Abstract
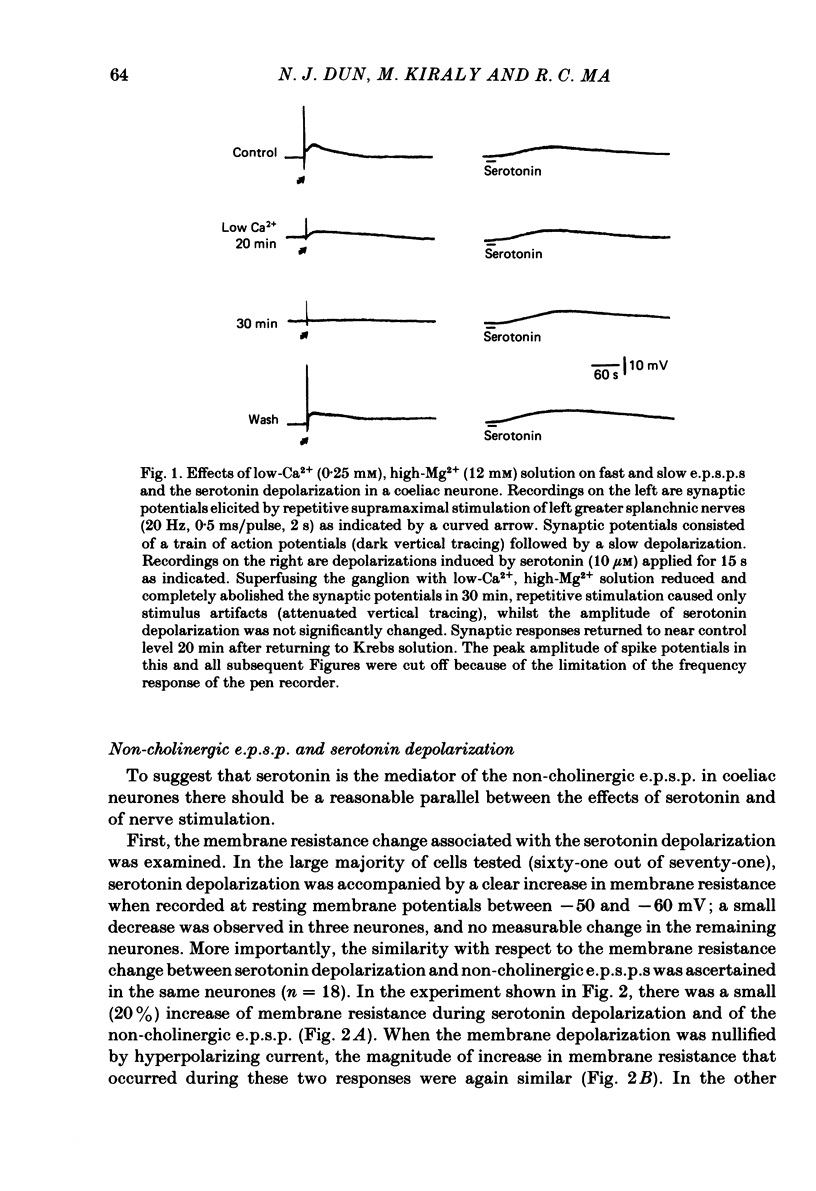
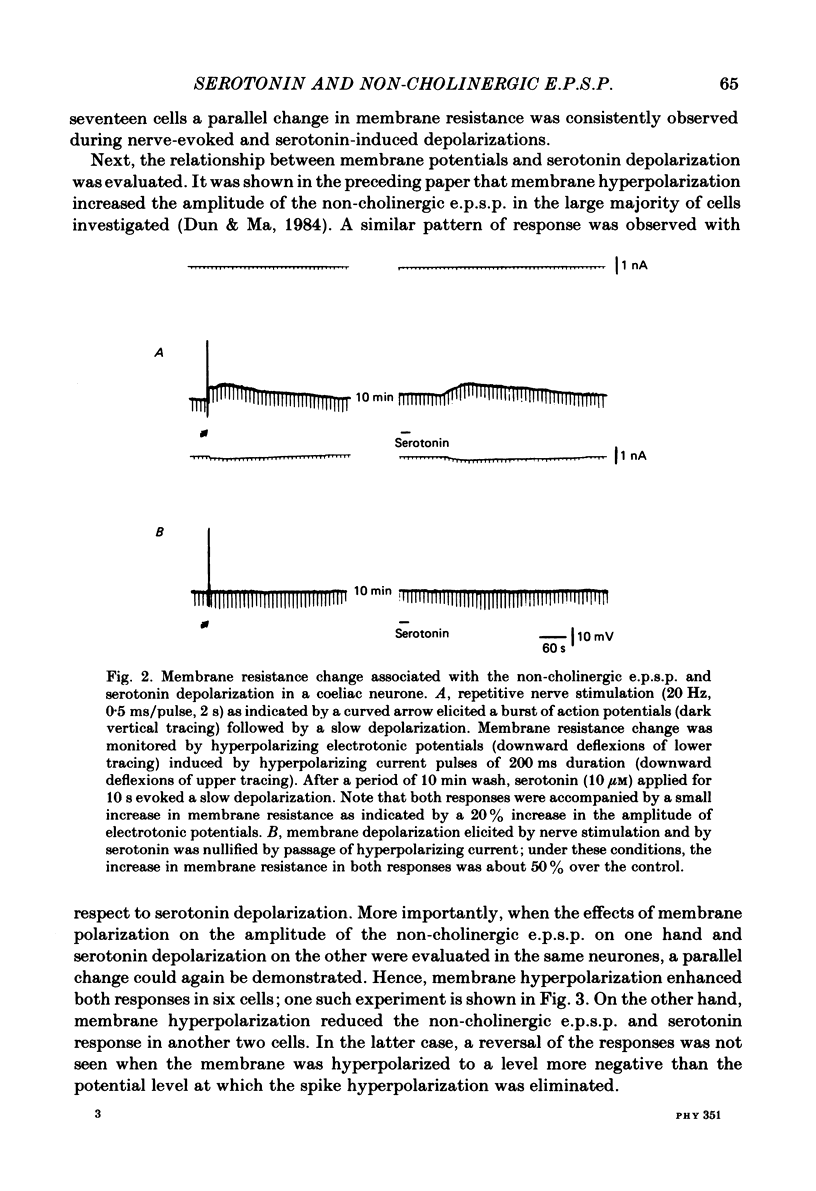
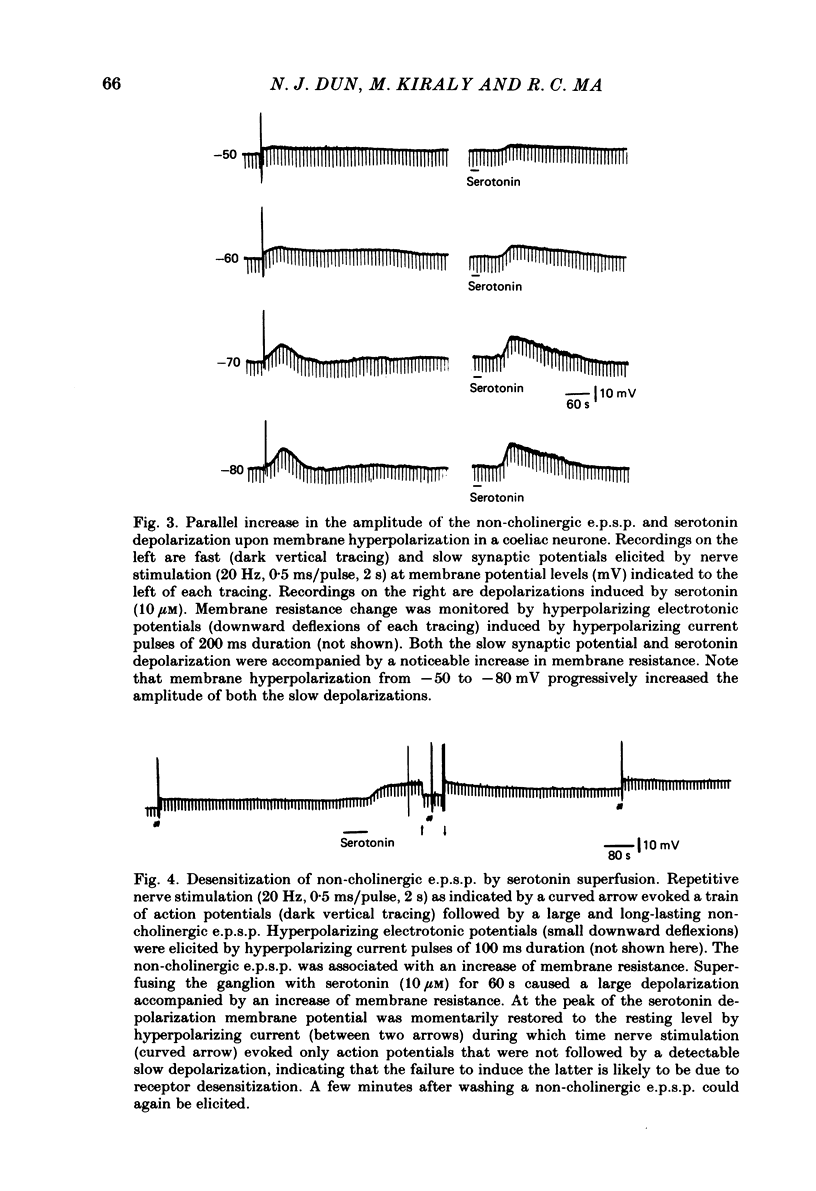
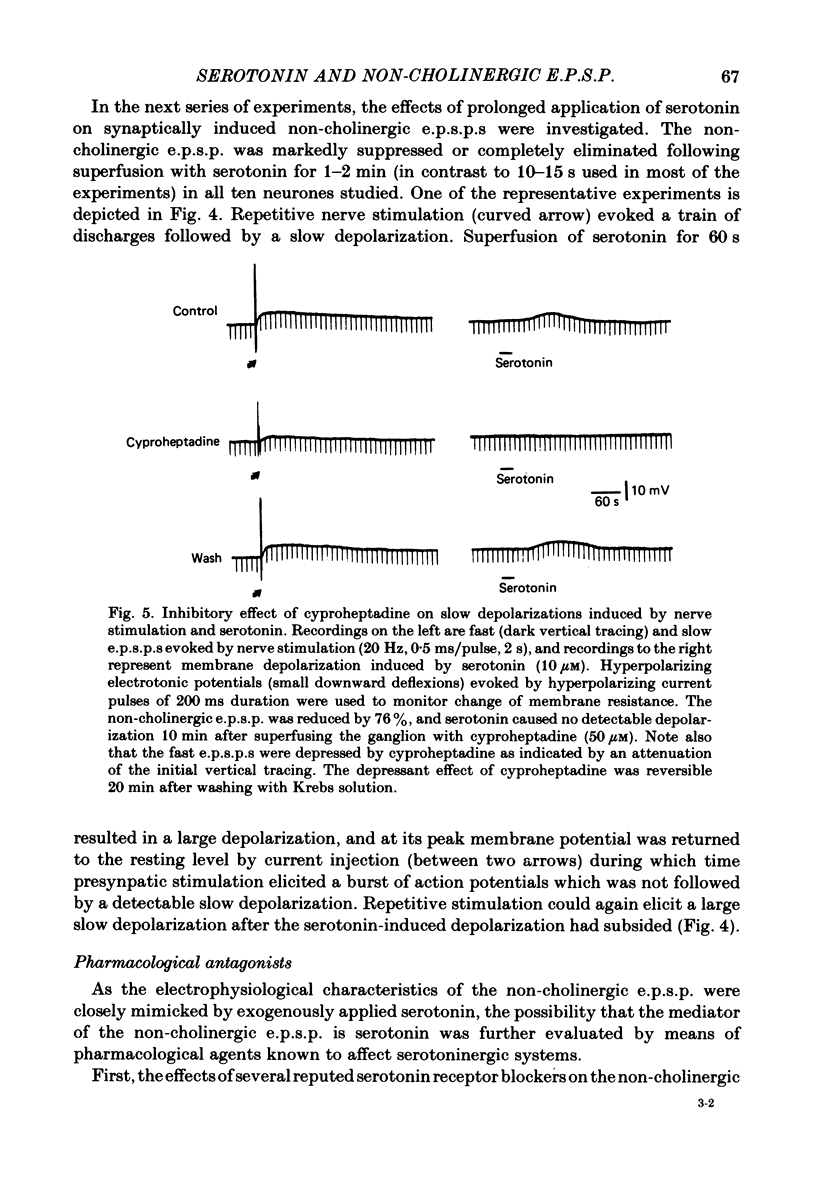
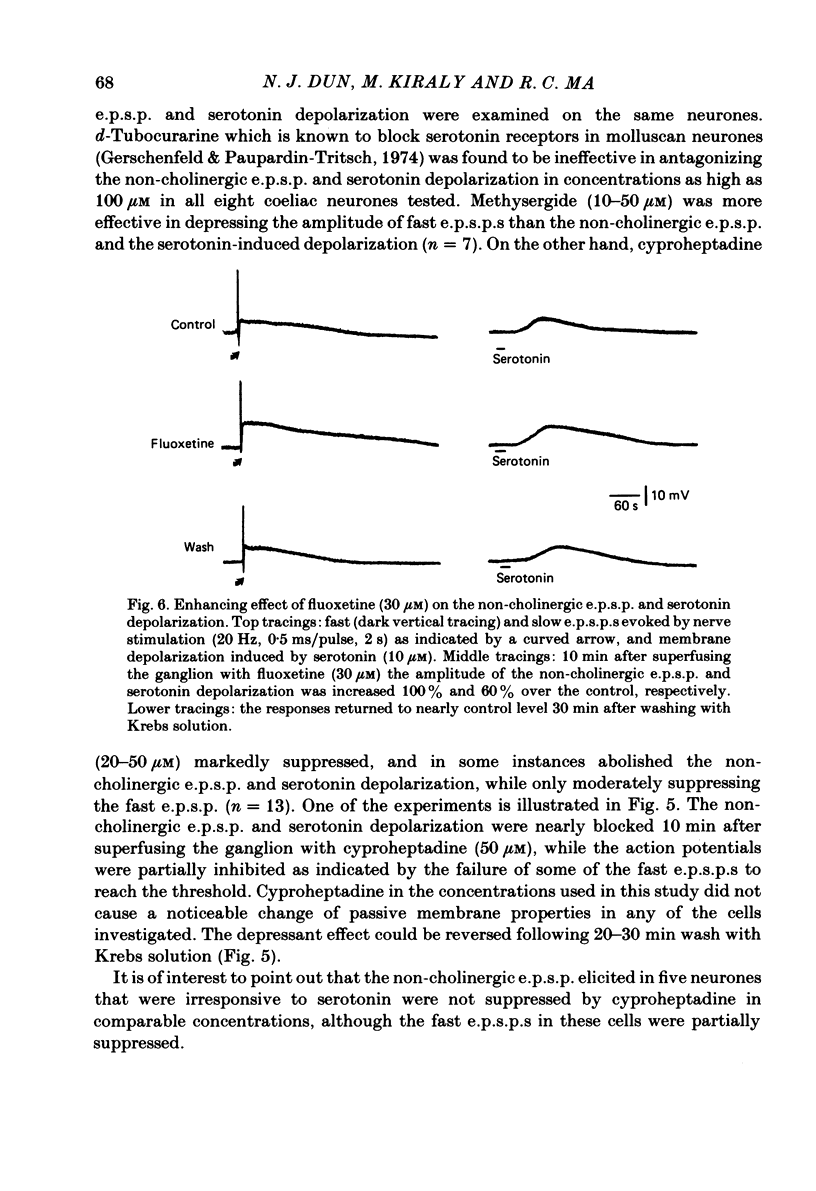
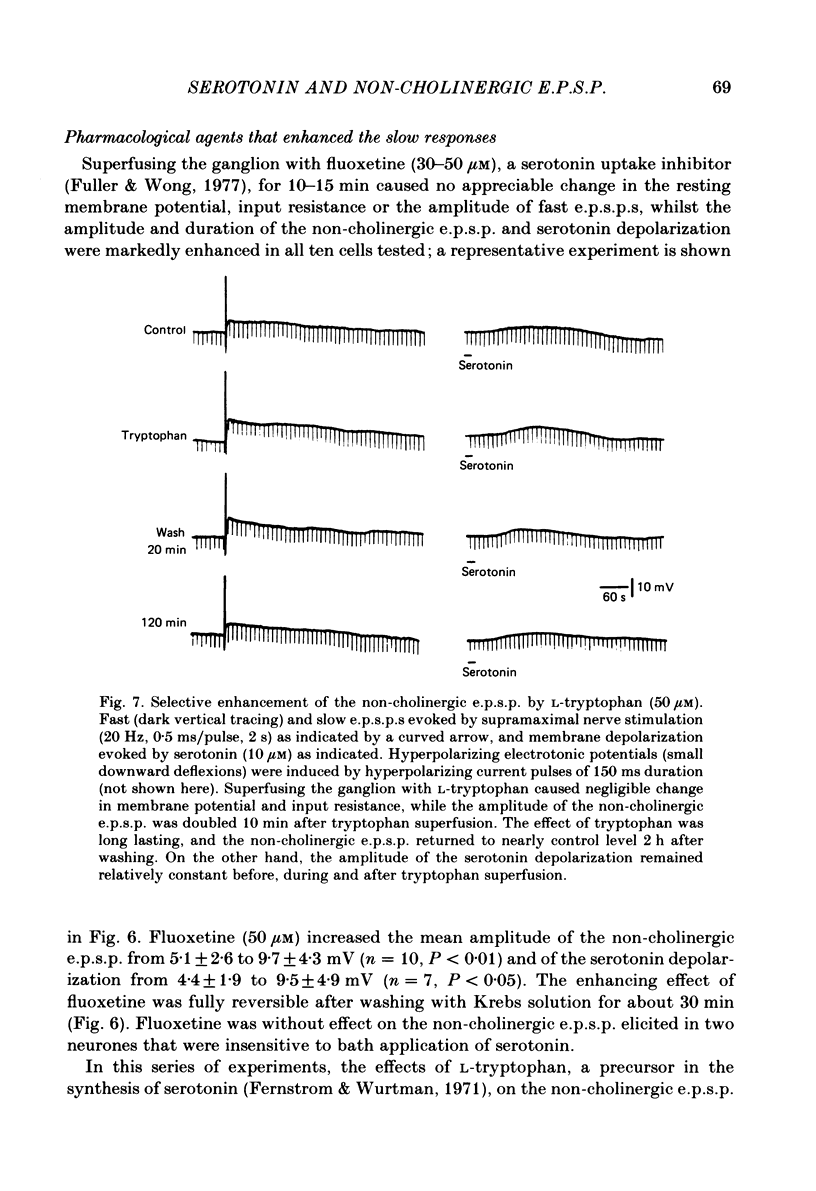
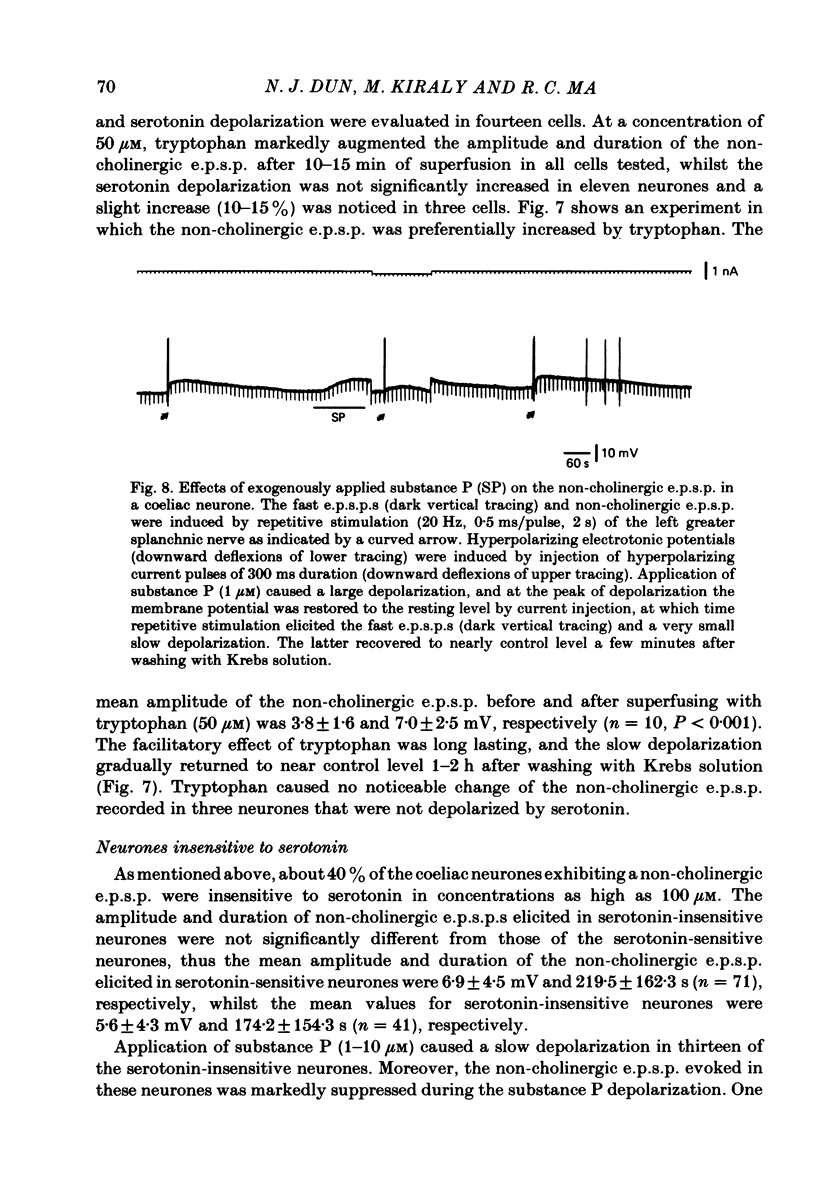
The nature of the putative transmitter(s) mediating the non-cholinergic excitatory post-synaptic potential (e.p.s.p.) described in the preceding paper was investigated by means of electrophysiological, pharmacological and immunohistochemical methods. Serotonin (1-10 microM) when applied by superfusion caused a slow depolarization that closely mimicked the synaptic response in about 60% of the coeliac neurones that exhibited a non-cholinergic e.p.s.p. The serotonin depolarization evoked in low-Ca2+, high-Mg2+ solution or in a Krebs solution containing cholinergic antagonists was quantitatively similar to that elicited in normal Krebs solution. When compared in the same neurones the membrane resistance change during the course of the serotonin depolarization and of the non-cholinergic e.p.s.p., as well as their respective responses to conditioning polarization, were similar. The non-cholinergic e.p.s.p. was reversibly abolished during serotonin-induced depolarization; the blockade persisted when the membrane potential was restored to the resting level by hyperpolarizing current. The serotonin depolarization as well as the non-cholinergic e.p.s.p. were reversibly suppressed by cyproheptadine (20-50 microM), a serotonin antagonist, and enhanced by fluoxetine (30-50 microM), a serotonin reuptake inhibitor. On the other hand, pre-treating the ganglia with L-tryptophan (50 microM), a precursor of serotonin, preferentially augmented the synaptically induced response. A portion of the neurones (15%) were depolarized by substance P (1 microM) which also reversibly desensitized the non-cholinergic e.p.s.p. elicited in these neurones. The remaining neurones (25%) were insensitive to either serotonin or substance P, and the non-cholinergic e.p.s.p.s elicited in these cells were likewise not appreciably affected by these two agents. Furthermore, cyproheptadine, fluoxetine and L-tryptophan had no significant effect on the non-cholinergic e.p.s.p.s elicited in serotonin-insensitive neurones. Using the immunohistofluorescent techniques, dense but unevenly distributed serotonin immunoreactive nerve fibres could be observed surrounding many coeliac neurones. Immunoreactivity was not observed in the ganglia incubated with antisera pre-absorbed with excess serotonin. Collectively our results suggest that serotonin is the mediator of non-cholinergic e.p.s.p.s. elicited in about 60% of coeliac neurones sampled in this study, and that in the remaining neurones the slow depolarization may be generated by substance P and/or some unknown transmitter(s).
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Asher I. M. Histochemical fluorescence of raphe neurons: selective enhancement by tryptophan. Science. 1971 Jun 11;172(3988):1159–1161. doi: 10.1126/science.172.3988.1159. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Elfvin L. G. Structural studies on the connectivity of the inferior mesenteric ganglion of the guinea pig. J Auton Nerv Syst. 1982 May;5(3):265–278. doi: 10.1016/0165-1838(82)90070-4. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Bornstein M. B. Synthesis of serotonin by neurons of the myenteric plexus in situ and in organotypic tissue culture. Brain Res. 1977 Jun 3;128(1):125–139. doi: 10.1016/0006-8993(77)90240-2. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Actions of substance P on sympathetic neurons. Neuropharmacology. 1979 Feb;18(2):215–218. doi: 10.1016/0028-3908(79)90064-9. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Evidence for a presynaptic inhibitory action of 5-hydroxytryptamine in a mammalian sympathetic ganglion. J Pharmacol Exp Ther. 1981 Jun;217(3):714–718. [PubMed] [Google Scholar]
- Dun N. J., Kiraly M. Capsaicin causes release of a substance P-like peptide in guinea-pig inferior mesenteric ganglia. J Physiol. 1983 Jul;340:107–120. doi: 10.1113/jphysiol.1983.sp014752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Ma R. C. Slow non-cholinergic excitatory potentials in neurones of the guinea-pig coeliac ganglia. J Physiol. 1984 Jun;351:47–60. doi: 10.1113/jphysiol.1984.sp015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom J. D. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev. 1983 Apr;63(2):484–546. doi: 10.1152/physrev.1983.63.2.484. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971 Jul 9;173(3992):149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- Fuller R. W. Pharmacology of central serotonin neurons. Annu Rev Pharmacol Toxicol. 1980;20:111–127. doi: 10.1146/annurev.pa.20.040180.000551. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Wong D. T. Inhibition of serotonin reuptake. Fed Proc. 1977 Jul;36(8):2154–2158. [PubMed] [Google Scholar]
- GYERMEK L. 5-hydroxytryptamine antagonists. Pharmacol Rev. 1961 Sep;13:399–439. [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. J., Aghajanian G. K. Serotonin receptors in the brain. Fed Proc. 1977 Jul;36(8):2159–2164. [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Schultzberg M., Goldstein M., Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977 Aug 19;132(1):29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. G., Simmons M. A., Dun N. J. Enkephalinergic modulation of non-cholinergic transmission in mammalian prevertebral ganglia. Brain Res. 1982 Mar 4;235(1):185–191. doi: 10.1016/0006-8993(82)90211-6. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Dun N. J., Karczmar A. G. Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science. 1982 Aug 20;217(4561):739–741. doi: 10.1126/science.6179162. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Konishi S., Tsunoo A., Otsuka M. Enkephalins presynaptically inhibit cholinergic transmission in sympathetic ganglia. Nature. 1979 Nov 29;282(5738):515–516. doi: 10.1038/282515a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors and their physiological significance. Fed Proc. 1983 Feb;42(2):213–217. [PubMed] [Google Scholar]
- Schultzberg M. Bombesin-like immunoreactivity in sympathetic ganglia. Neuroscience. 1983;8(2):363–374. doi: 10.1016/0306-4522(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Terenius L., Elfvin L. G., Lundberg J. M., Brandt J., Elde R. P., Goldstein M. Enkephalin immunoreactive nerve fibres and cell bodies in sympathetic ganglia of the guinea-pig and rat. Neuroscience. 1979;4(2):249–270. doi: 10.1016/0306-4522(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]



