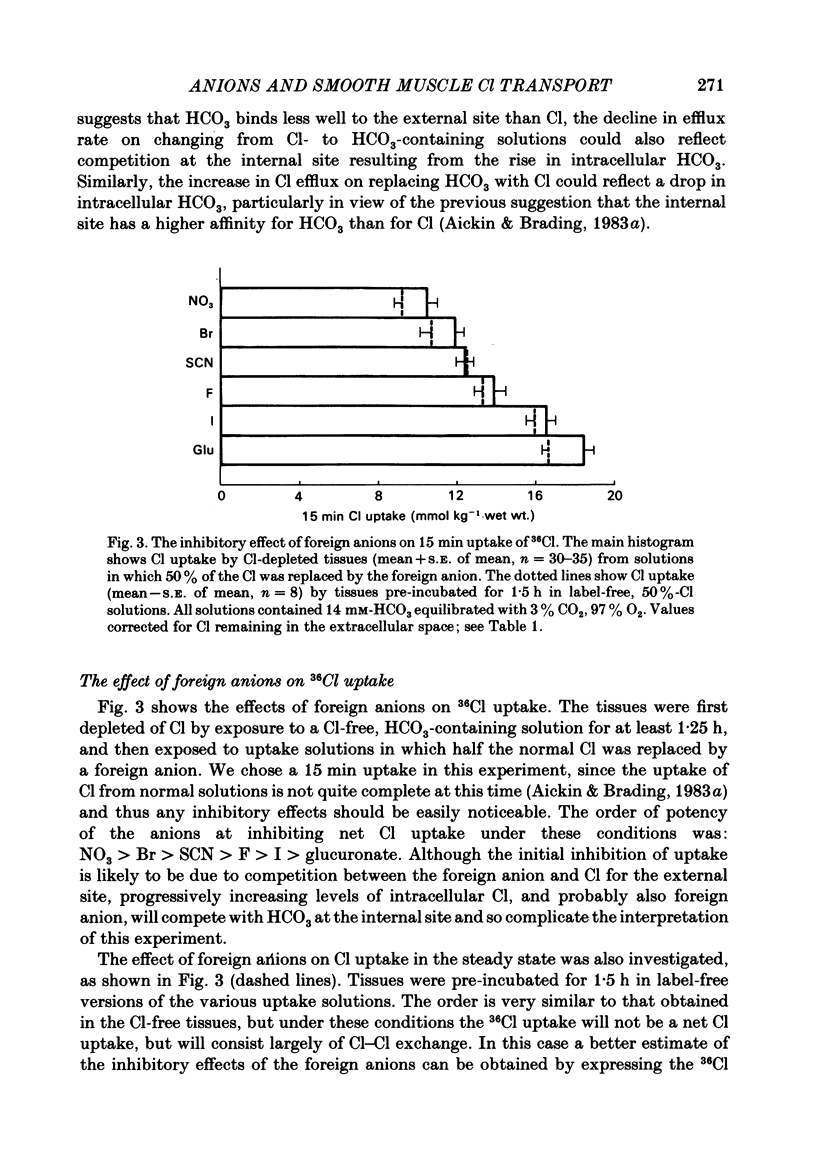
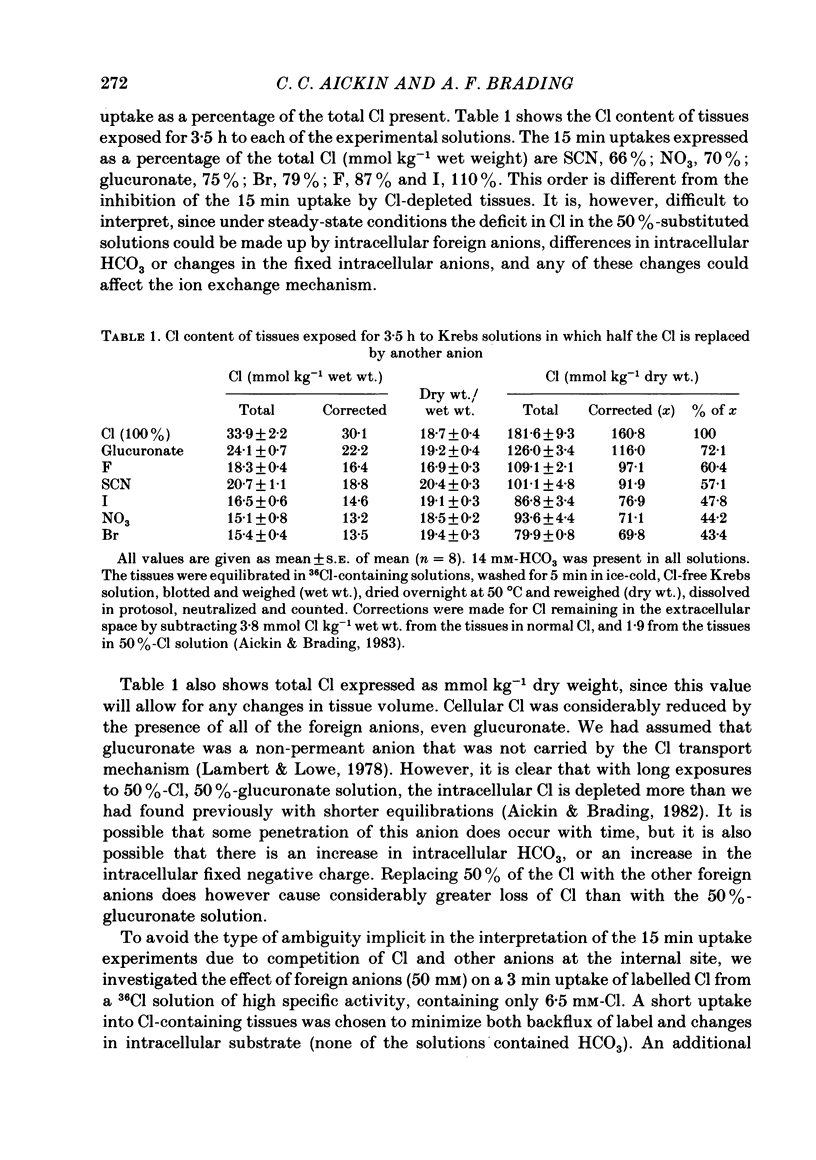
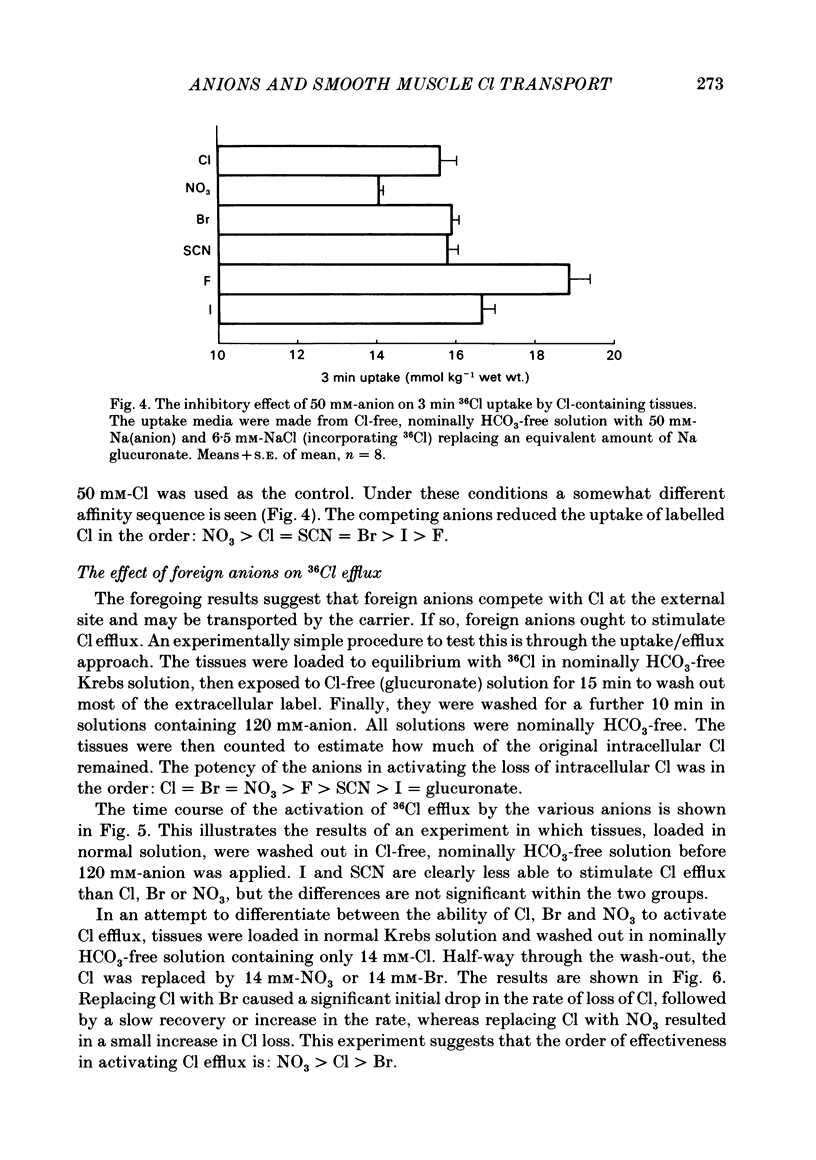
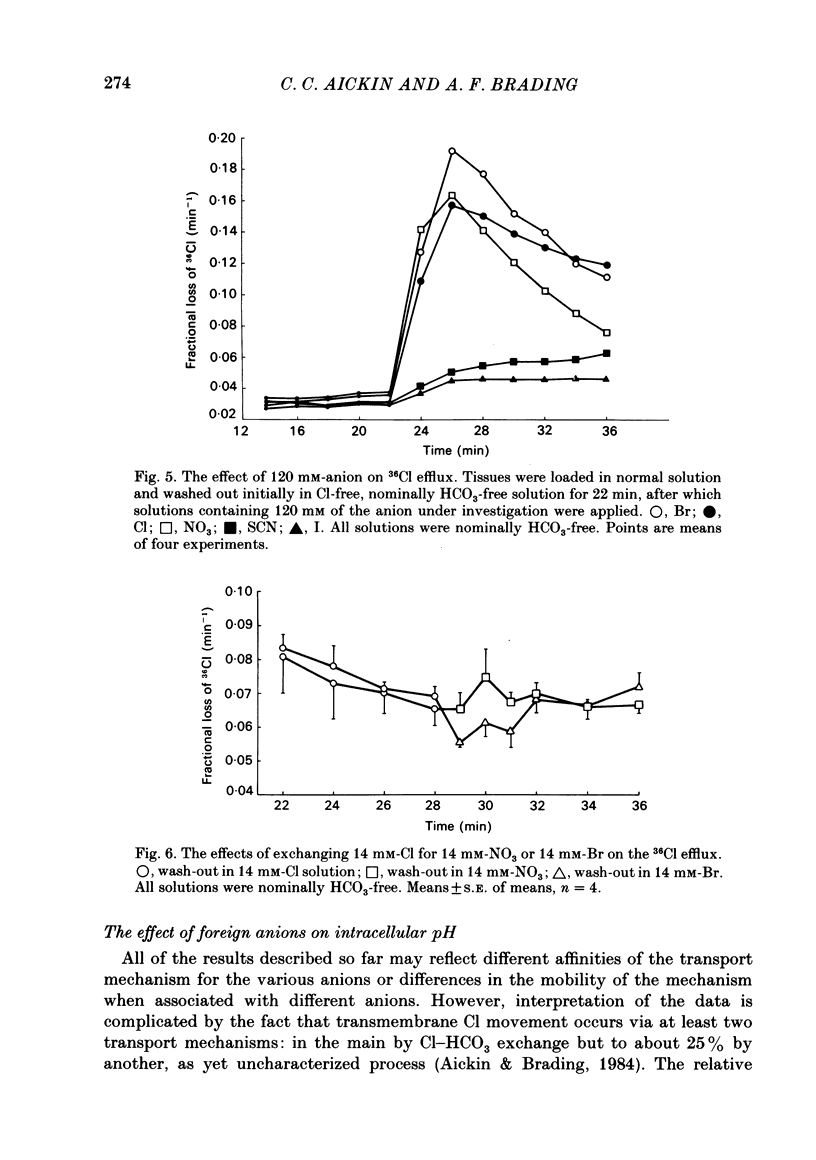
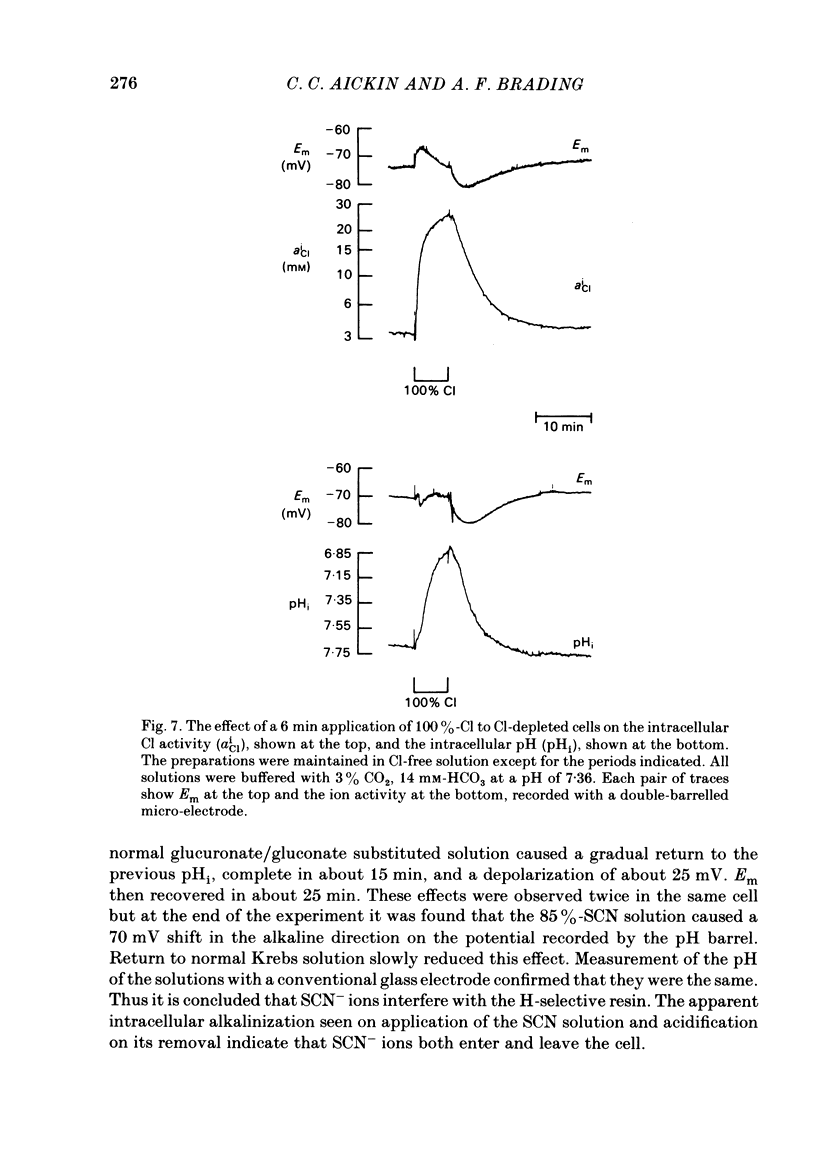
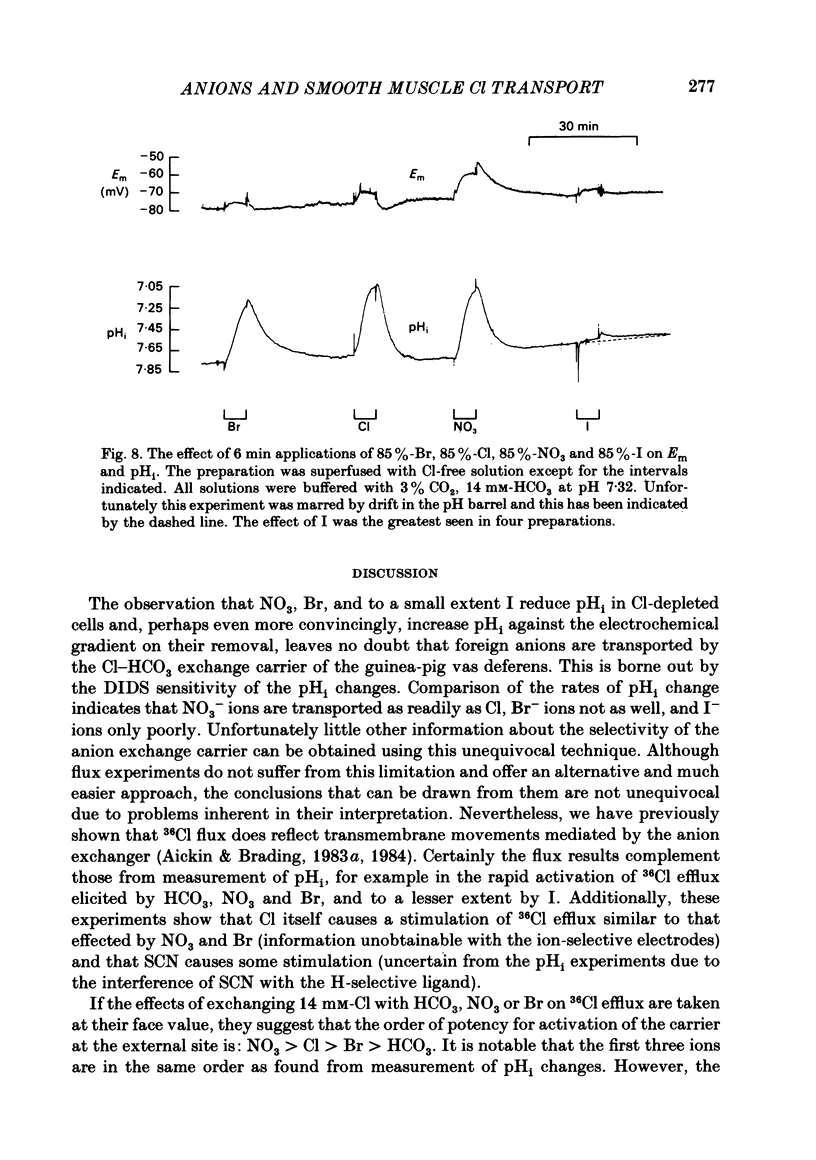
The selectivity of the external site of the Cl transporting mechanism in the guinea-pig vas deferens has been investigated by measurement of 36Cl uptake and efflux and by direct measurement of intracellular pH. Replacing 50% of the Cl in normal Krebs solution inhibited the 15 min uptake of 36Cl in the order NO3 greater than Br greater than SCN greater than F greater than I greater than glucuronate, both in Cl-depleted tissues and tissues pre-incubated in the 50%-Cl solutions (steady-state uptake). After 3 h incubation in these solutions, the total cellular Cl was reduced by the anions in the order Br greater than NO3 greater than I greater than SCN greater than F greater than glucuronate. Br, NO3 and I reduced the cellular Cl to less than 50% of normal, suggesting that they are actively taken up by the cells. The ability of foreign anions to inhibit a 3 min uptake of high specific activity, low concentration Cl (6.5 mM) suggests an apparent affinity series of NO3 greater than Cl = SCN = Br greater than I greater than F at the external site. Addition of NO3, Cl, Br, HCO3, F, SCN or I to a Cl-free, nominally HCO3-free bathing solution accelerated 36Cl efflux. The first four mentioned were powerful stimulants, the other three less potent. However, the exact position of HCO3 in the sequence is uncertain. The rapidity with which CO2 crosses the membrane and forms HCO3 intracellularly may allow competition between HCO3 and Cl at the internal site and so distort the result. The action of F is also questionable since this ion drastically reduces the divalent cation activity and is a metabolic inhibitor. Measurement of intracellular pH provided conclusive evidence that Cl, NO3, Br and I can exchange with HCO3 across the cell membrane. This exchange is as rapid with NO3 as with Cl but slower with Br and considerably slower with I. The results also indicate that SCN ions cross the cell membrane. It is concluded that Cl, HCO3, Br and NO3 are all translocated by the exchange carrier. I and perhaps SCN also interact with the transport mechanism, but the translocation rate is then greatly reduced. The precise order of the affinity of these anions remains uncertain but the following sequence: NO3 greater than Cl = SCN = Br greater than I greater than F is considered to be the most likely.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. The role of chloride-bicarbonate exchange in the regulation of intracellular chloride in guinea-pig vas deferens. J Physiol. 1984 Apr;349:587–606. doi: 10.1113/jphysiol.1984.sp015175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983 Mar;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Ashoori F., Tomita T. Mechanical response to noradrenaline in calcium-free solution in the rat vas deferens. J Physiol. 1983 May;338:165–178. doi: 10.1113/jphysiol.1983.sp014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Effects of halides and bicarbonate on chloride transport in human red blood cells. J Gen Physiol. 1976 Feb;67(2):223–234. doi: 10.1085/jgp.67.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Fröhlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. Evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol. 1979 Sep;74(3):351–374. doi: 10.1085/jgp.74.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO M., KOSTYUK P. G., OSHIMA T. Further study on anion permeability of inhibitory post-synaptic membrane of cat motoneurones. J Physiol. 1962 Oct;164:150–156. doi: 10.1113/jphysiol.1962.sp007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Law F. Y., Tarshis T., Furuya W. Effects of the transport site conformation on the binding of external NAP-taurine to the human erythrocyte anion exchange system. Evidence for intrinsic asymmetry. J Gen Physiol. 1984 May;83(5):683–701. doi: 10.1085/jgp.83.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Mann N. A. Use of niflumic acid to determine the nature of the asymmetry of the human erythrocyte anion exchange system. J Gen Physiol. 1984 May;83(5):703–725. doi: 10.1085/jgp.83.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A., Lowe A. G. Chloride/bicarbonate exchange in human erythrocytes. J Physiol. 1978 Feb;275:51–63. doi: 10.1113/jphysiol.1978.sp012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Cohen C. J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflugers Arch. 1981 Apr;390(1):96–98. doi: 10.1007/BF00582719. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Bicarbonate exchange through the human red cell membrane determined with [14C] bicarbonate. J Physiol. 1979 Sep;294:521–539. doi: 10.1113/jphysiol.1979.sp012944. [DOI] [PMC free article] [PubMed] [Google Scholar]


