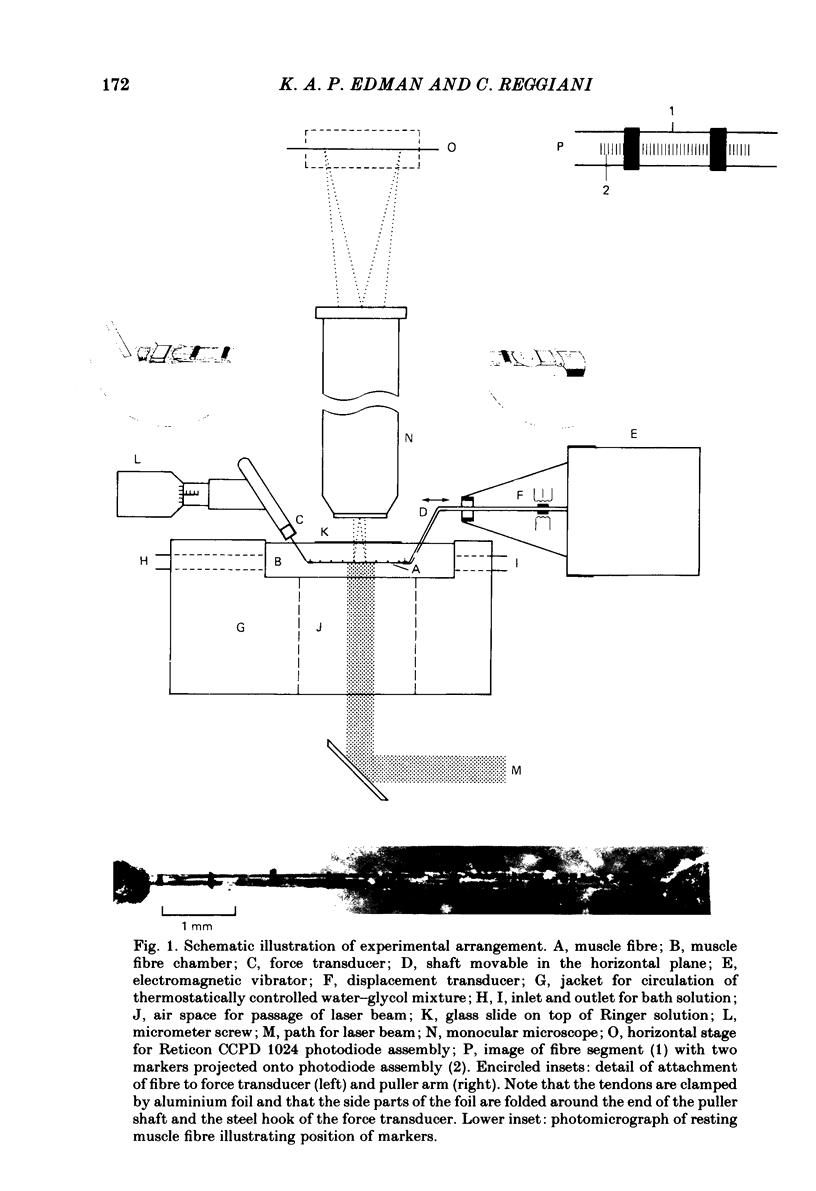
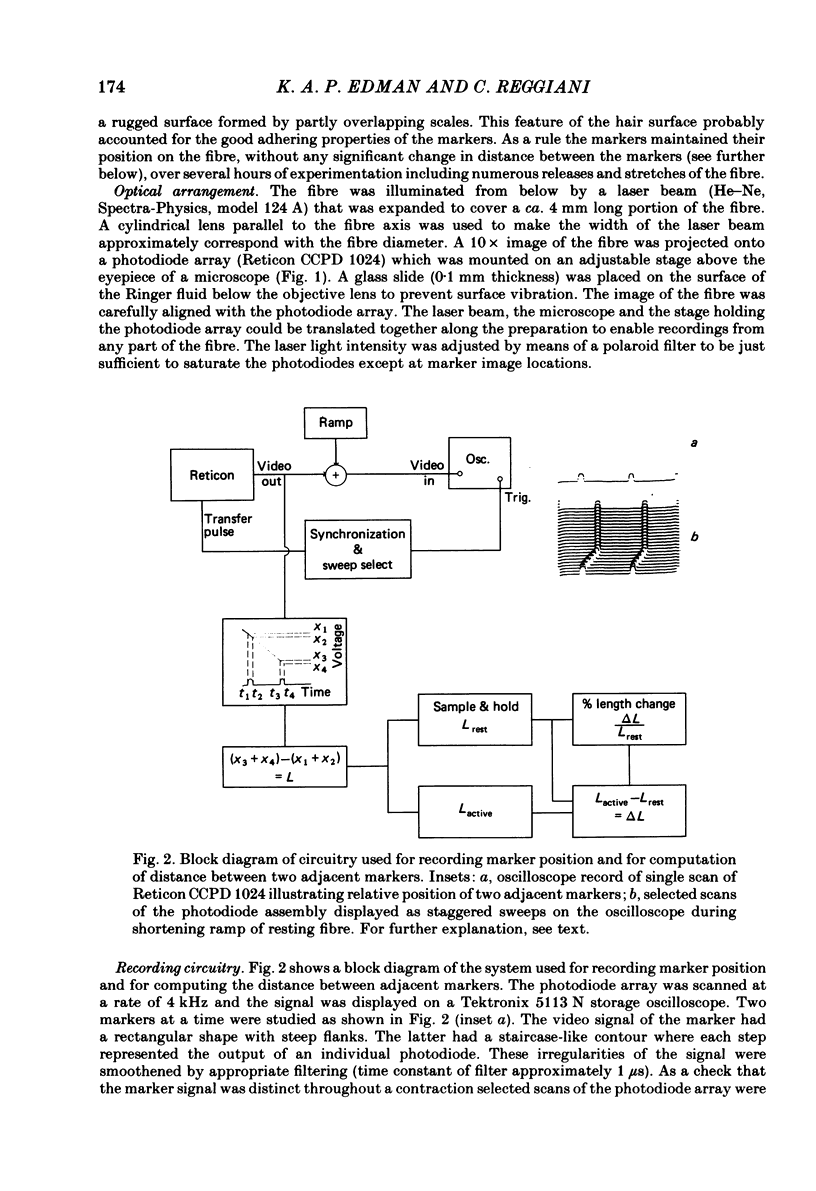
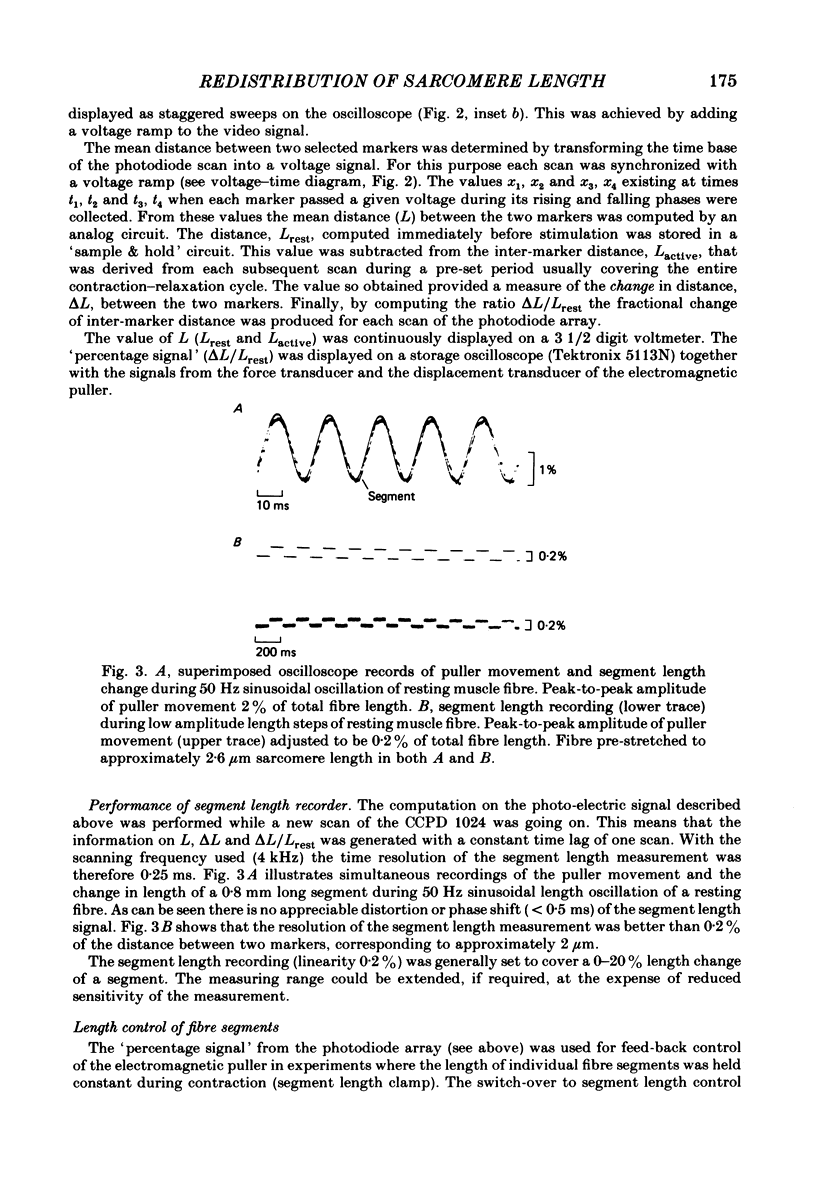
Abstract
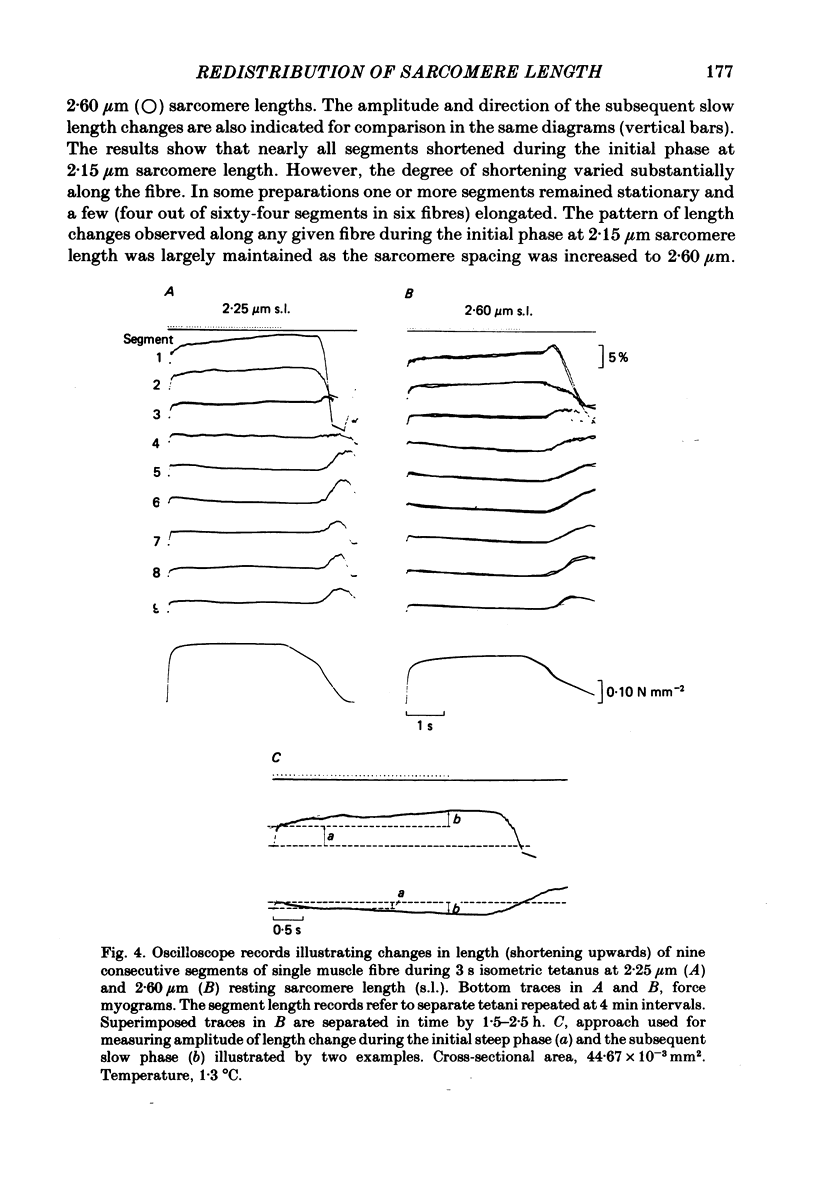
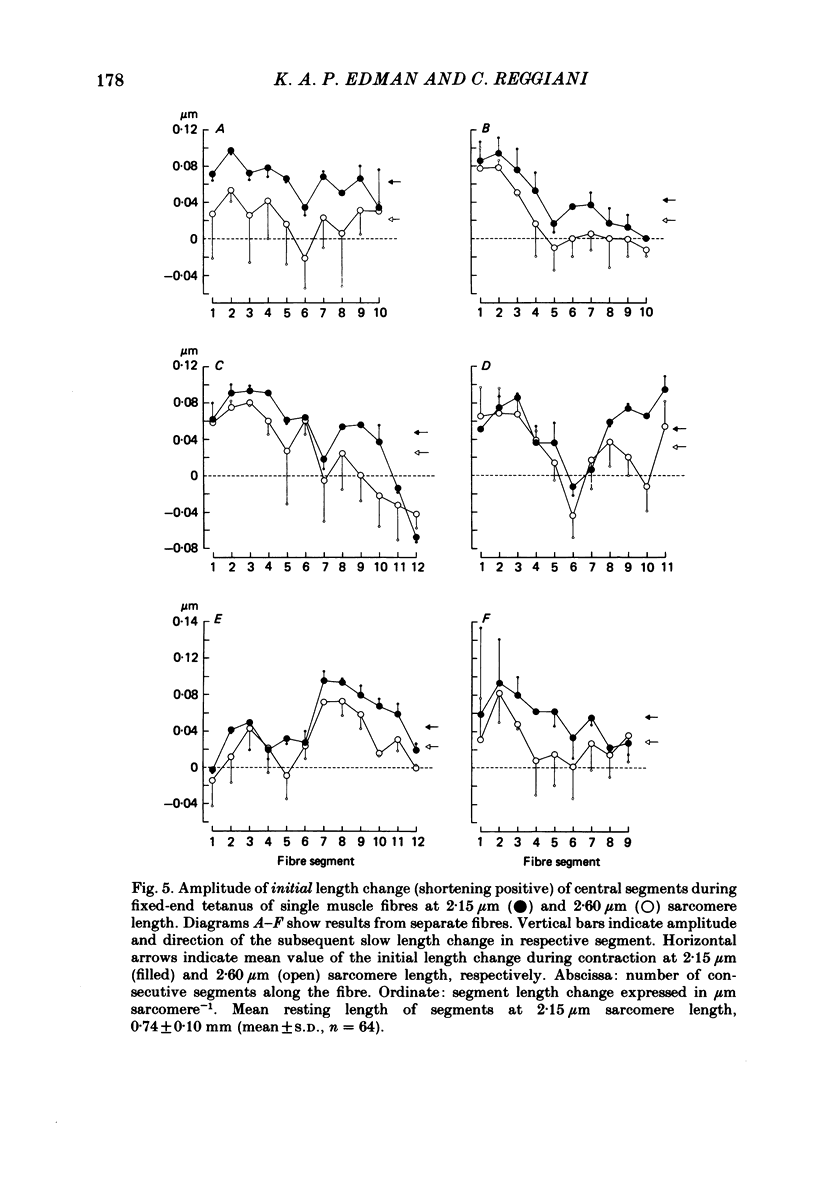
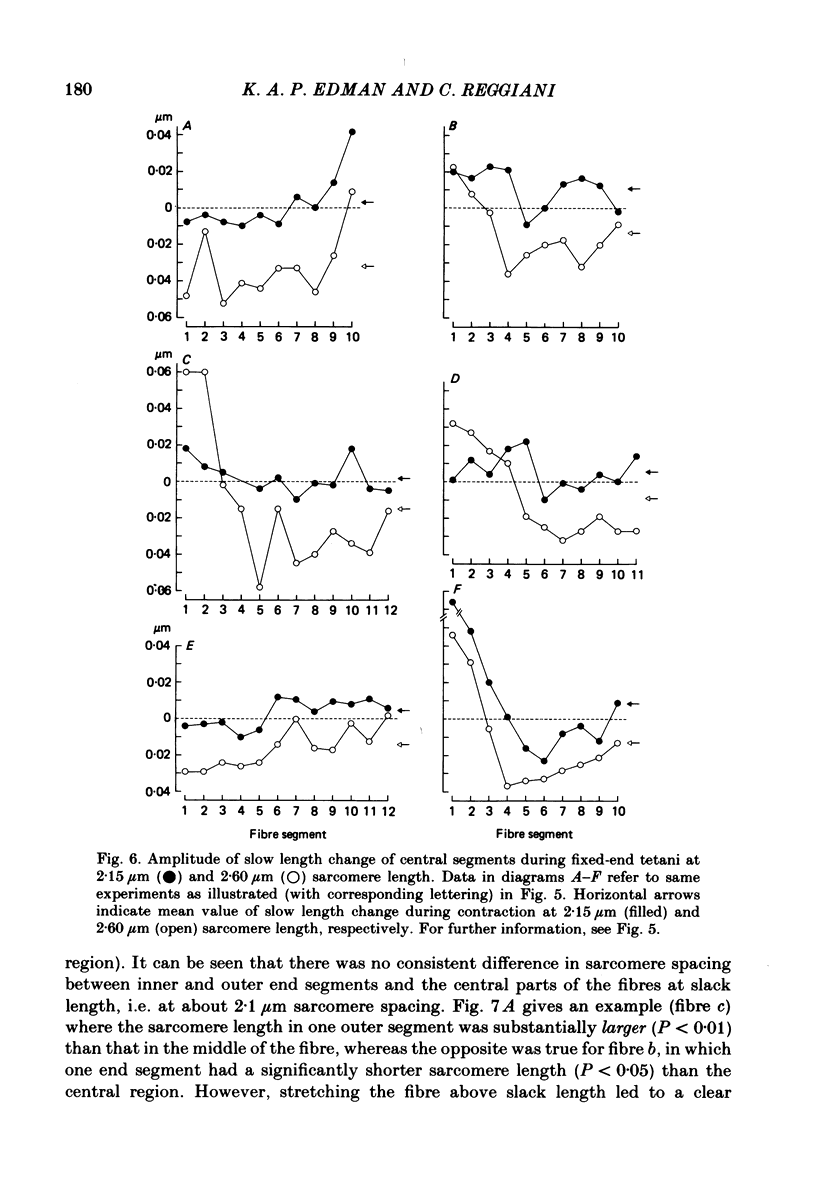
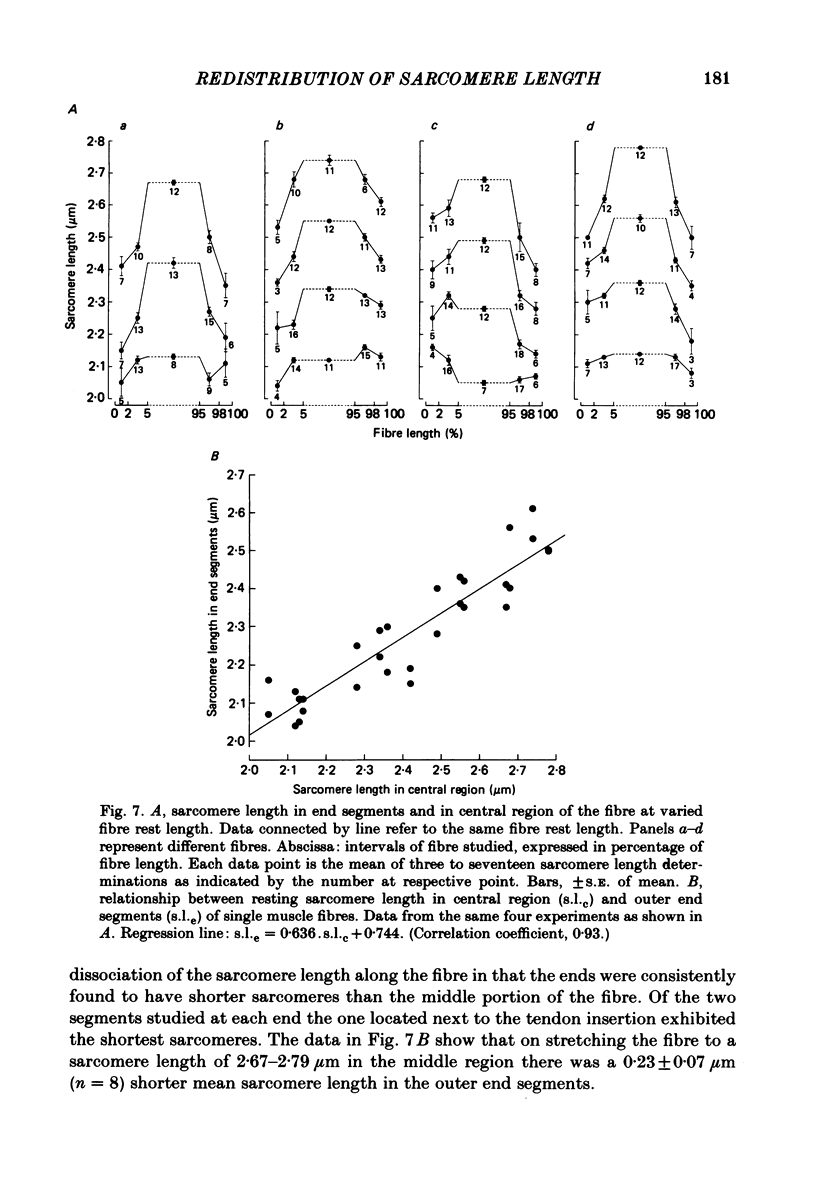
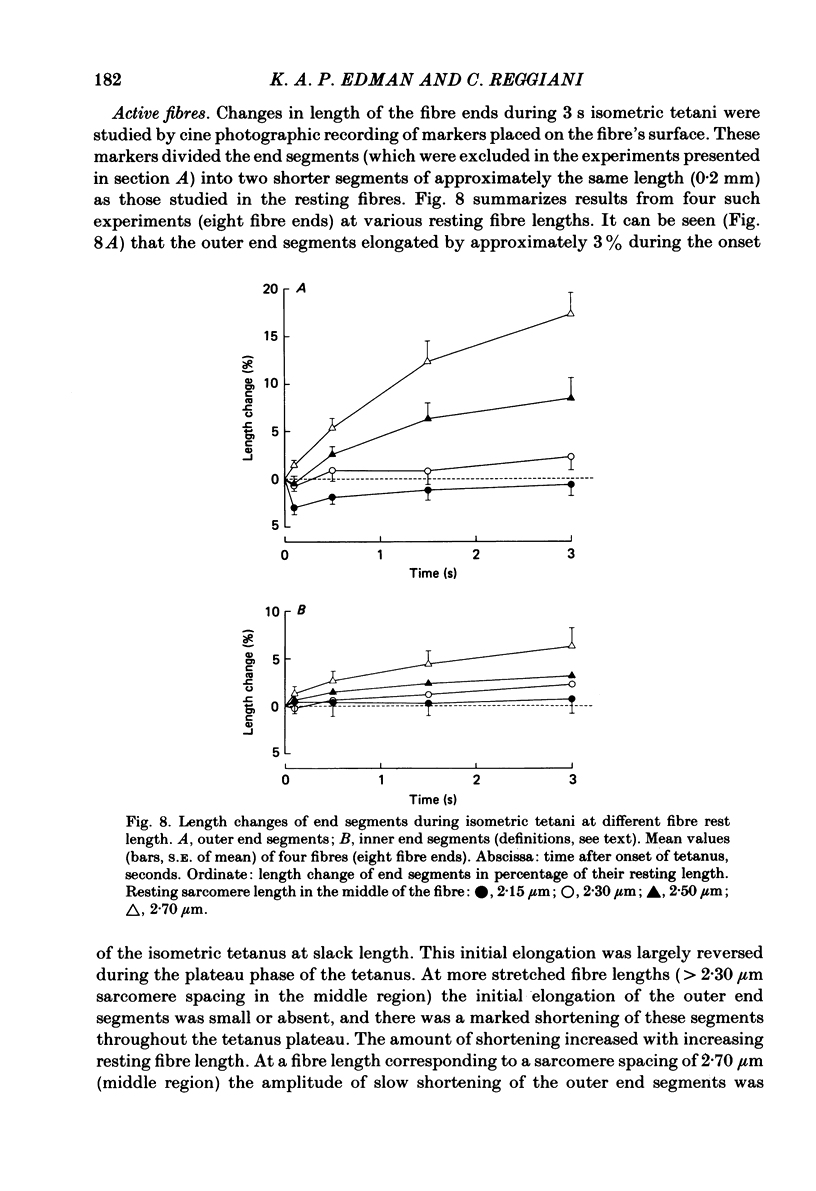
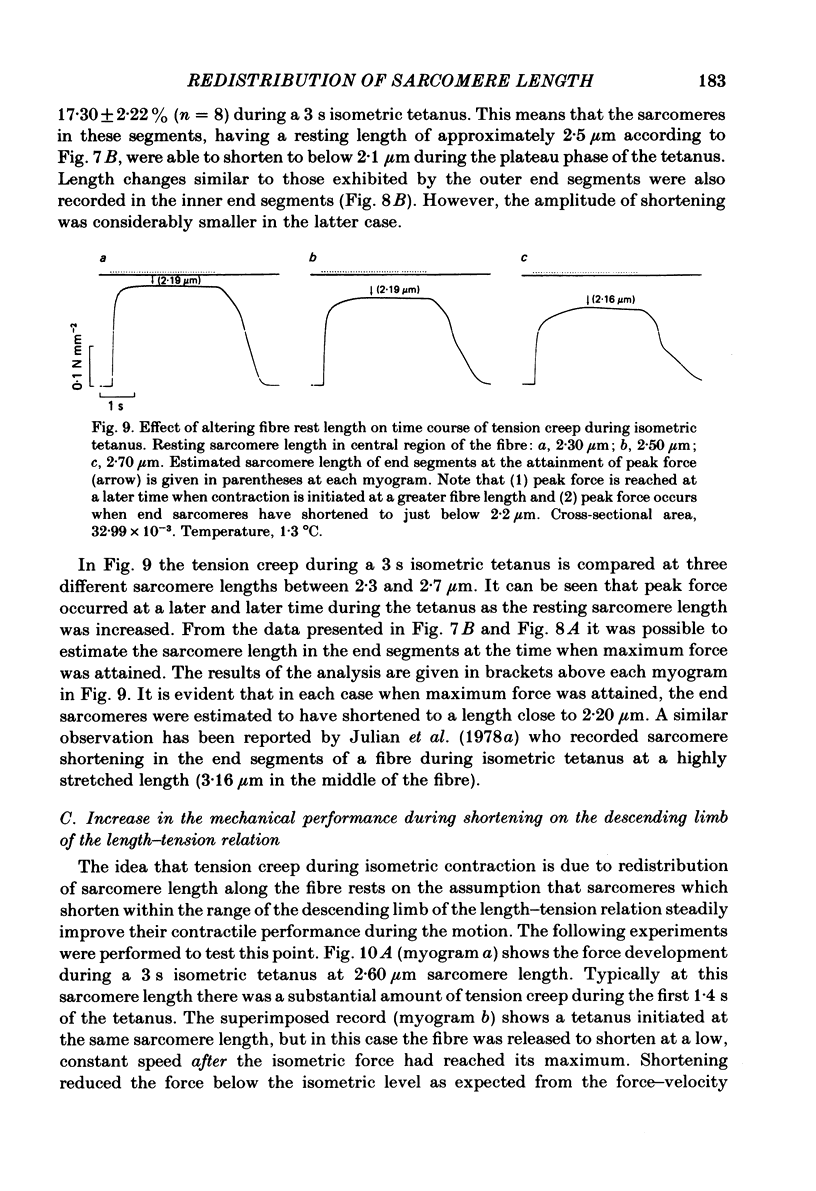
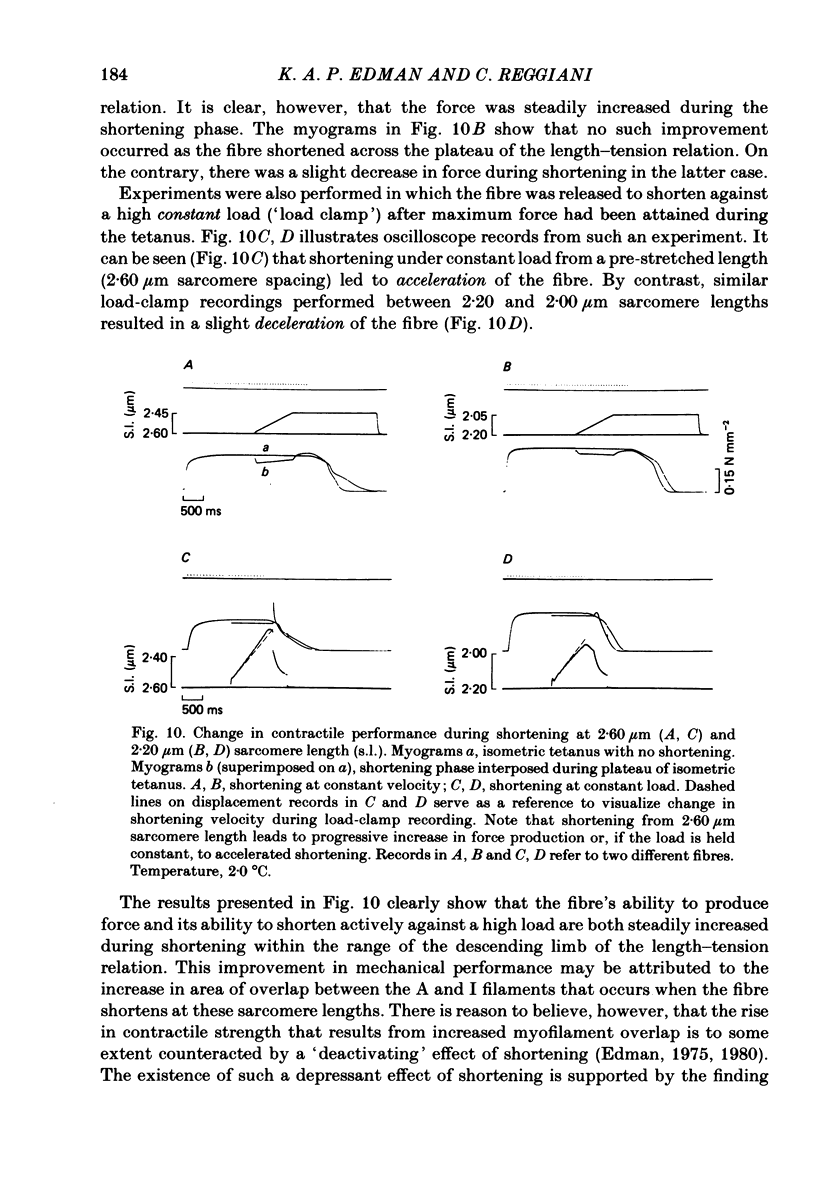
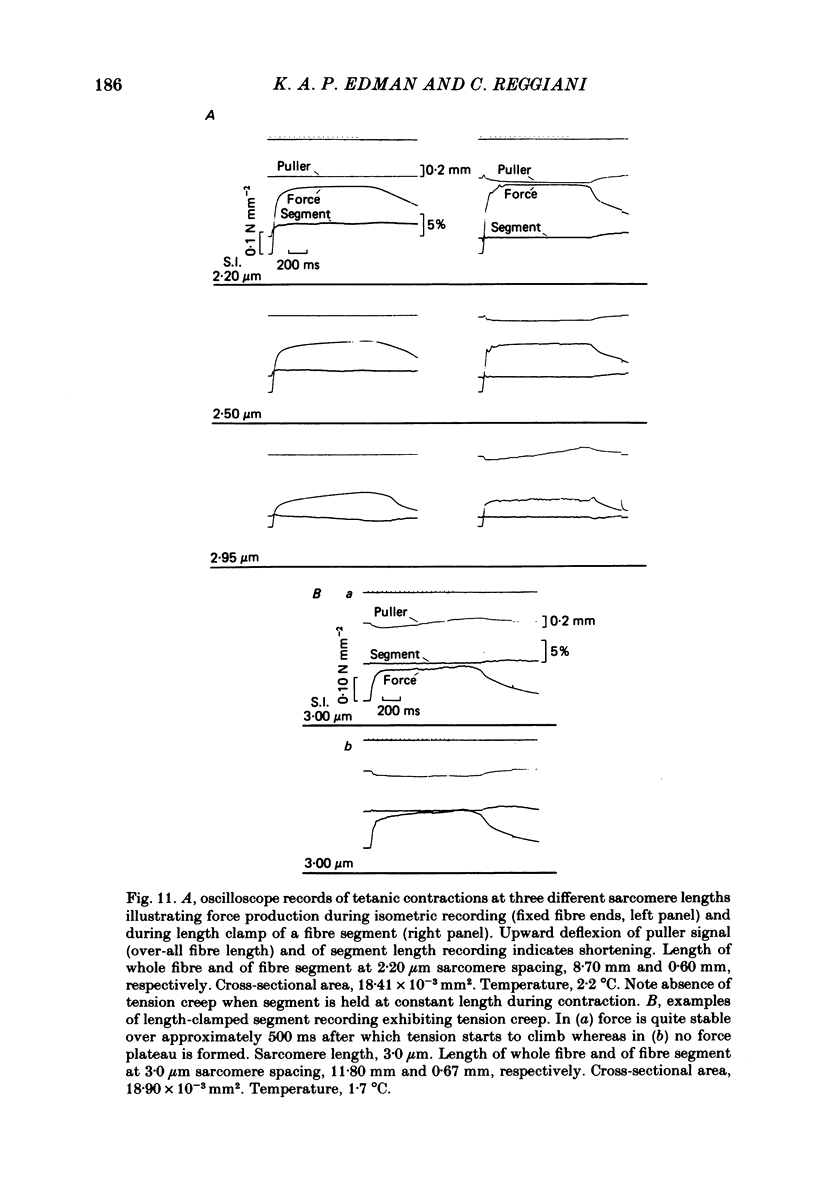
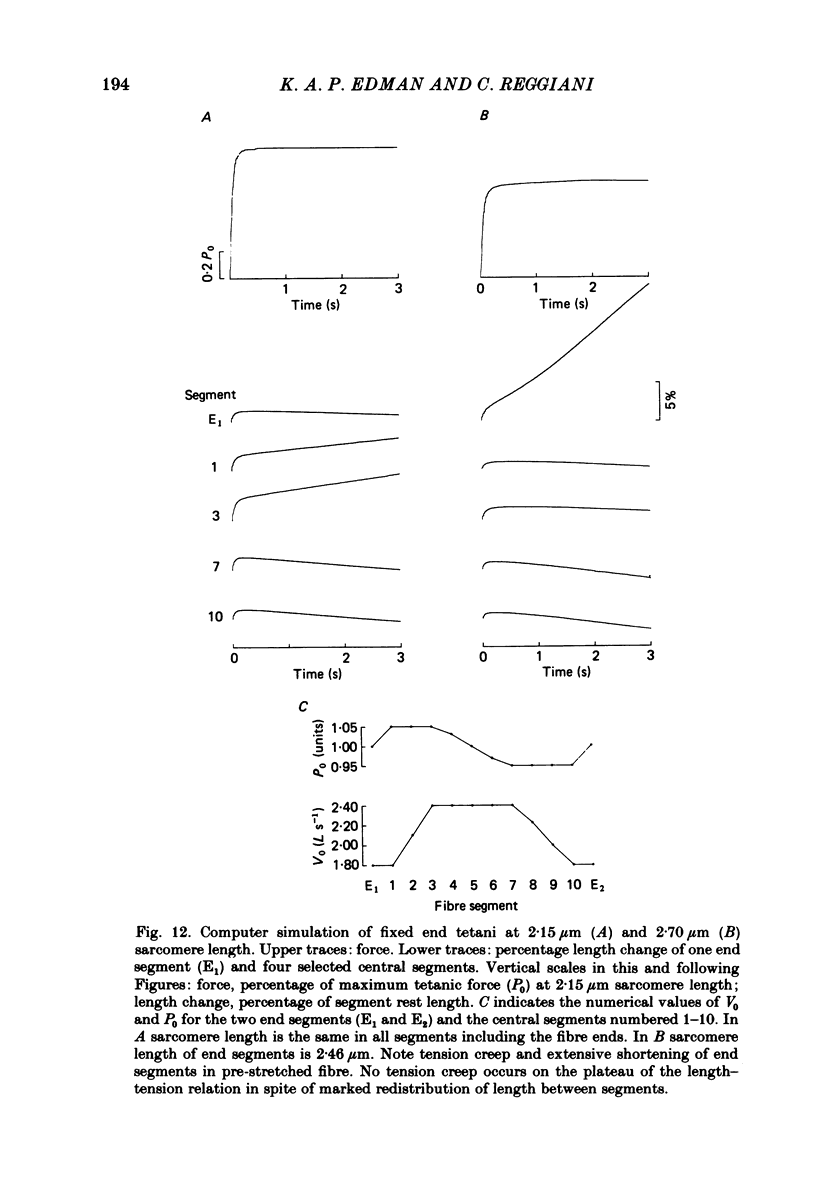
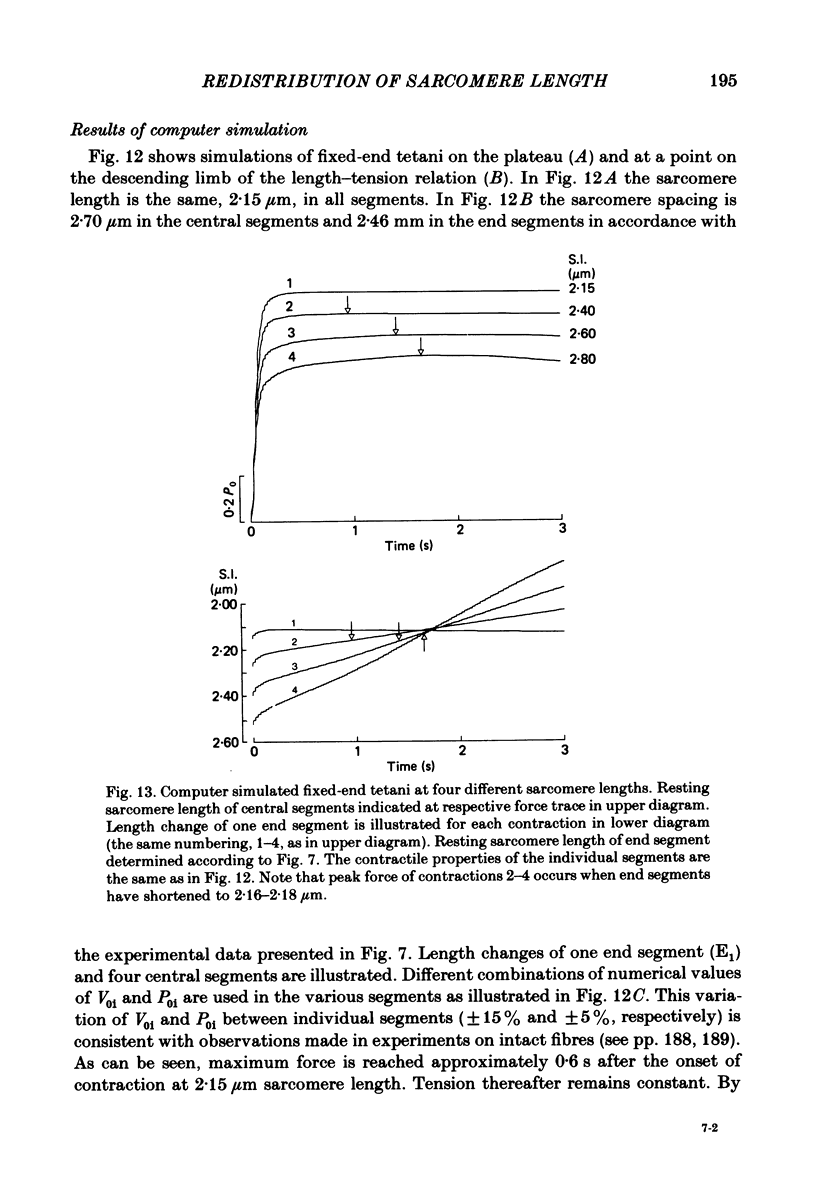
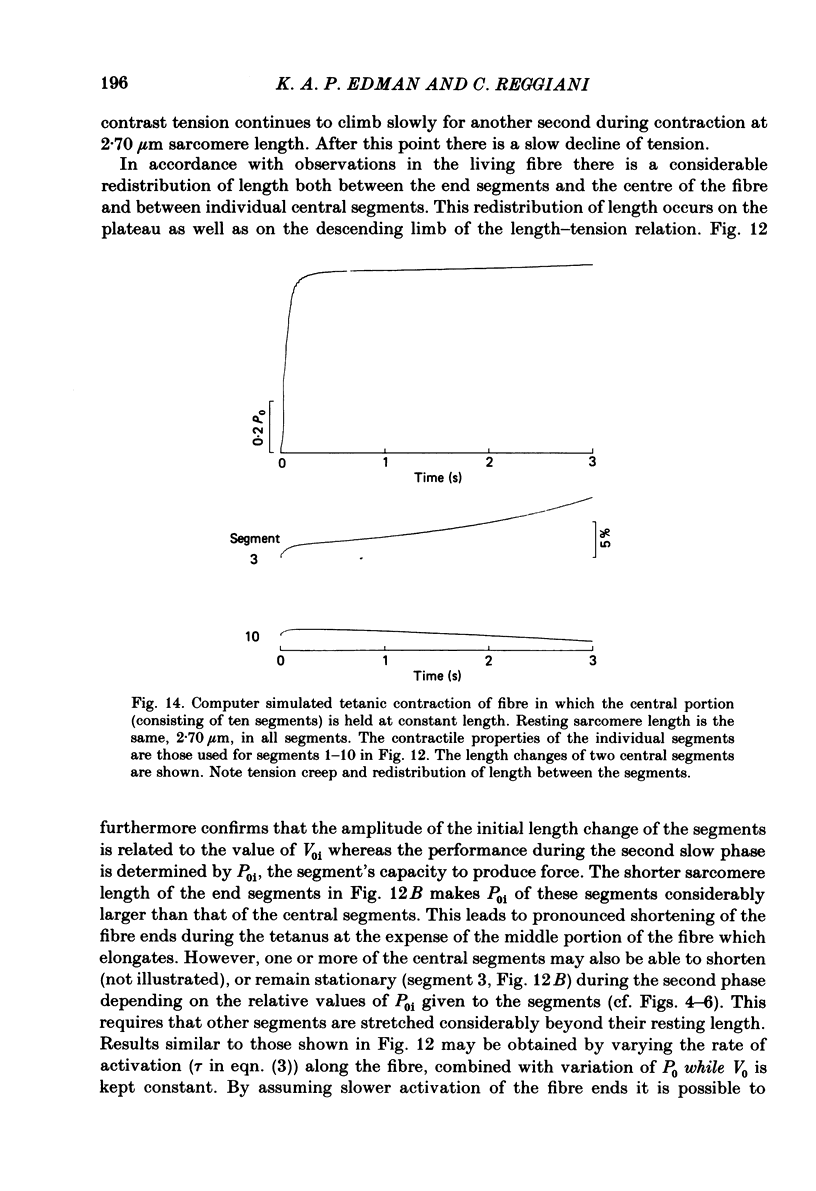
Changes in length of successive 0.5-0.8 mm segments along single muscle fibres of Rana temporaria were recorded during 3 s isometric (fixed fibre ends) tetani at 2.15 and 2.60 micron sarcomere length. The measurements were performed by means of a photo-electric detector system which recorded the distance between opaque markers (ca. 60 microns in width) that were attached to the upper surface of the fibre. The segment length change had an initial rapid phase (1) which coincided with the steep rise of force and a subsequent slow phase (2) which coincided with the upper, rounded portion of the force myogram and the 'plateau' of the tetanus. At 2.15 micron sarcomere length the majority of the central segments (comprising approximately 90% of the fibre) shortened to various degrees during phase 1. A considerable redistribution of length occurred during phase 2 in that some segments shortened at the expense of others which were forcibly stretched. The central region, taken as a whole, shortened by 0.1-0.5% during phase 2. The end segments were consistently found to elongate during phase 1. However, they were able to hold the tension, without further elongation, during phase 2. The pattern of length changes within the central region of the fibre observed at 2.15 micron sarcomere spacing remained largely the same after increasing the sarcomere length to 2.60 micron. However, in contrast to the situation at 2.15 micron sarcomere length there was an over-all (0.4-1.5%) elongation of the central region of the fibre during phase 2 at the great fibre length. This elongation of the central region was associated with marked shortening of the end segments. The sarcomere length of the end segments (s.1.e) was compared to that of the central region of the fibre (s.l.c) at various fibre rest lengths. There was no significant difference between s.l.e and s.l.c when the fibre was just taut, i.e. at approximately 2.1 micron sarcomere length. The following relationship between s.l.e and s.l.c was found to apply for values of s.l.c ranging between 2.2 and 2.7 micron: s.l.e = 0.636 s.l.c + 0.744 (correlation coefficient, 0.93). The possibility was explored that redistribution of sarcomere length along the fibre causes the slow climb of force ('tension creep') that occurs during a tetanus at great (greater than 2.2 micron) sarcomere lengths. Tension creep could be reproduced, after peak force had been attained, during an isometric tetanus by releasing the fibre to shorten within the range 2.6-2.3 micron sarcomere length.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., AUBERT X. M. The force exerted by active striated muscle during and after change of length. J Physiol. 1952 May;117(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. M., Hill L. Mercuric chloride in alcohol and chloroform used as a rapidly acting fixative for contracting muscle fibres. J Microsc. 1982 Mar;125(Pt 3):319–336. doi: 10.1111/j.1365-2818.1982.tb00348.x. [DOI] [PubMed] [Google Scholar]
- CARLSEN F., KNAPPEIS G. G., BUCHTHAL F. Ultrastructure of the resting and contracted striated muscle fiber at different degrees of stretch. J Biophys Biochem Cytol. 1961 Oct;11:95–117. doi: 10.1083/jcb.11.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna G. A., Citterio G. Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol. 1974 May;239(1):1–14. doi: 10.1113/jphysiol.1974.sp010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEZE J. B. The mechanical properties of the semitendinosus muscle at lengths greater than its length in the body. J Physiol. 1961 Sep;158:154–164. [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Depression of mechanical performance by active shortening during twitch and tetanus of vertebrate muscle fibres. Acta Physiol Scand. 1980 May;109(1):15–26. doi: 10.1111/j.1748-1716.1980.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. J Gen Physiol. 1981 Oct;78(4):365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol. 1978 Aug;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Cross-bridge detachment and sarcomere 'give' during stretch of active frog's muscle. J Physiol. 1978 Mar;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Sollins M. R. The mechanism for vertebrate striated muscle contraction. Circ Res. 1978 Jan;42(1):2–14. doi: 10.1161/01.res.42.1.2. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R., Moss R. L. Sarcomere length non-uniformity in relation to tetanic responses of stretched skeletal muscle fibres. Proc R Soc Lond B Biol Sci. 1978 Jan 24;200(1138):109–116. doi: 10.1098/rspb.1978.0009. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Mochon S., Julian F. J. A quantitative model of intersarcomere dynamics during fixed-end contractions of single frog muscle fibers. Biophys J. 1982 Aug;39(2):189–196. doi: 10.1016/S0006-3495(82)84507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H. The cross-bridge theory. Physiol Rev. 1983 Jul;63(3):1049–1113. doi: 10.1152/physrev.1983.63.3.1049. [DOI] [PubMed] [Google Scholar]
- Sugi H., Tsuchiya T. Enhancement of mechanical performance in frog muscle fibres after quick increases in load. J Physiol. 1981;319:239–252. doi: 10.1113/jphysiol.1981.sp013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H. E., Iwazumi T., Pollack G. H. The sarcomere length-tension relation in skeletal muscle. J Gen Physiol. 1978 Oct;72(4):565–592. doi: 10.1085/jgp.72.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]