Abstract
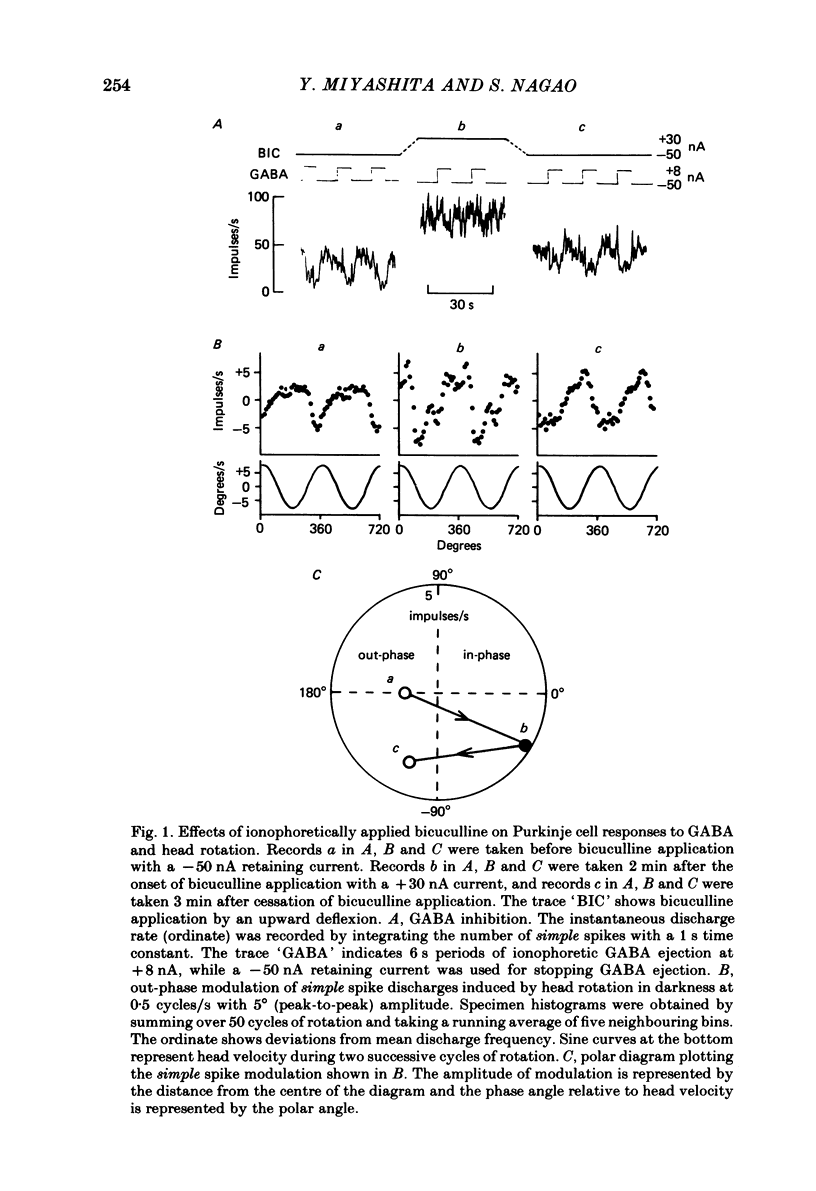
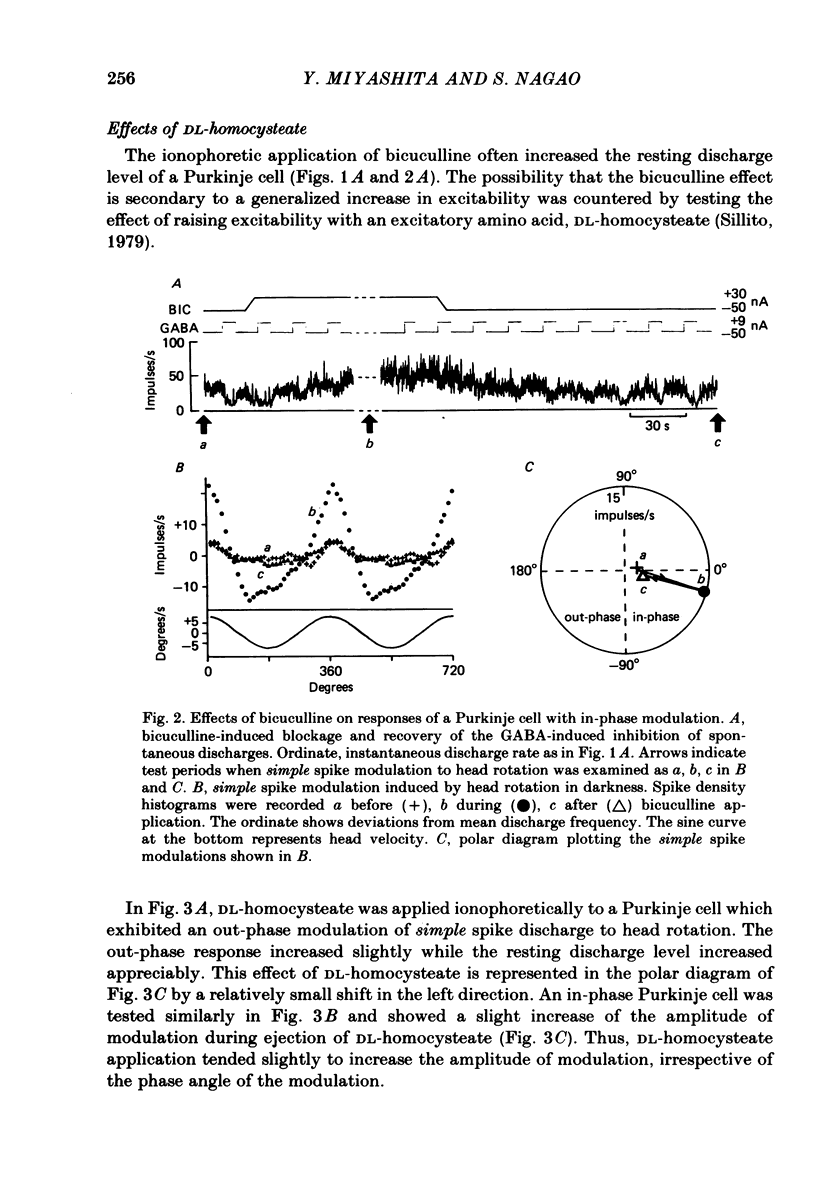
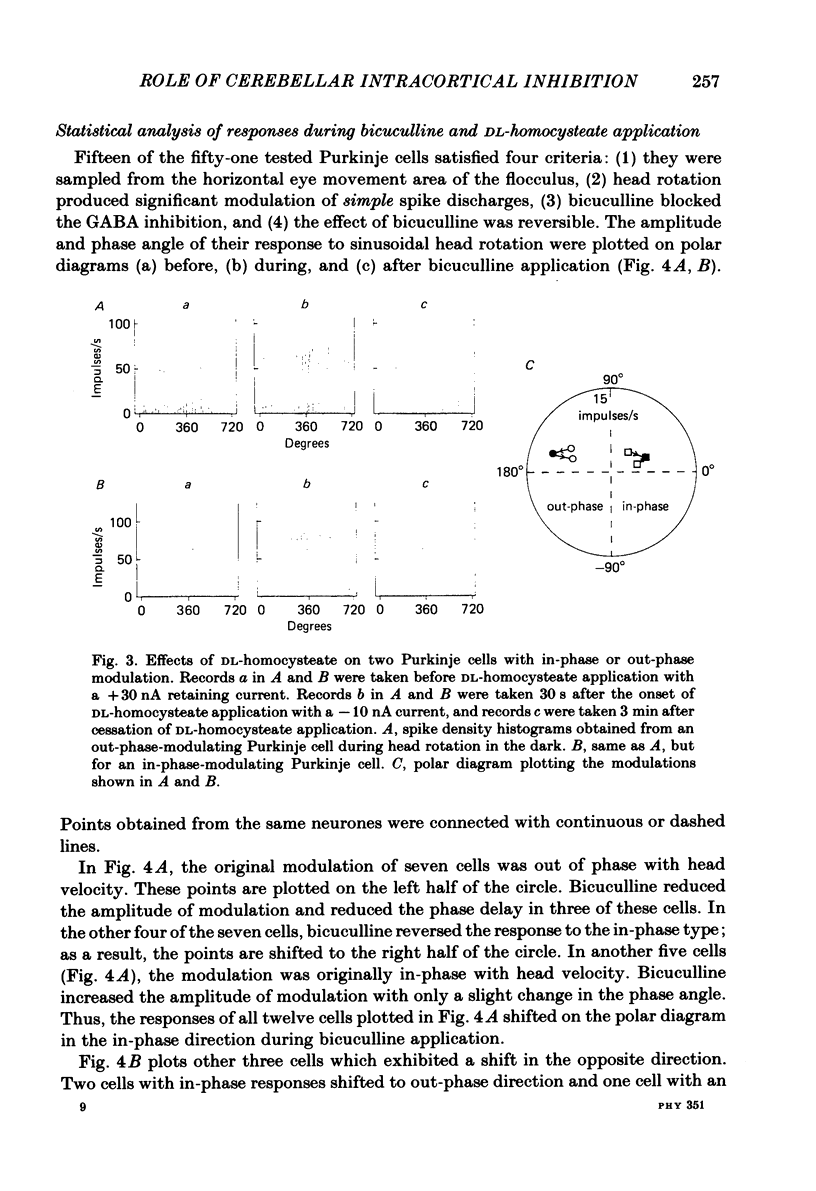
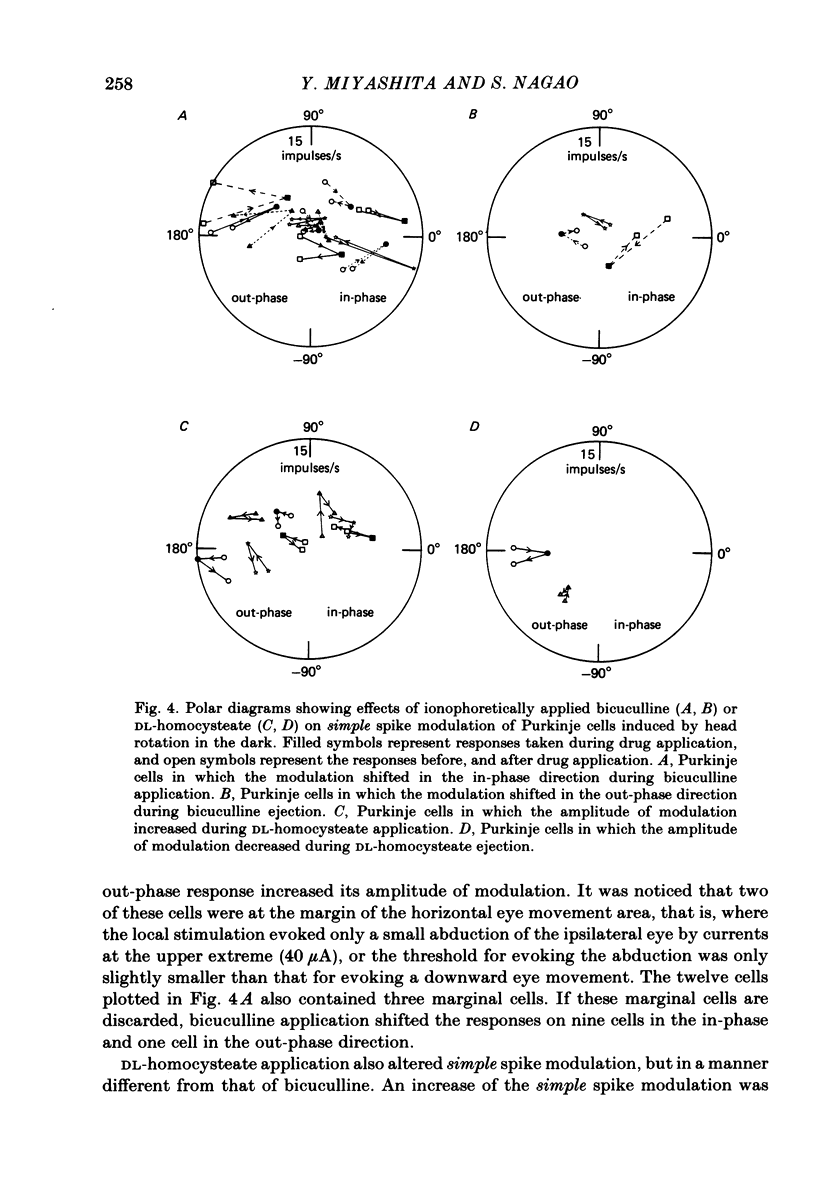
Ionophoretic application of bicuculline, an antagonist of gamma-aminobutyric acid (GABA), was used to examine the contribution of intracortical inhibition to vestibular responses of Purkinje cells in the cerebellar flocculus of alert rabbits. Purkinje cells were sampled extracellularly (with triple-barrelled micropipettes) from the floccular area where electrical stimulation through the micro-electrode evoked abduction of the ipsilateral eye, indicating its close functional relationship to the horizontal vestibulo-ocular reflex. These cells exhibited frequency modulation of simple spike discharges in-phase or out-phase with sinusoidal head rotation (0.5 cycles/s, 5 degrees peak-to-peak) in the horizontal plane. Bicuculline was ejected ionophoretically through one barrel with a 20-60 nA current. The pharmacological effectiveness of the ejected bicuculline was confirmed for each Purkinje cell by its blocking action upon the depressant action of GABA applied ionophoretically through another barrel. Bicuculline usually shifted the simple spike modulation in the in-phase direction: it reduced the amplitude of out-phase modulation in three cells, converted out-phase modulation to the in-phase type in four cells, and increased in-phase modulation in five cells. In three other cells, however, bicuculline shifted the modulation in the out-phase direction. Because bicuculline application usually increased the resting discharge level of a Purkinje cell, ionophoretic application of DL-homocysteate was used in ten Purkinje cells to control for the effect of a generalized increase in excitability. In contrast to bicuculline, DL-homocysteate generally induced a slight increase of the simple spike modulation regardless of the phase relationship. Since frequency modulation of the simple spike discharges of flocculus Purkinje cells is presumed to contribute to the control of vestibulo-ocular reflexes, these results point to an important functional role of intracortical post-synaptic inhibition in the cerebellar cortex.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R., Precht W., Llinás R. Cerebellar modulatory action on the vestibulo-trochlear pathway in the cat. Exp Brain Res. 1972;15(4):364–385. doi: 10.1007/BF00234124. [DOI] [PubMed] [Google Scholar]
- Batini C., Ito M., Kado R. T., Jastreboff P. J., Miyashita Y. Interaction between the horizontal vestibulo-ocular reflex and optokinetic response in rabbits. Exp Brain Res. 1979 Sep;37(1):1–15. doi: 10.1007/BF01474249. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971 Oct 8;33(1):57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D. The effect of bicuculline upon synaptic inhibition in the cerebral and cerebellar corticles of the cat. Brain Res. 1971 Nov;34(2):301–321. doi: 10.1016/0006-8993(71)90283-6. [DOI] [PubMed] [Google Scholar]
- Dufossé M., Ito M., Jastreboff P. J., Miyashita Y. A neuronal correlate in rabbit's cerebellum to adaptive modification of the vestibulo-ocular reflex. Brain Res. 1978 Jul 21;150(3):611–616. doi: 10.1016/0006-8993(78)90825-9. [DOI] [PubMed] [Google Scholar]
- Frederickson R. C., Neuss M., Morzorati S. L., McBride W. J. A comparison of the inhibitory effects of taurine and GABA on identified Purkinje cells and other neurons in the cerebellar cortex of the rat. Brain Res. 1978 Apr 21;145(1):117–126. doi: 10.1016/0006-8993(78)90800-4. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Highstein S. M., Ito M. Cerebellar inhibitory control of the vestibulo-ocular reflex investigated in rabbit 3rd nucleus. Exp Brain Res. 1972 Apr 27;14(5):511–526. doi: 10.1007/BF00236593. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B., Ito M., Yagi N. Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res. 1975 Apr 4;87(1):66–72. doi: 10.1016/0006-8993(75)90780-5. [DOI] [PubMed] [Google Scholar]
- Highstein S. M. Synaptic linkage in the vestibulo-ocular and cerebello-vestibular pathways to the VIth nucleus in the rabbit. Exp Brain Res. 1973;17(3):301–314. doi: 10.1007/BF00234668. [DOI] [PubMed] [Google Scholar]
- Ito M., Jastreboff P. J., Miyashita Y. Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Exp Brain Res. 1982;45(1-2):233–242. doi: 10.1007/BF00235783. [DOI] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972 May 12;40(1):81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. 1977 Mar;265(3):833–854. doi: 10.1113/jphysiol.1977.sp011747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Orlov I., Yamamoto M. Topographical representation of vestibulo-ocular reflexes in rabbit cerebellar flocculus. Neuroscience. 1982 Jul;7(7):1657–1664. doi: 10.1016/0306-4522(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Jastreboff P. W. Evaluation and statistical judgement of neural responses to sinusoidal stimulation in cases with superimposed drift and noise. Biol Cybern. 1979 Jun;33(2):113–120. doi: 10.1007/BF00355259. [DOI] [PubMed] [Google Scholar]
- Kawamura H., Provini L. Depression of cerebellar Purkinje cells by microiontophoretic application of GABA and related amino acids. Brain Res. 1970 Dec 1;24(2):293–304. doi: 10.1016/0006-8993(70)90108-3. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Precht W. Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience. 1979;4(11):1599–1613. doi: 10.1016/0306-4522(79)90023-x. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978 May;41(3):733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Barber R., Vaughn J. E., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res. 1974 Aug 23;76(3):377–391. doi: 10.1016/0006-8993(74)90815-4. [DOI] [PubMed] [Google Scholar]
- Neverov V. P., Sterc J., Bures J. Electrophysiological correlates of the reversed postoptokinetic nystagmus in the rabbit: activity of vestibular and floccular neurons. Brain Res. 1980 May 12;189(2):355–367. doi: 10.1016/0006-8993(80)90096-7. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Sakai Y. Augmentation by chlordiazepoxide of the inhibitory effects of taurine, beta-alanine and gamma-aminobutyric acid on spike discharges in guinea-pig cerebellar slices. Br J Pharmacol. 1979 Feb;65(2):277–285. doi: 10.1111/j.1476-5381.1979.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Sakai Y. Localization of sensitive sites to taurine, gamma-aminobutyric acid, glycine and beta-alanine in the molecular layer of guinea-pig cerebellar slices. Br J Pharmacol. 1980 Jul;69(3):407–413. doi: 10.1111/j.1476-5381.1980.tb07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. A. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976 Sep;39(5):954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C., Privat A., Drian M. J. Localization of ( 3 H)GABA in tissue culture of rat cerebellum using electron microscopy radioautography. Brain Res. 1972 Oct 13;45(1):302–308. doi: 10.1016/0006-8993(72)90242-9. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]



