Abstract
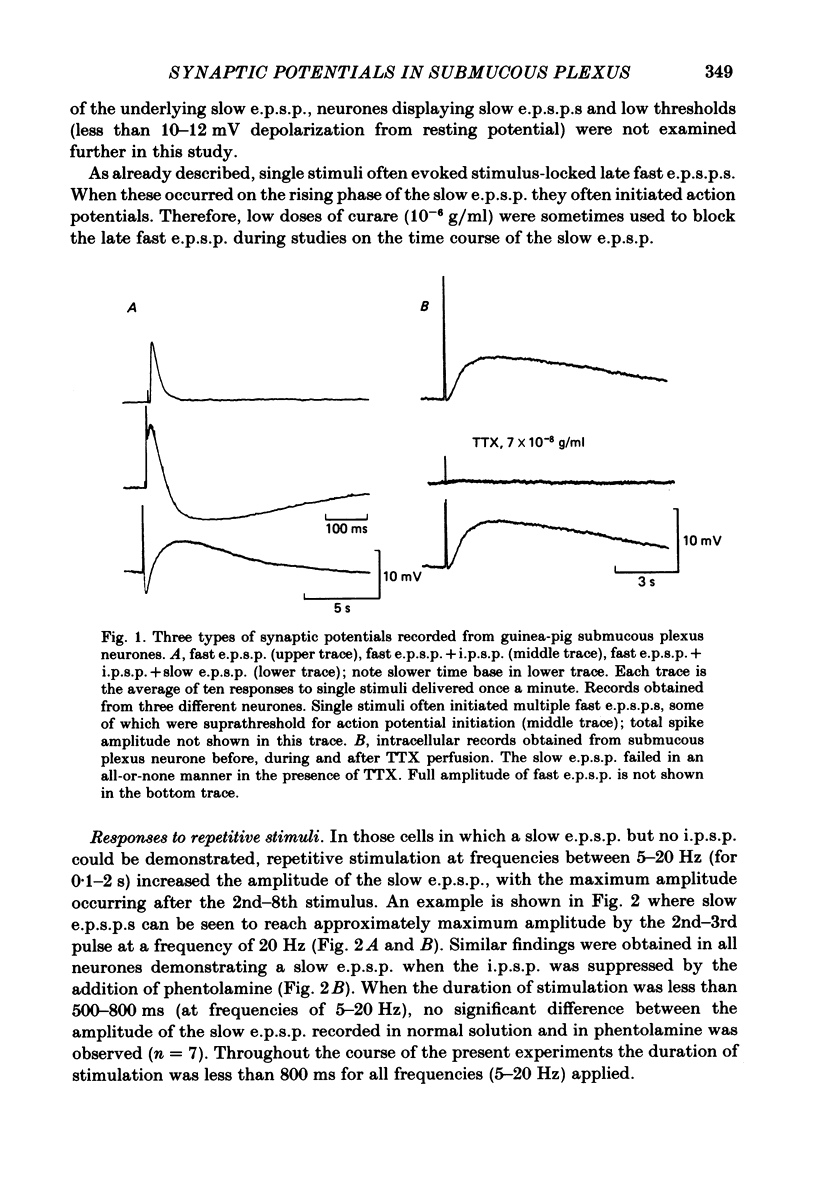
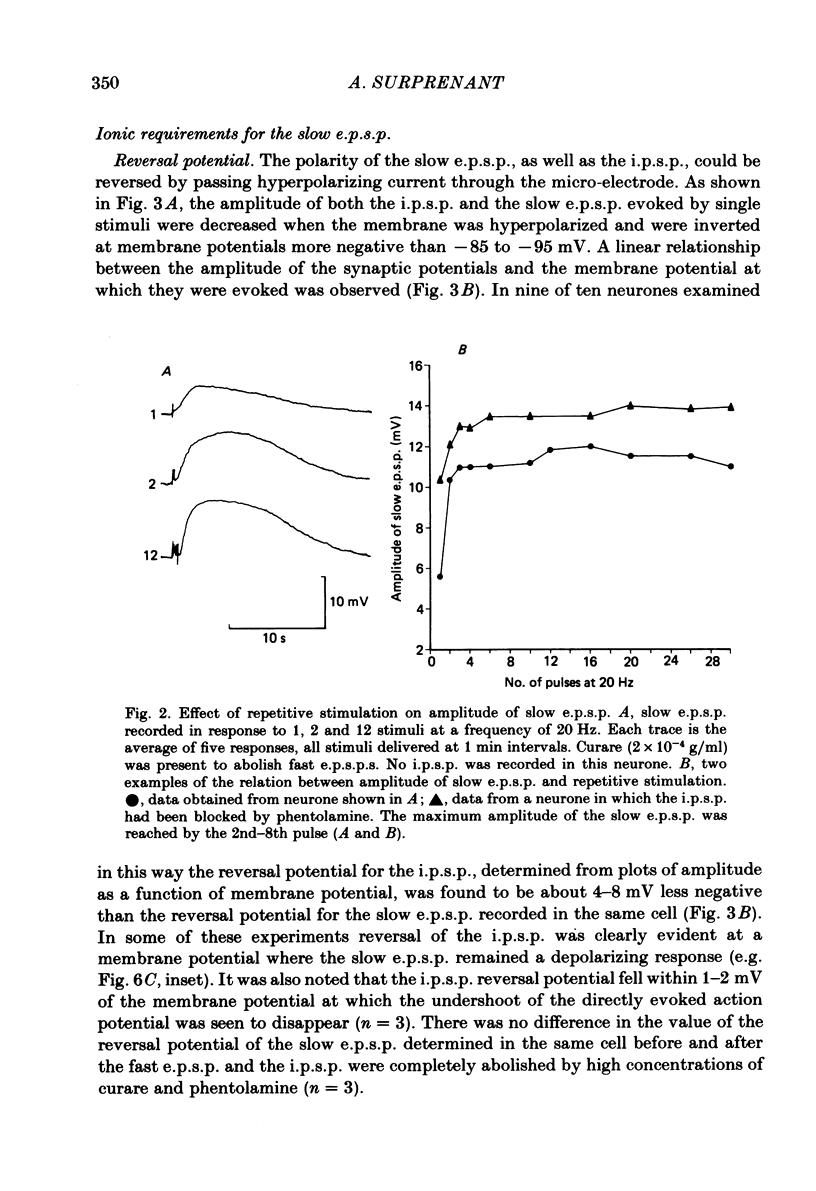
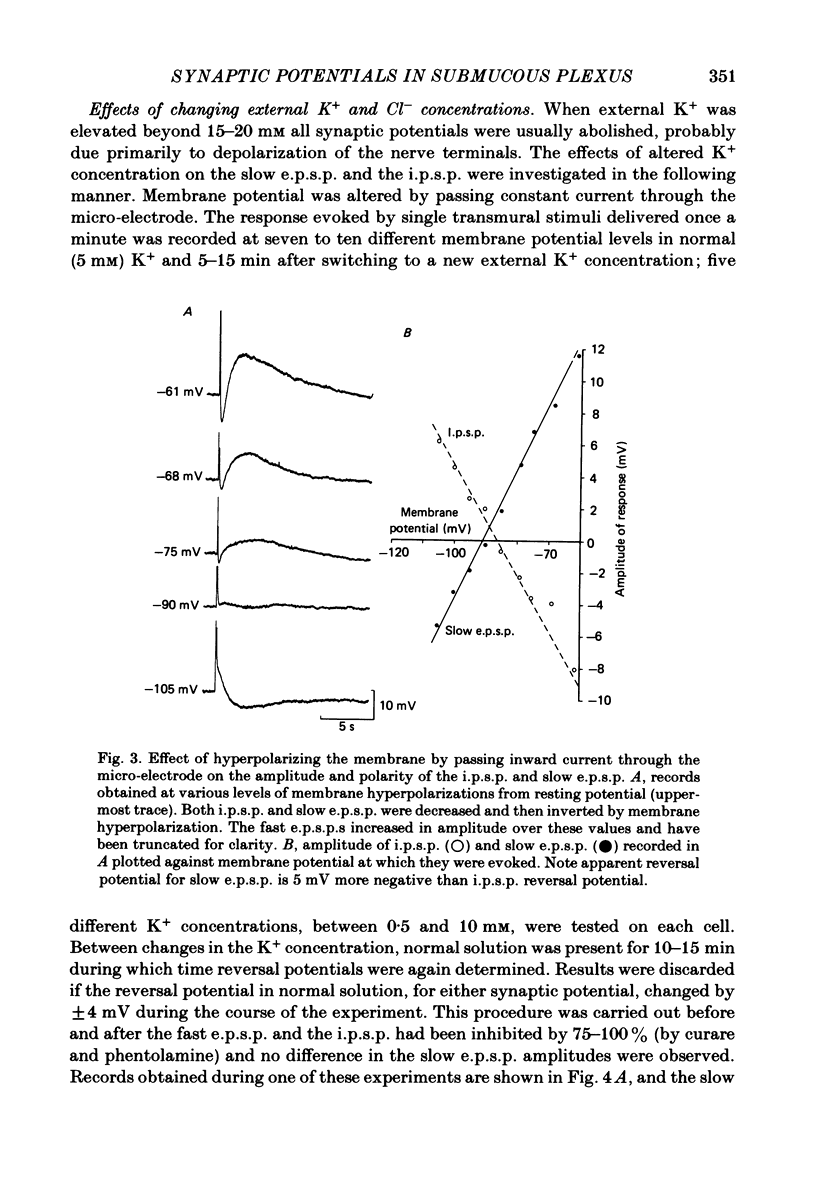
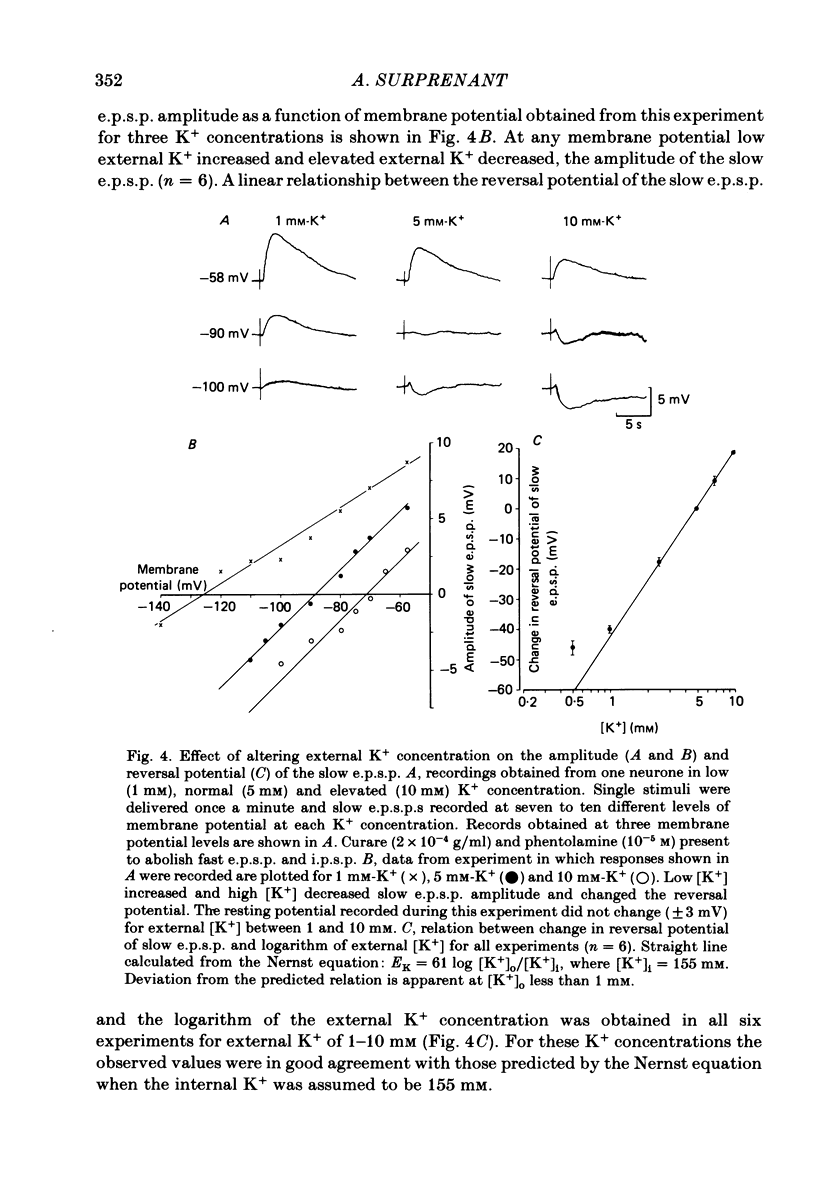
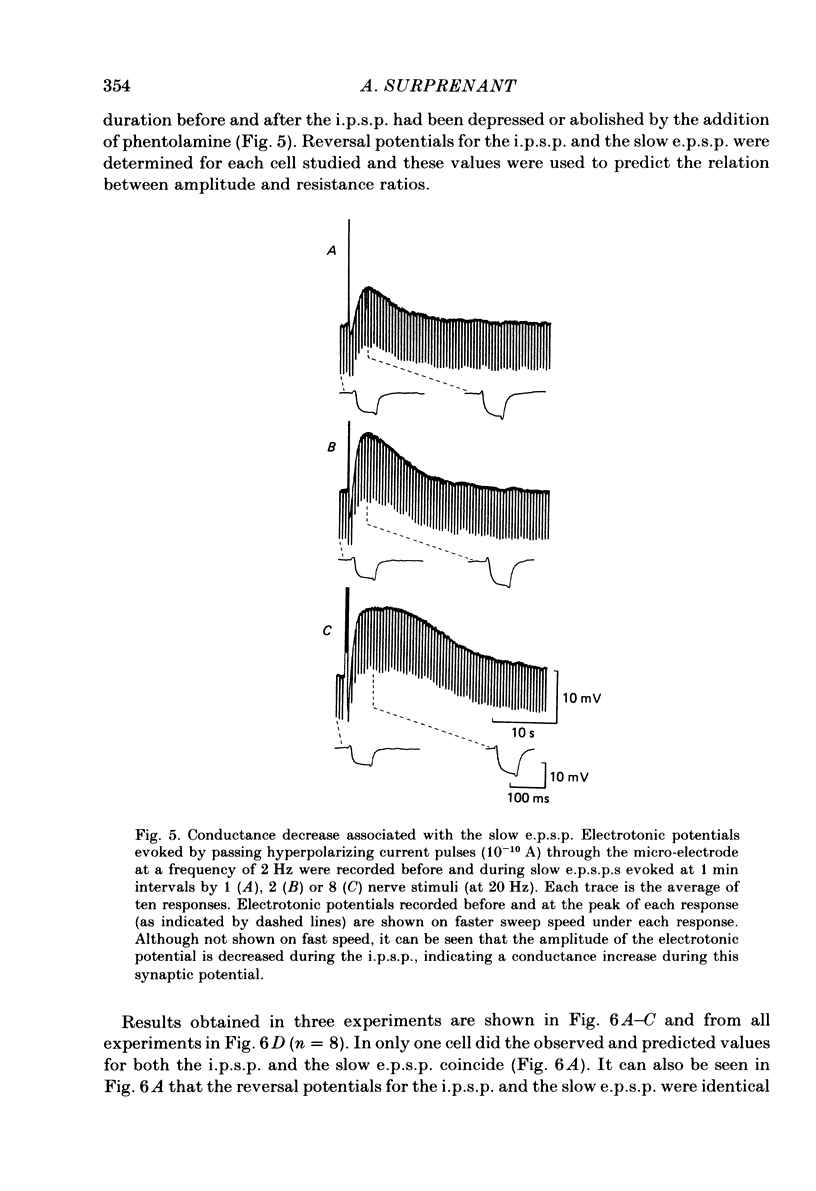
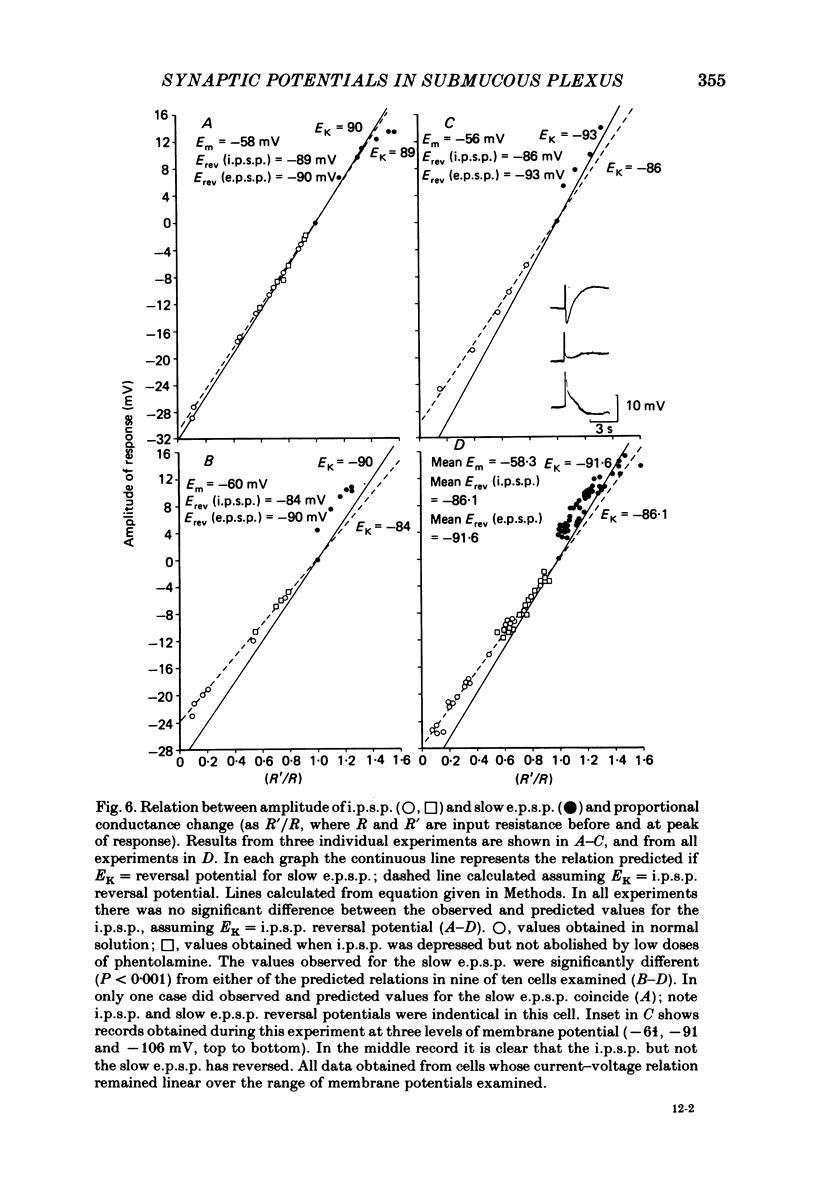
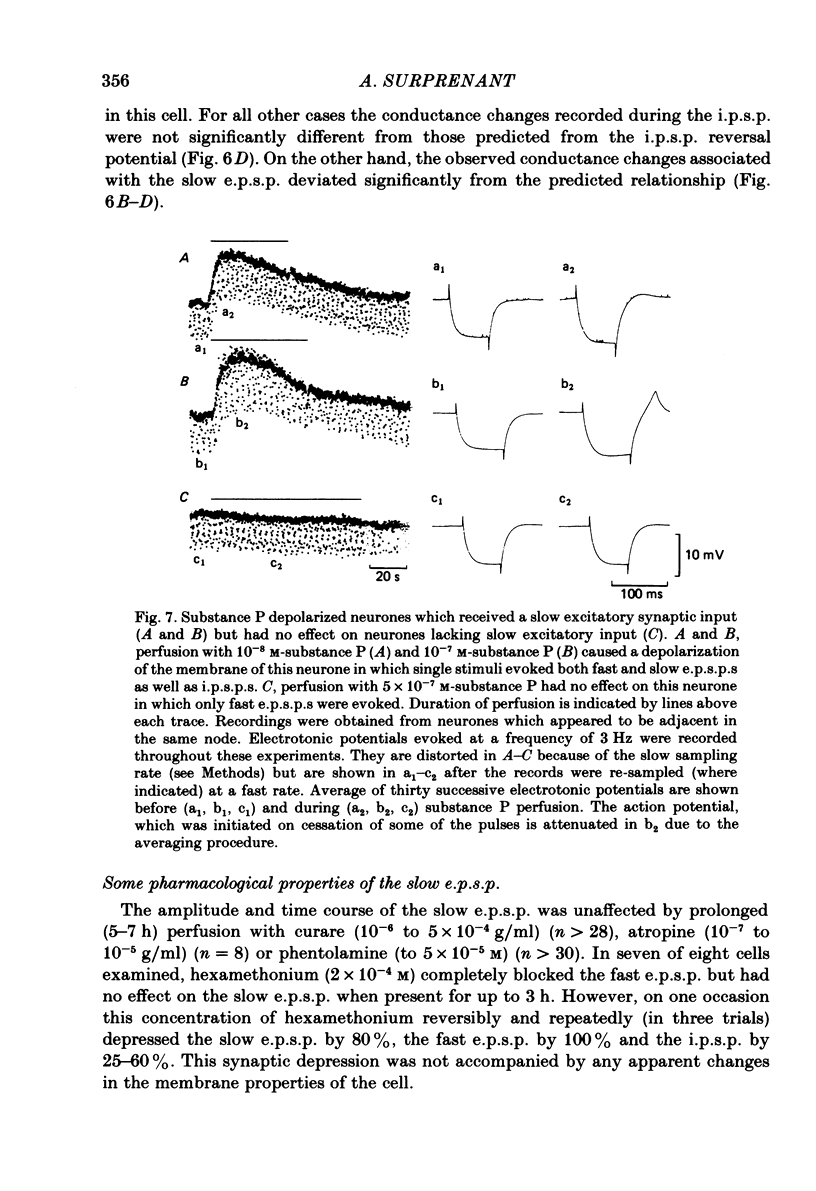
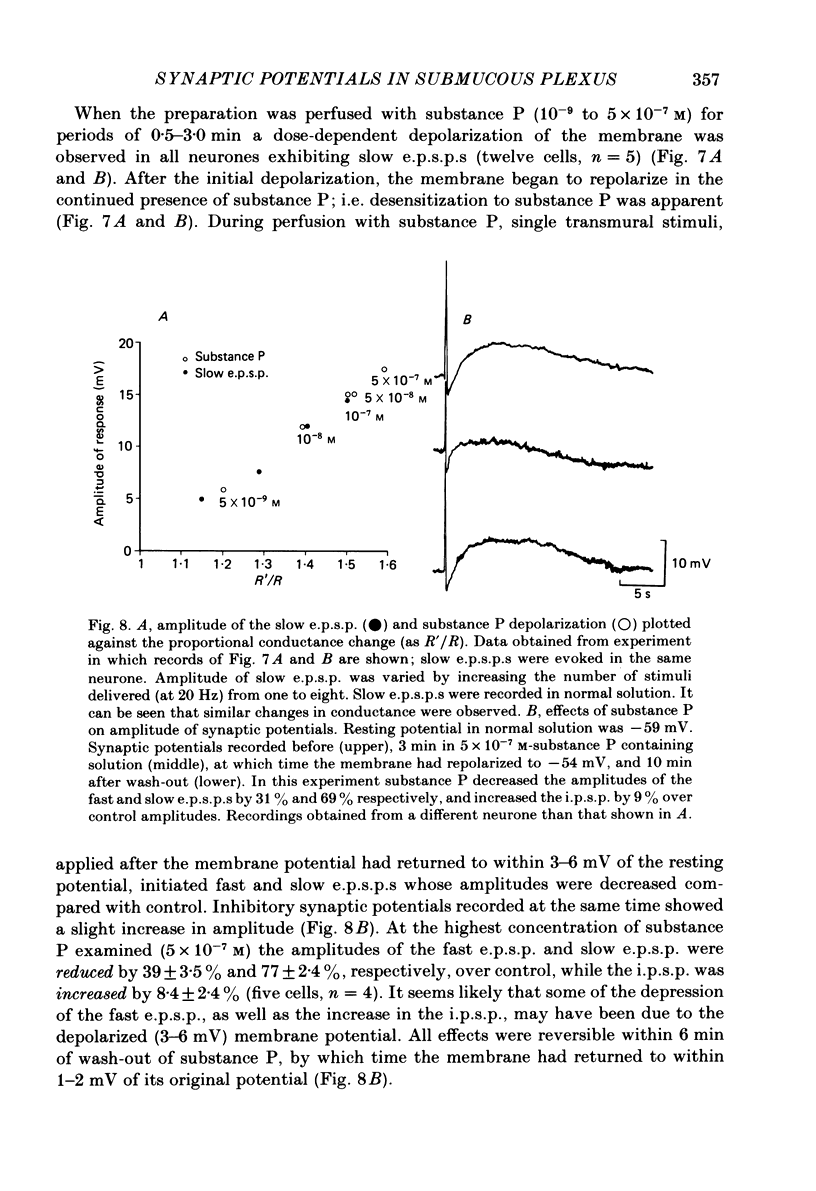
Intracellular recordings made from neurones of guinea-pig submucous plexus revealed three types of synaptic input: cholinergic excitatory synaptic potentials (fast e.p.s.p.s) of 50-80 ms duration, inhibitory synaptic potentials (i.p.s.p.s) of 1 s duration, and non-cholinergic, non-adrenergic slow e.p.s.p.s which lasted for 15-20 s. A single stimulus was sufficient to elicit the slow e.p.s.p. in all neurones in which this synaptic input was present. Slow e.p.s.p.s were recorded in those neurones which also displayed i.p.s.p.s. Both the i.p.s.p. and the slow e.p.s.p. appeared in an all-or-none fashion and were not affected by alterations in the stimulus strength. The inhibitory as well as the slow excitatory synaptic potentials reversed close to the K+ equilibrium potential, indicating that the i.p.s.p. is due to an activation of K+ conductance while the slow e.p.s.p. is due to its inactivation. Evidence is presented which suggests the slow e.p.s.p. may be generated at a synapse located some distance from the soma, presumably at a dendritic location. Only those cells which showed slow e.p.s.p.s responded to substance P with a depolarization which mimicked the slow e.p.s.p.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costa M., Furness J. B. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jul;294(1):47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silinsky E. M. Interaction between inhibitory and excitatory synaptic potentials at a peripheral neurone. J Physiol. 1976 Aug;259(3):647–663. doi: 10.1113/jphysiol.1976.sp011487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McKirdy H. C. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Jul;249(2):369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Mechanisms of peristalsis. Br Med Bull. 1979 Sep;35(3):263–268. doi: 10.1093/oxfordjournals.bmb.a071587. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Polak J. M., Van Noorden S., Bloom S. R., Burnstock G. Peptide-containing neurones connect the two ganglionated plexuses of the enteric nervous system. Nature. 1980 Jan 24;283(5745):391–393. doi: 10.1038/283391a0. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., Morita K., North R. A. Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. J Physiol. 1981 Nov;320:175–186. doi: 10.1113/jphysiol.1981.sp013942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A., Williams J. T. The action of substance P on neurons of the myenteric plexus of the guinea-pig small intestine. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1163):191–208. doi: 10.1098/rspb.1979.0101. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Katayama Y. Evidence that substance P is a neurotransmitter in the myenteric plexus. Nature. 1980 Sep 11;287(5778):151–152. doi: 10.1038/287151a0. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. The action of 5-hydroxytryptamine and possible 5-hydroxytryptamine antagonists on neurones of the guinea-pig submucous plexus. Gen Pharmacol. 1981;12(4):281–284. doi: 10.1016/0306-3623(81)90059-8. [DOI] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. The calcium-dependent slow after-hyperpolarization in myenteric plexus neurones with tetrodotoxin-resistant action potentials. Br J Pharmacol. 1973 Dec;49(4):709–711. doi: 10.1111/j.1476-5381.1973.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Two types of neurones lacking synaptic input in the submucous plexus of guinea-pig small intestine. J Physiol. 1984 Jun;351:363–378. doi: 10.1113/jphysiol.1984.sp015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D. Electrical activity from single neurons in Auerbach's plexus. Am J Physiol. 1970 Jul;219(1):159–169. doi: 10.1152/ajplegacy.1970.219.1.159. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Intracellular study of tonic-type enteric neurons in guinea pig small intestine. J Neurophysiol. 1979 Mar;42(2):569–581. doi: 10.1152/jn.1979.42.2.569. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]


