Abstract
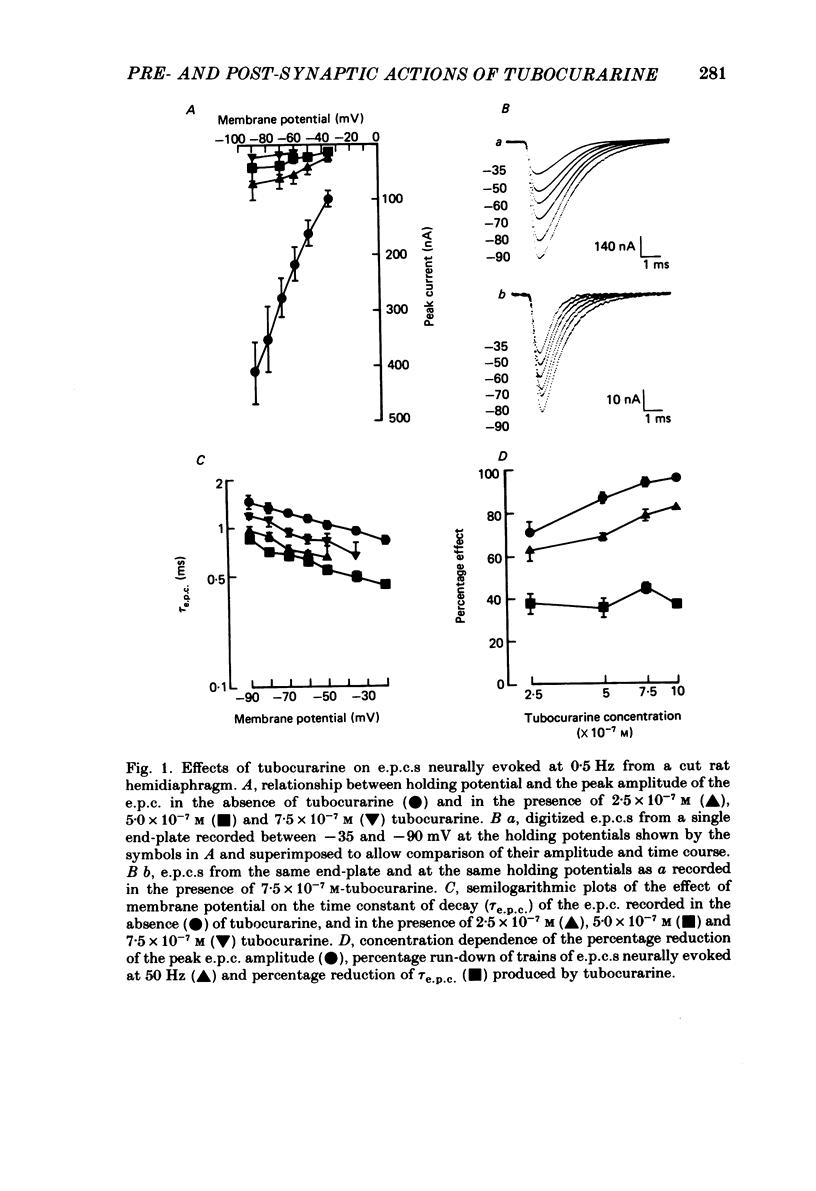
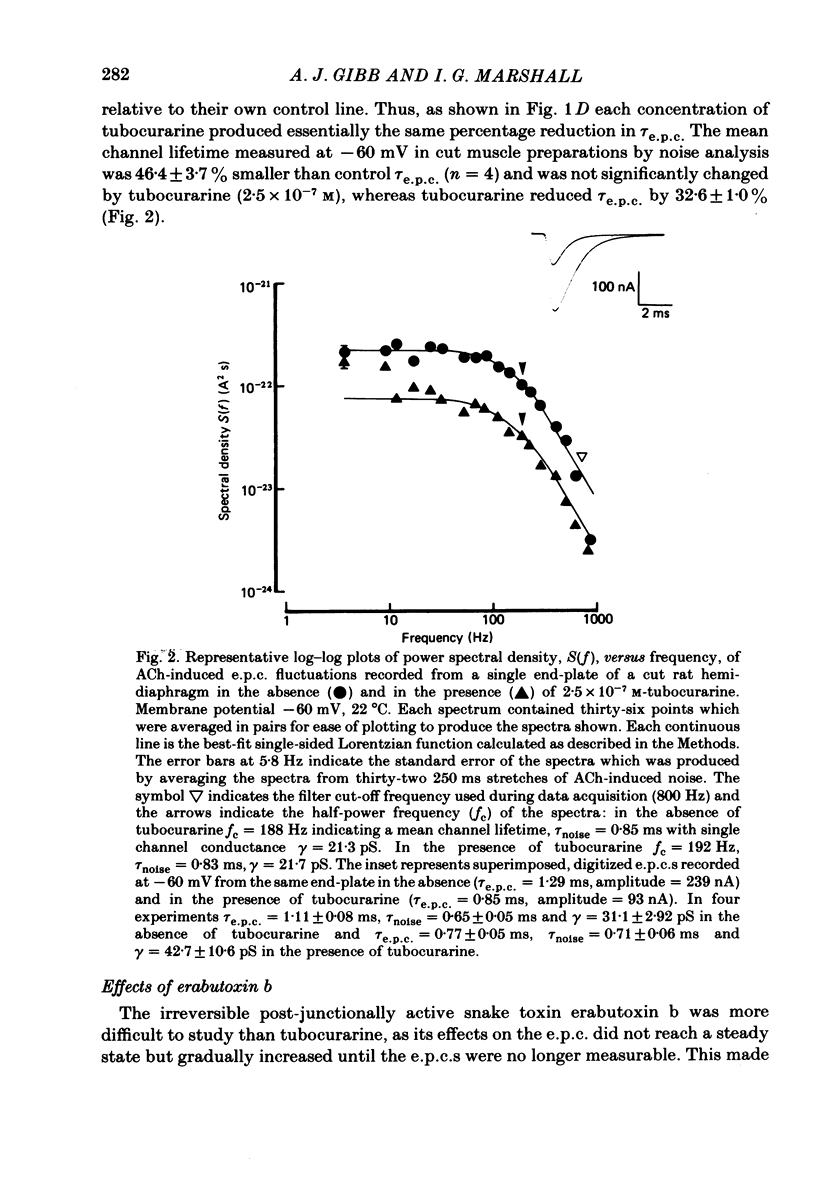
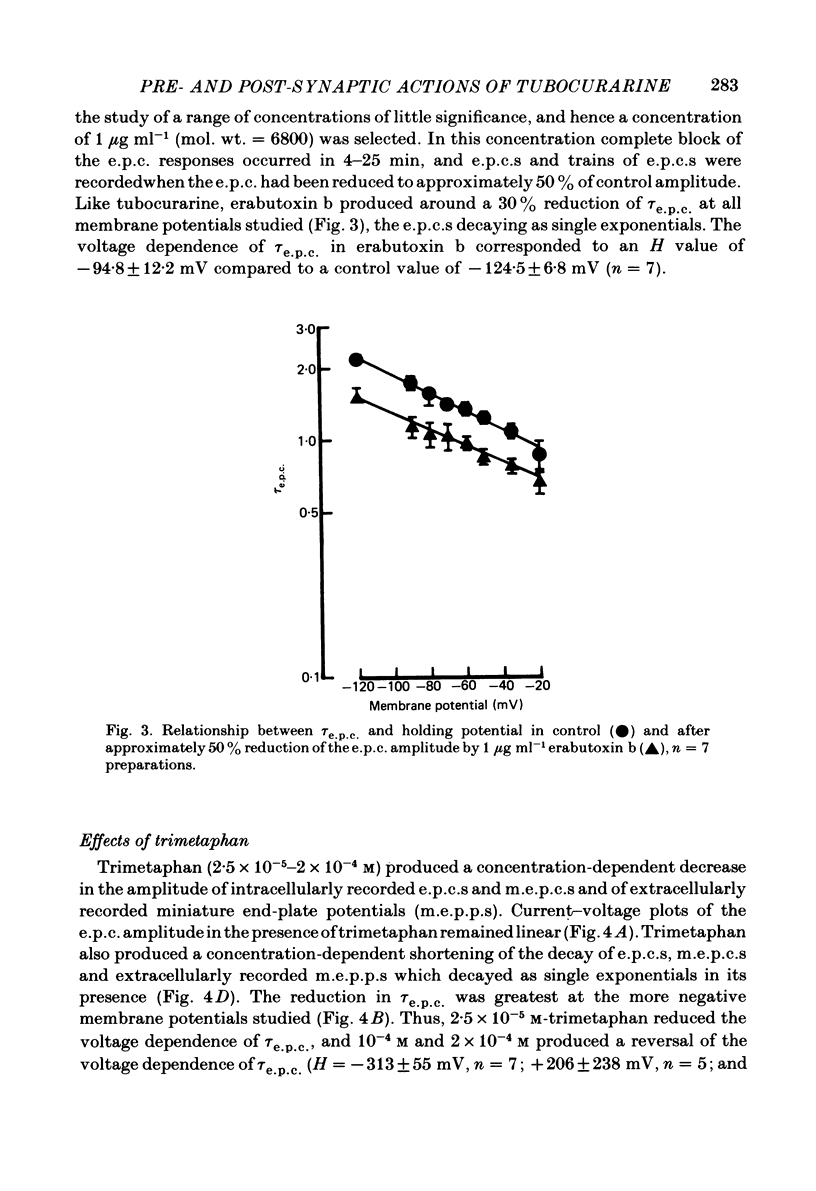
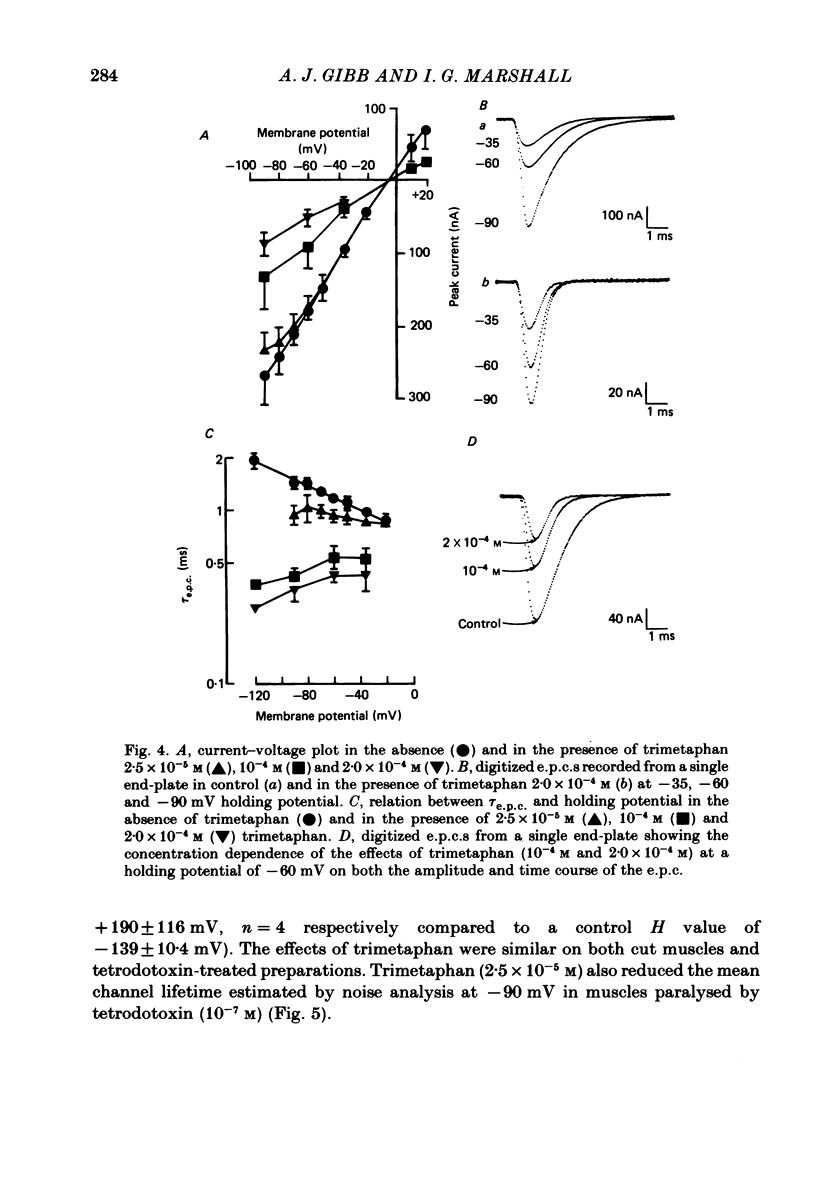
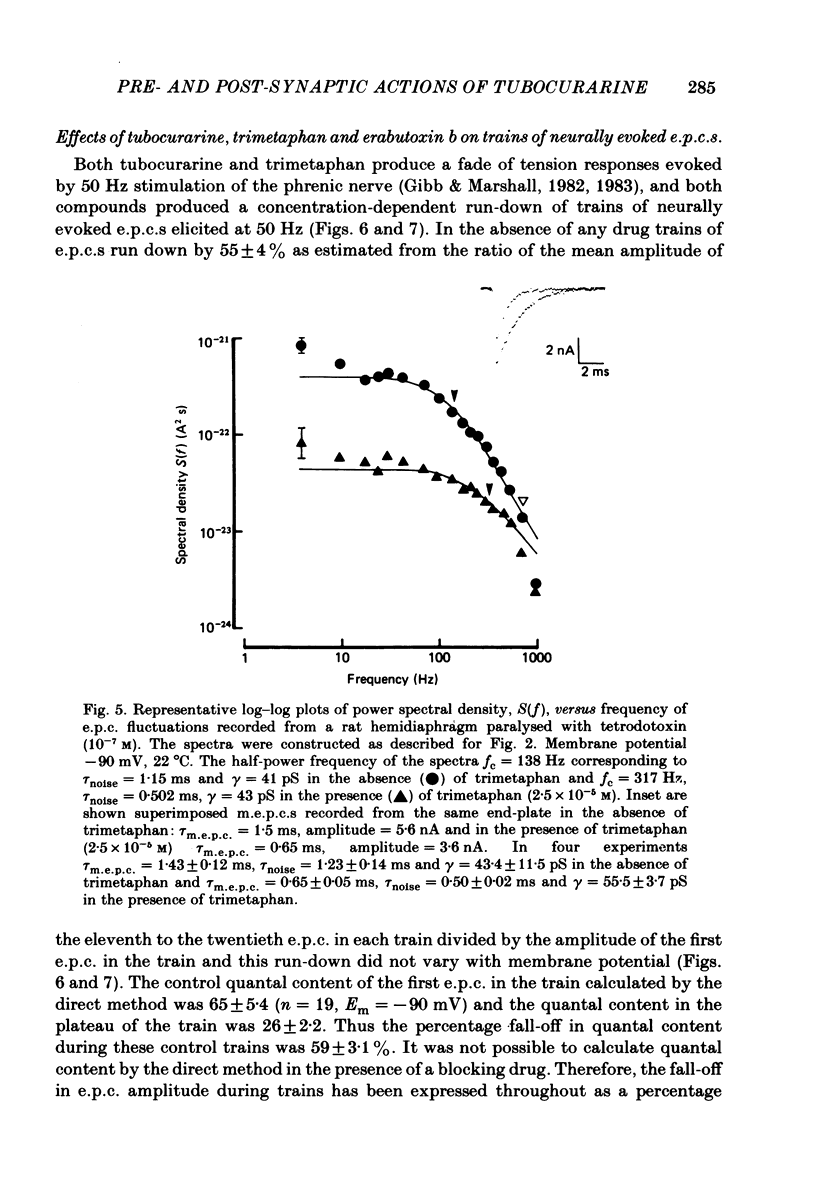
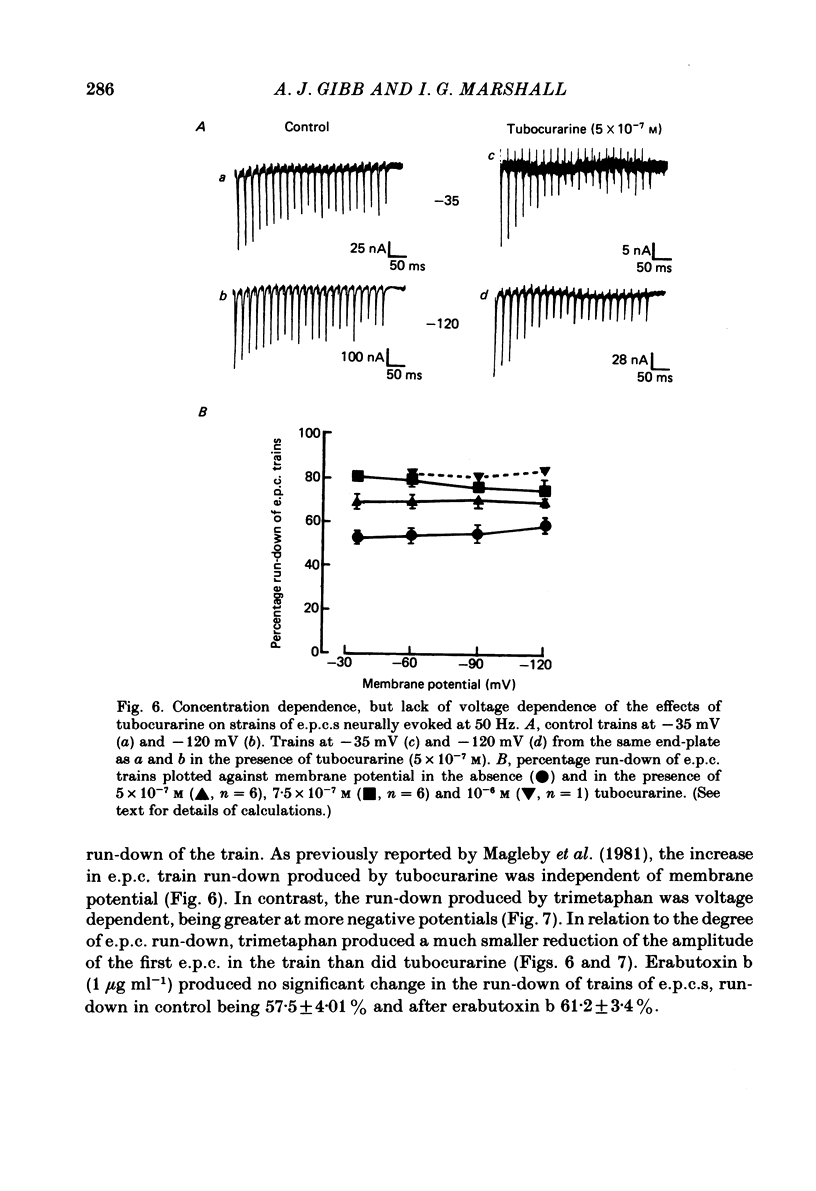
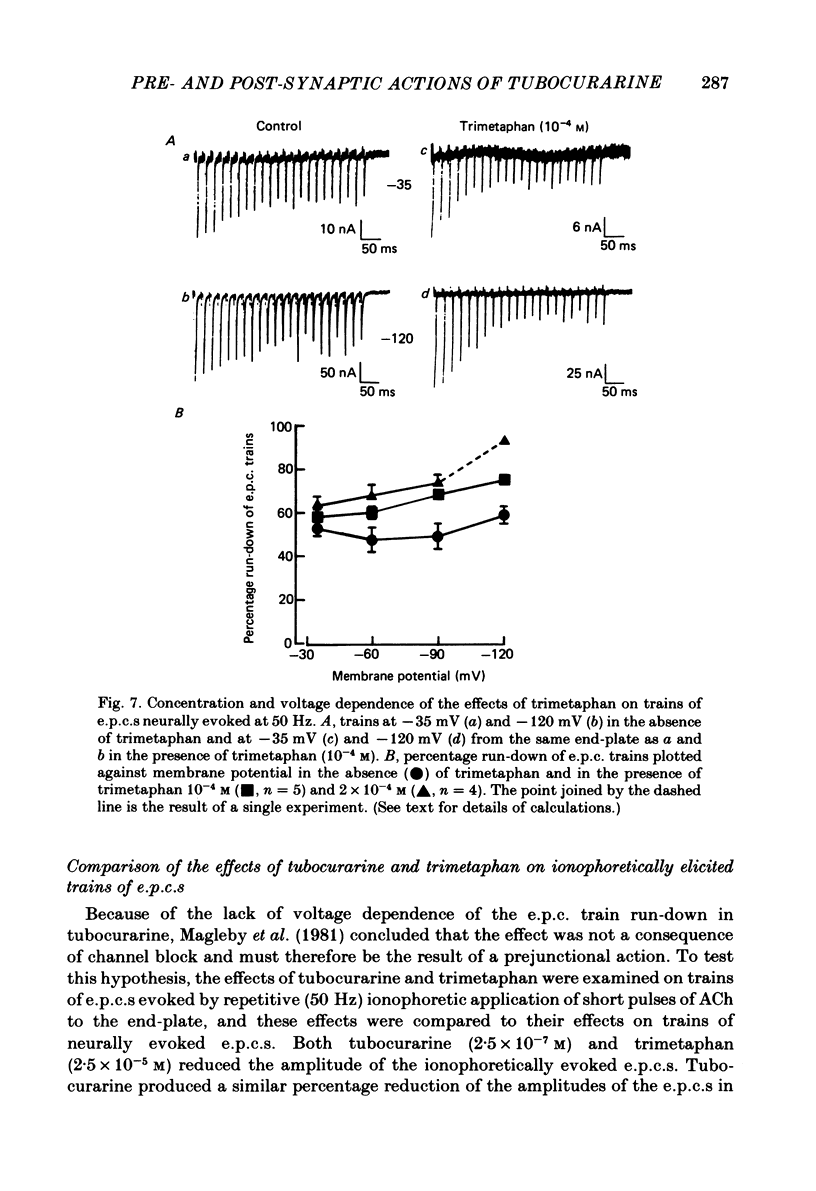
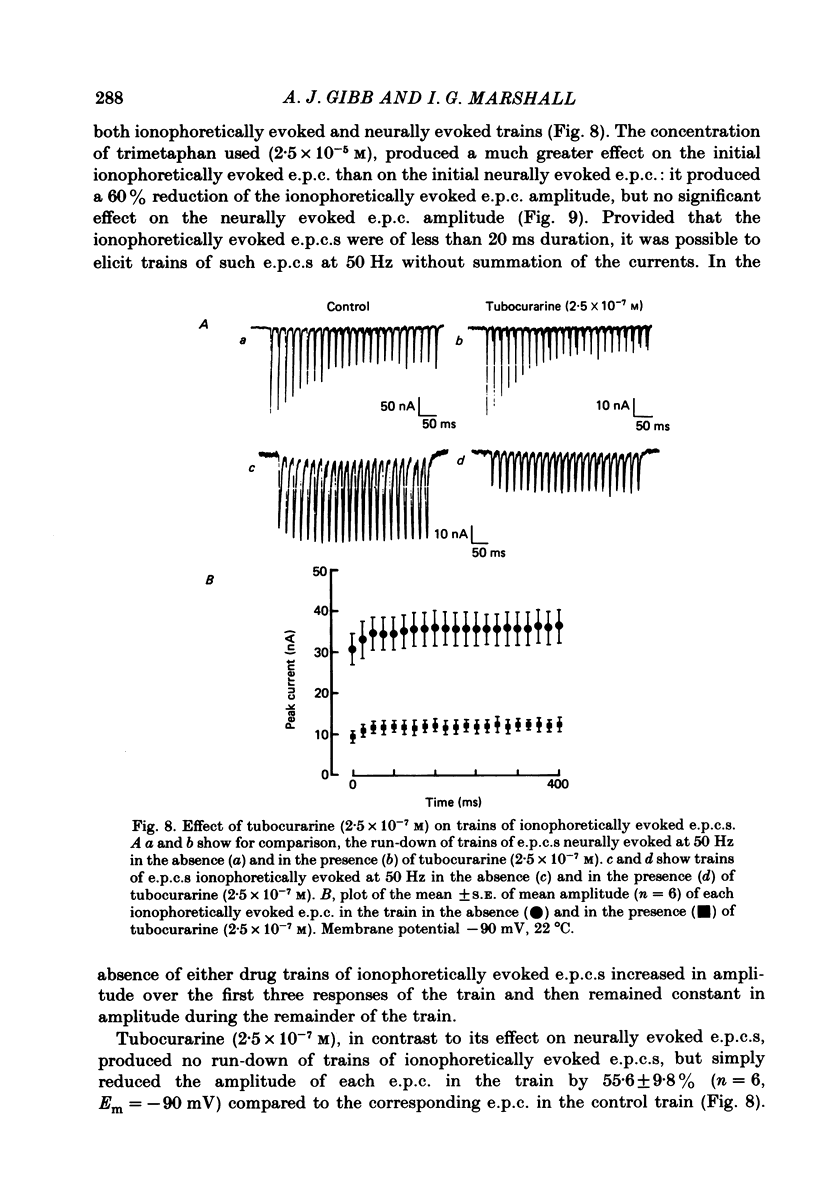
The effects of tubocurarine and trimetaphan have been examined at voltage-clamped rat diaphragm neuromuscular junctions during (a) single and repetitive stimulation of the phrenic nerve in cut muscles and (b) repetitive ionophoretic application of acetylcholine (ACh). Tubocurarine (2.5 X 10(-7)-10(-6)M) produced a concentration-dependent reduction in the amplitude of neurally evoked end-plate currents (e.p.c.s). It also reduced their time constant of decay (tau e.p.c.) in a manner that was independent of membrane potential, and not markedly dependent on the tubocurarine concentration. Likewise the snake alpha-neurotoxin, erabutoxin b, reduced the e.p.c. amplitude and produced a voltage-independent shortening of tau e.p.c. Estimates of mean channel lifetime (tau noise) from ACh-induced e.p.c. fluctuations revealed that (a) tau noise was 46.4 +/- 3.7% shorter than tau e.p.c. measured at the same end-plate. At these same end-plates in the presence of tubocurarine (2.5 X 10(-7)M) tau e.p.c. was 32.6 +/- 1.0% shorter than the control tau e.p.c. but tubocurarine did not change tau noise, (b) trimetaphan (2.5 X 10(-5)-2 X 10(-4)M) produced a concentration-dependent and voltage-dependent reduction of tau e.p.c., and a concentration-dependent reduction of peak e.p.c. amplitude. Trimetaphan (2.5 X 10(-5)M) produced a 50% reduction of tau noise. (a) Both tubocurarine and trimetaphan produced concentration-dependent increases in the run-down of trains of neurally evoked e.p.c.s (50 Hz, 0.4 s). This effect did not vary with membrane potential in tubocurarine, but was voltage dependent when induced by trimetaphan. (b) Erabutoxin b reduced the e.p.c. amplitude but did not produce any increase in the run-down of trains of neurally evoked e.p.c.s. During 50 Hz repetitive ionophoretic application of ACh, tubocurarine (2.5 X 10(-7)M) reduced the amplitude of each current in the train without inducing any run-down of the current amplitudes. This effect was not dependent on the membrane potential. In contrast trimetaphan (2.5 X 10(-5)M) induced a voltage-dependent run-down of trains of ionophoretically evoked e.p.c.s. We conclude that tubocurarine and erabutoxin b reduce the e.p.c. amplitude by blocking the post-junctional ACh receptor. Tubocurarine produces tetanic rundown of e.p.c.s. by a prejunctional mechanism, whereas the effects of trimetaphan during single and repetitive stimulation are at least partly due to block of the open ion channel associated with the ACh receptor.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- Barstad J. A., Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pbarmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):373–390. [PubMed] [Google Scholar]
- Beam K. G. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J Physiol. 1976 Jun;258(2):301–322. doi: 10.1113/jphysiol.1976.sp011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber L. C. The prejunctional actions of some non-depolarizing blocking drugs. Br J Pharmacol. 1973 Jan;47(1):109–116. doi: 10.1111/j.1476-5381.1973.tb08163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980 Dec;59(12):935–943. [PubMed] [Google Scholar]
- Bowman W. C., Webb S. N. Tetanic fade during partial transmission failure produced by non-depolarizing neuromuscular blocking drugs in the cat. Clin Exp Pharmacol Physiol. 1976 Nov-Dec;3(6):545–555. doi: 10.1111/j.1440-1681.1976.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Characteristics of the acetylcholine-operated channel at twitch and slow fibre neuromuscular junctions of the garter snake. J Physiol. 1981 Jan;310:145–158. doi: 10.1113/jphysiol.1981.sp013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F. Acetylcholine receptor. Br J Anaesth. 1982 Feb;54(2):115–130. doi: 10.1093/bja/54.2.115. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R., Bradley R. J., Müller K. D. Drug-receptor interaction at the frog neuromuscular junction. Prog Brain Res. 1979;49:213–223. doi: 10.1016/S0079-6123(08)64635-X. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head S. D. Temperature and end-plate currents in rat diaphragm. J Physiol. 1983 Jan;334:441–459. doi: 10.1113/jphysiol.1983.sp014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F., Miyamoto M. Reduction of transmitter release by D-tubocurarine. Nature. 1969 Aug 2;223(5205):531–533. doi: 10.1038/223531a0. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Salpeter M. M. Absence of [125I] alpha-bungarotoxin binding to motor nerve terminals of frog, lizard and mouse muscle. J Neurosci. 1983 Feb;3(2):326–331. doi: 10.1523/JNEUROSCI.03-02-00326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Volle R. L., Henderson E. G. An attempt to distinguish between the actions of neuromuscular blocking drugs on the acetylcholine receptor and on its associated ionic channel. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5003–5007. doi: 10.1073/pnas.77.8.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleheil G. Transmitter release in the rat diaphragm during tetanic nerve stimulation. Experientia. 1965 Jun 15;21(6):344–346. doi: 10.1007/BF02144707. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S., Terrar D. A. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981 Mar;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Electrophysiological and chemical determination of acetylcholine release at the frog neuromuscular junction. J Physiol. 1983 Jan;334:245–254. doi: 10.1113/jphysiol.1983.sp014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. The methonium. Pharmacol Rev. 1952 Sep;4(3):219–253. [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDIC M., STRAUGHAN D. W. ANTIDROMIC ACTIVITY IN THE RAT PHRENIC NERVE-DIAPHRAGM PREPARATION. J Physiol. 1964 Sep;173:130–148. doi: 10.1113/jphysiol.1964.sp007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Colquhoun D., Rang H. P. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982 Jan;75(1):151–168. doi: 10.1111/j.1476-5381.1982.tb08768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. Local anesthetic alteration of miniature endplate currents and endplate current fluctuations. Biophys J. 1976 May;16(5):433–439. doi: 10.1016/S0006-3495(76)85699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. The kinetics of local anesthetic blockade of end-plate channels. Biophys J. 1982 Mar;37(3):625–631. [PMC free article] [PubMed] [Google Scholar]
- Shaker N., Eldefrawi A. T., Aguayo L. G., Warnick J. E., Albuquerque E. X., Eldefrawi M. E. Interactions of d-tubocurarine with the nicotinic acetylcholine receptor/channel molecule. J Pharmacol Exp Ther. 1982 Jan;220(1):172–177. [PubMed] [Google Scholar]
- Spivak C. E., Maleque M. A., Oliveira A. C., Masukawa L. M., Tokuyama T., Daly J. W., Albuquerque E. X. Actions of the histrionicotoxins at the ion channel of the nicotinic acetylcholine receptor and at the voltage-sensitive ion channels of muscle membranes. Mol Pharmacol. 1982 Mar;21(2):351–361. [PubMed] [Google Scholar]
- Wilson D. F. Influence of presynaptic receptors on neuromuscular transmission in rat. Am J Physiol. 1982 May;242(5):C366–C372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]


