Abstract
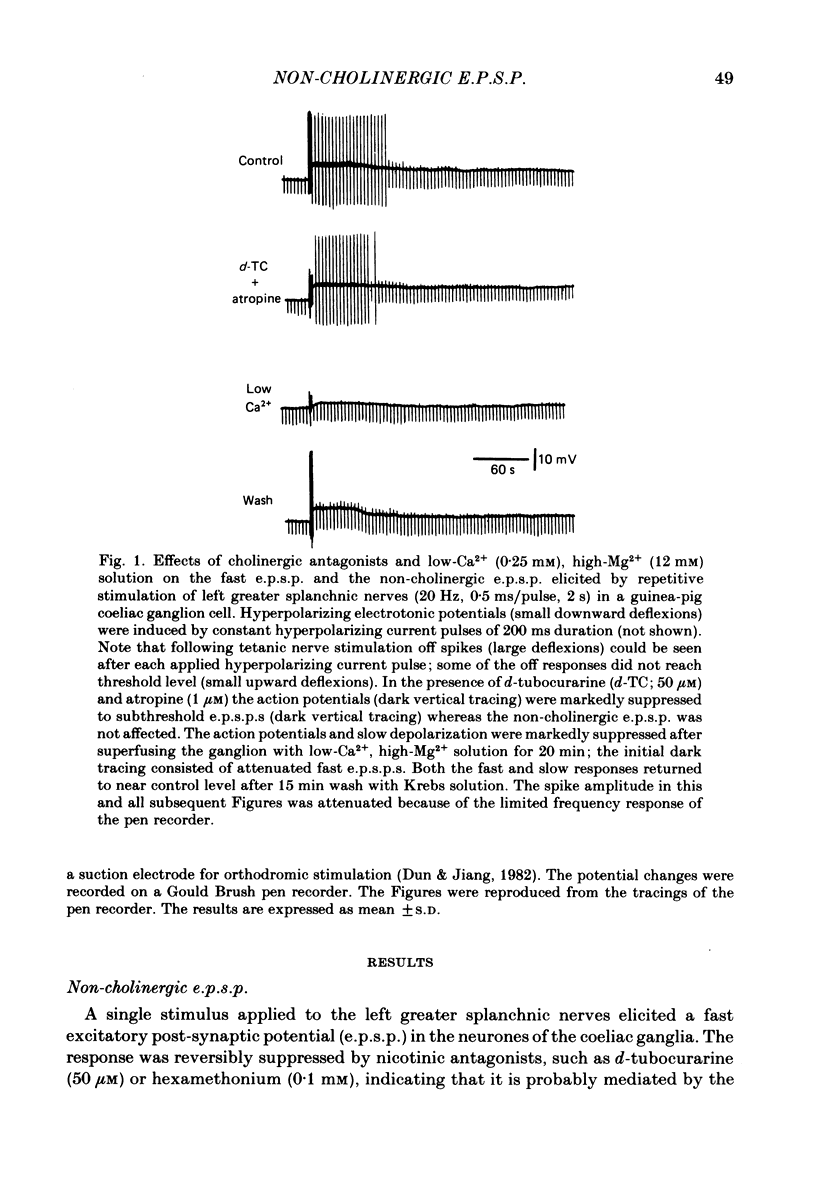
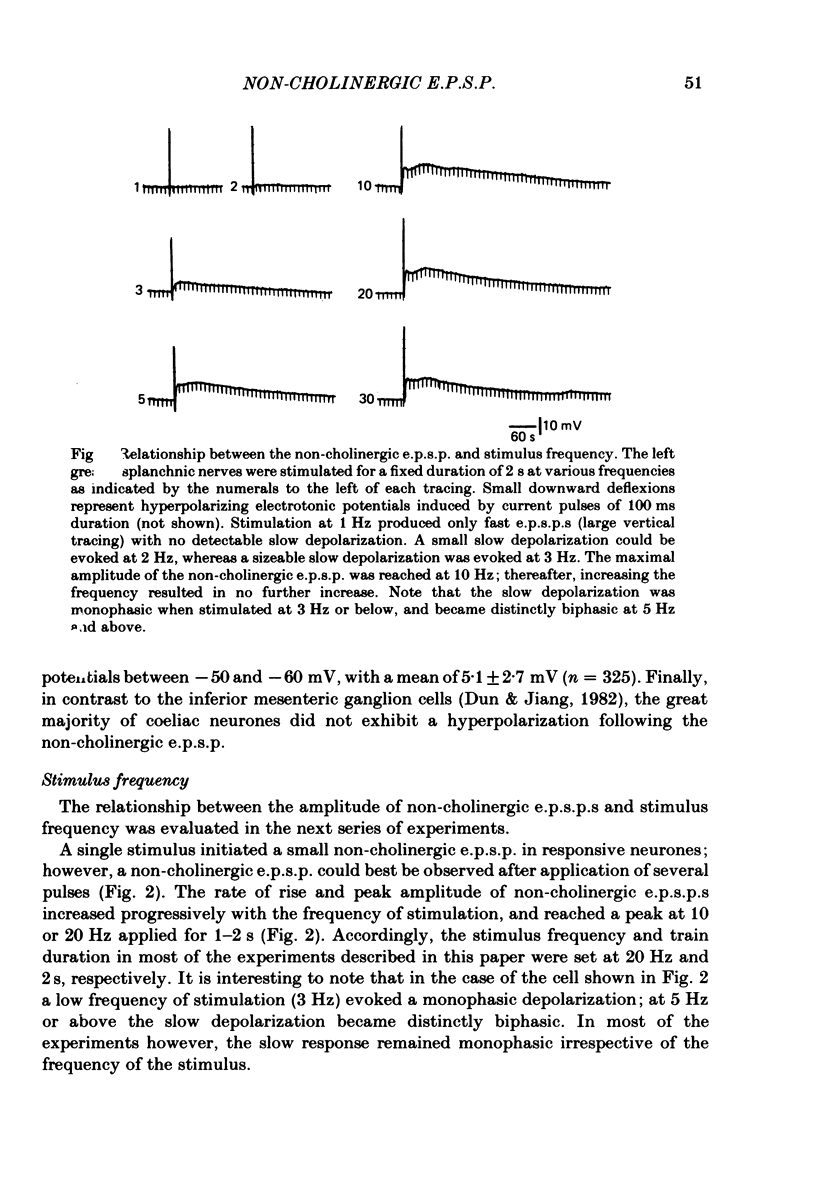
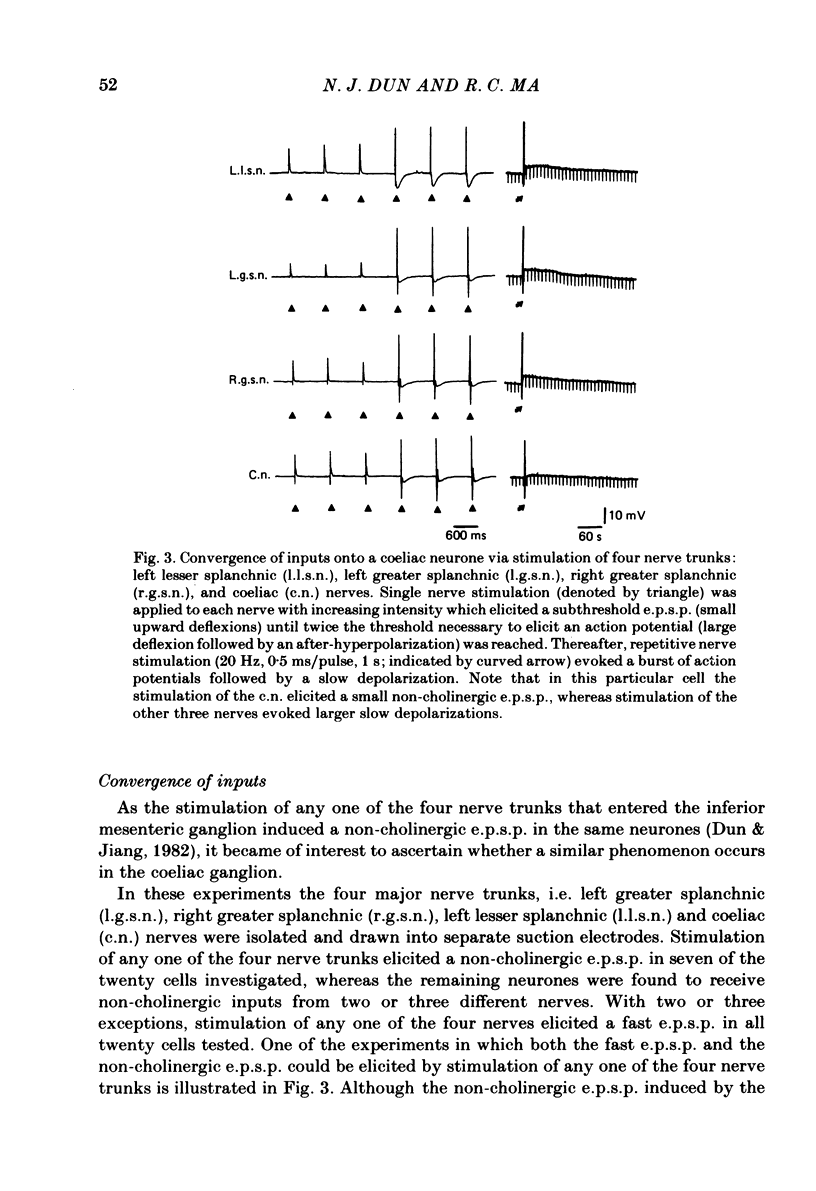
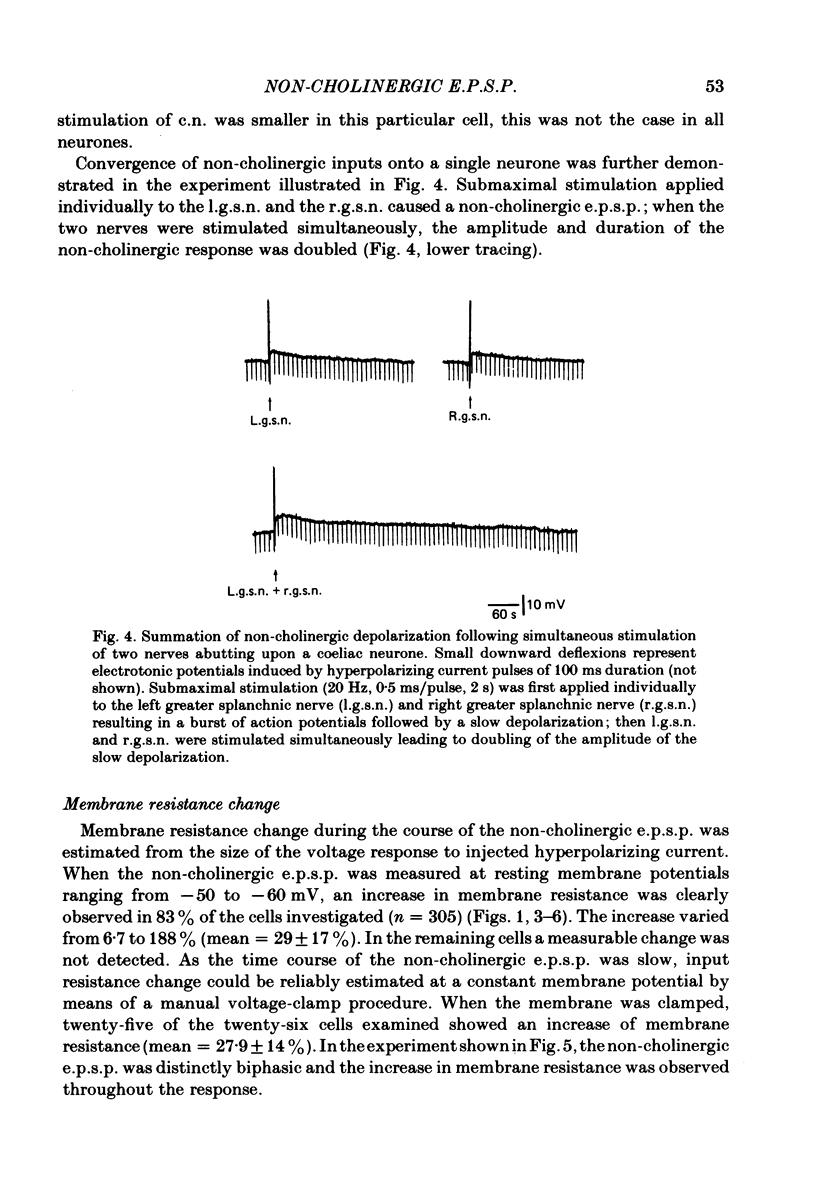
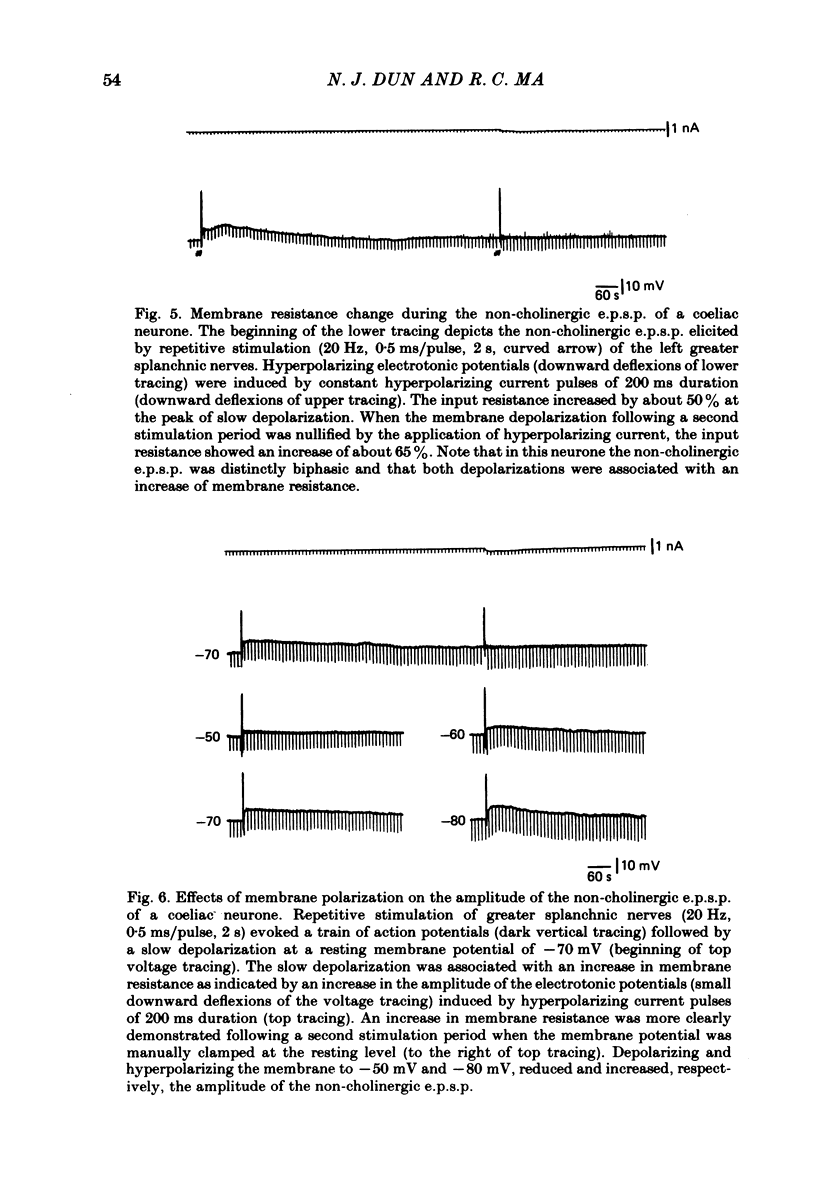
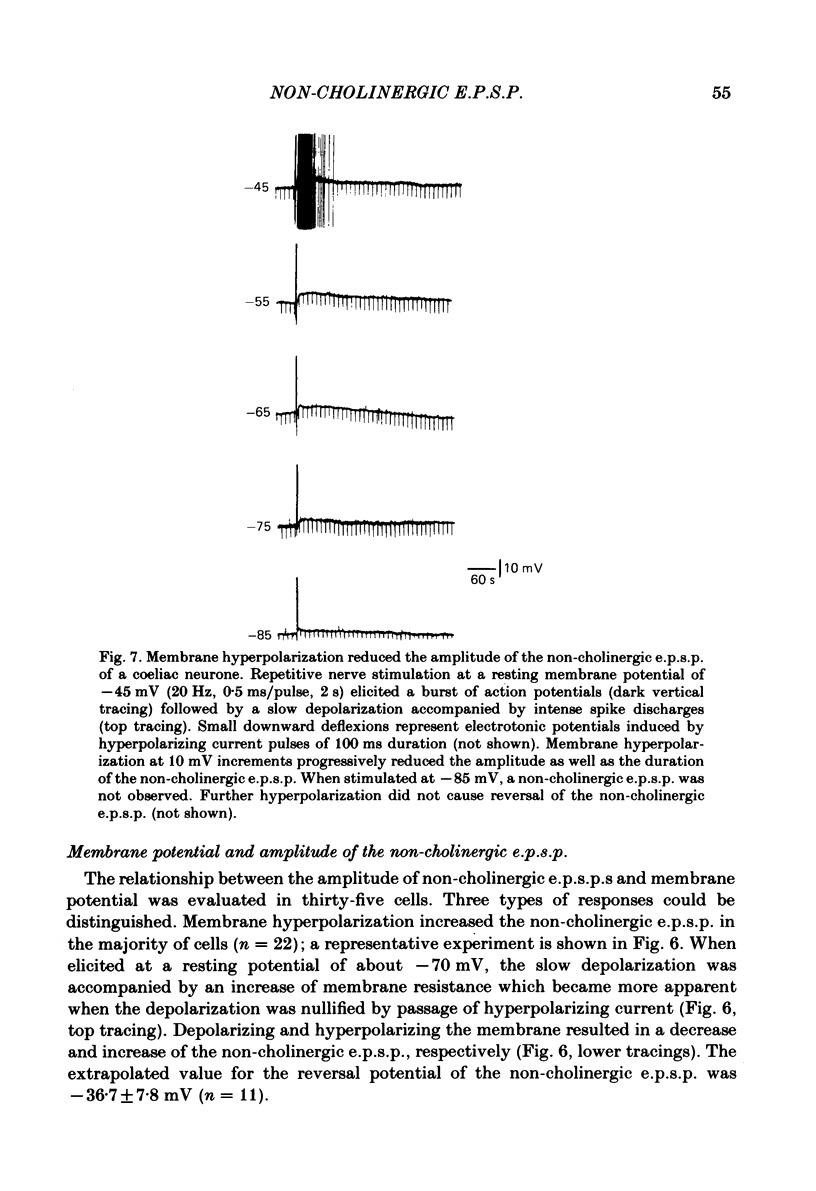
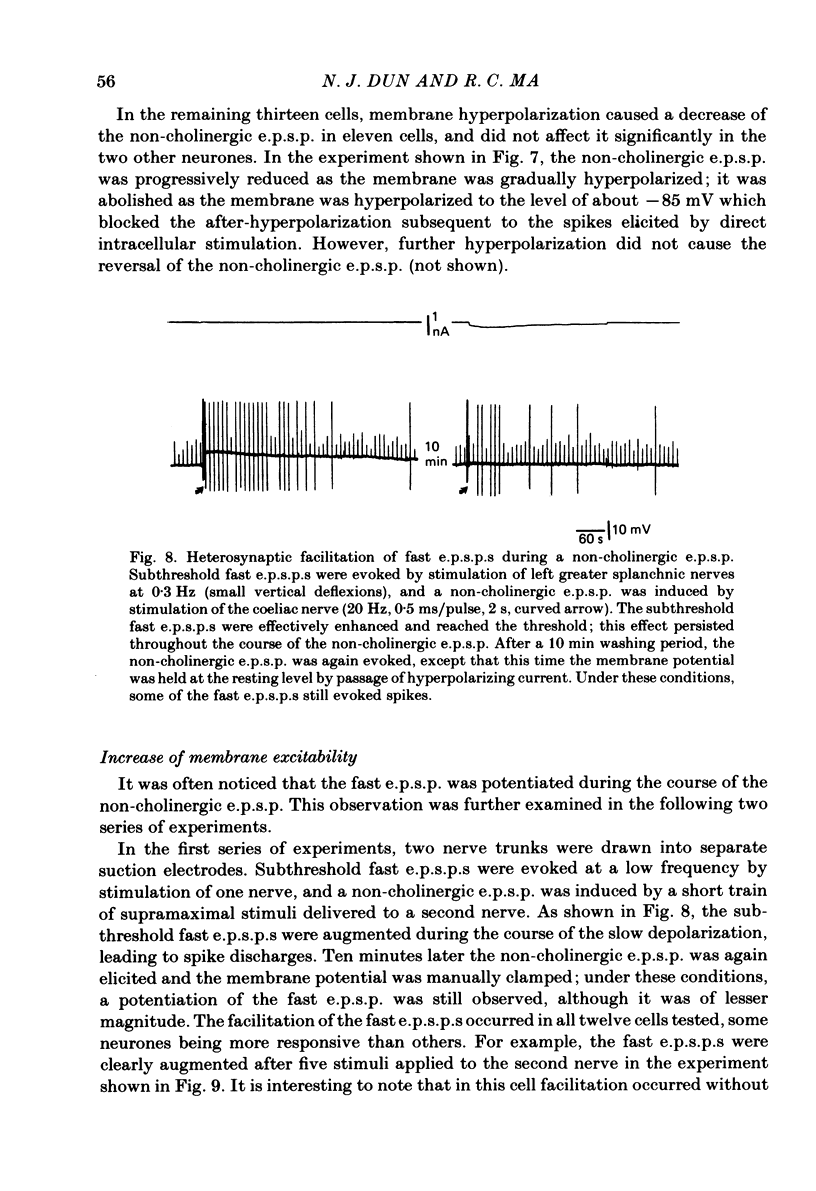
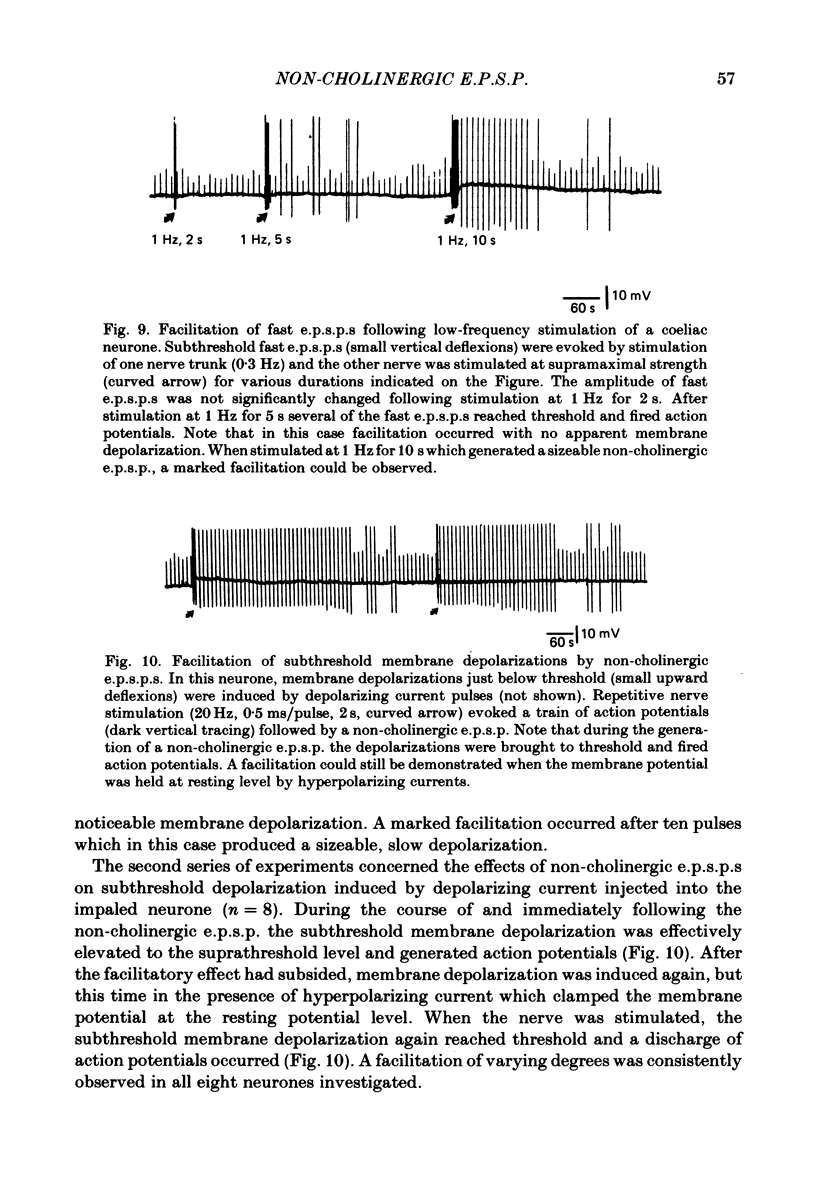
Intracellular recordings were made from neurones of the coeliac ganglia of the guinea-pig in vitro. In addition to the fast excitatory post-synaptic potential (e.p.s.p.) repetitive stimulation (10-20 Hz, 1-2 s) of the left greater splanchnic nerves elicited a slow depolarization in about 70% of the neurones examined. This depolarization lasted for minutes and was resistant to nicotinic and muscarinic antagonists; it was abolished reversibly in a low-Ca2+, high-Mg2+ solution. The response is henceforth termed non-cholinergic e.p.s.p. In about 10% of the neurones the non-cholinergic e.p.s.p. exhibited a biphasic pattern. The fast as well as the non-cholinergic e.p.s.p. could be effectively induced by stimulation of any of the several nerve trunks that enter the ganglion. Moreover, simultaneous stimulation of two separate nerves resulted in a much larger non-cholinergic e.p.s.p. than could be achieved by stimulation of a single nerve. When the membrane potential was manually clamped, the non-cholinergic e.p.s.p. was associated with an increase of membrane resistance in the large majority of cells tested. Membrane hyperpolarization generally caused an increase in the amplitude of the non-cholinergic e.p.s.p.; a decrease was observed in only a few cells. Subthreshold depolarizations induced by direct intracellular stimulation as well as fast e.p.s.p.s elicited by heterosynaptic nerve stimulation were facilitated during the course of a non-cholinergic e.p.s.p., often resulting in spike discharges. A potentiation of lesser magnitude occurred when the membrane potential was manually clamped during the course of the slow response, indicating that the facilitation may be attributed to both membrane depolarization and increased membrane resistance. These results indicate that the non-cholinergic e.p.s.p. constitutes an integral part of synaptic transmission in coeliac ganglia, and that its function may be to provide a mechanism for increasing the responsiveness of sympathetic neurones to incoming fast e.p.s.p.s.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol. 1982 Nov;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Elfvin L. G., Skirboll L., Emson P. Substance P-containing primary sensory neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience. 1982 Mar;7(3):647–654. doi: 10.1016/0306-4522(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Kiraly M. Capsaicin causes release of a substance P-like peptide in guinea-pig inferior mesenteric ganglia. J Physiol. 1983 Jul;340:107–120. doi: 10.1113/jphysiol.1983.sp014752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Kiraly M., Ma R. C. Evidence for a serotonin-mediated slow excitatory potential in the guinea-pig coeliac ganglia. J Physiol. 1984 Jun;351:61–76. doi: 10.1113/jphysiol.1984.sp015232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Dun N. J., Karczmar A. G. Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science. 1982 Aug 20;217(4561):739–741. doi: 10.1126/science.6179162. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Nishi S. Voltage-clamp analysis of peptidergic slow depolarizations in bullfrog sympathetic ganglion cells. J Physiol. 1982 Dec;333:305–313. doi: 10.1113/jphysiol.1982.sp014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L., Szurszewski J. H. Nerve pathways in celiac plexus of the guinea pig. Am J Physiol. 1979 Jul;237(1):E90–E97. doi: 10.1152/ajpendo.1979.237.1.E90. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. Substance P-immunoreactive peripheral branches of sensory neurons innervate guinea pig sympathetic neurons. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1668–1672. doi: 10.1073/pnas.79.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. Physiology of mammalian prevertebral ganglia. Annu Rev Physiol. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Konishi S., Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7(9):2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. Modulation of colonic motility by peripheral neural inputs to neurons of the inferior mesenteric ganglion. Gastroenterology. 1977 Aug;73(2):273–278. [PubMed] [Google Scholar]


