Abstract
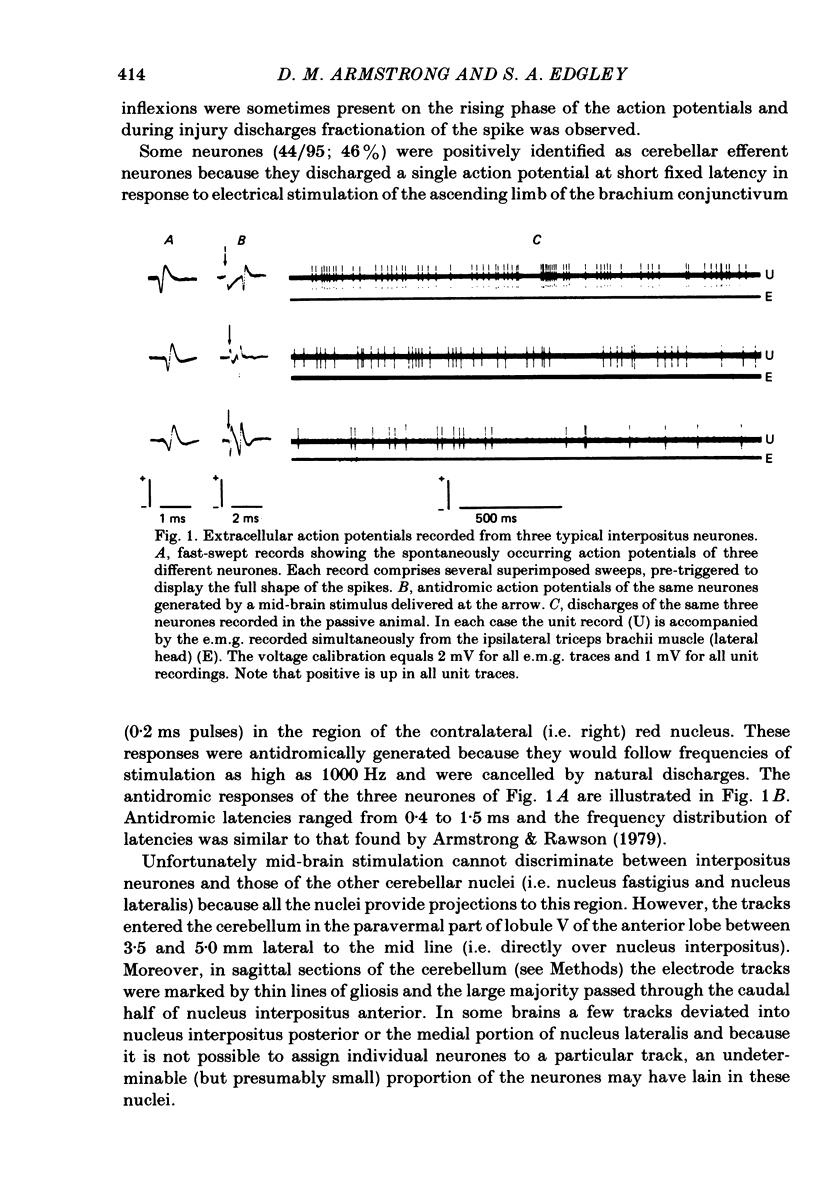
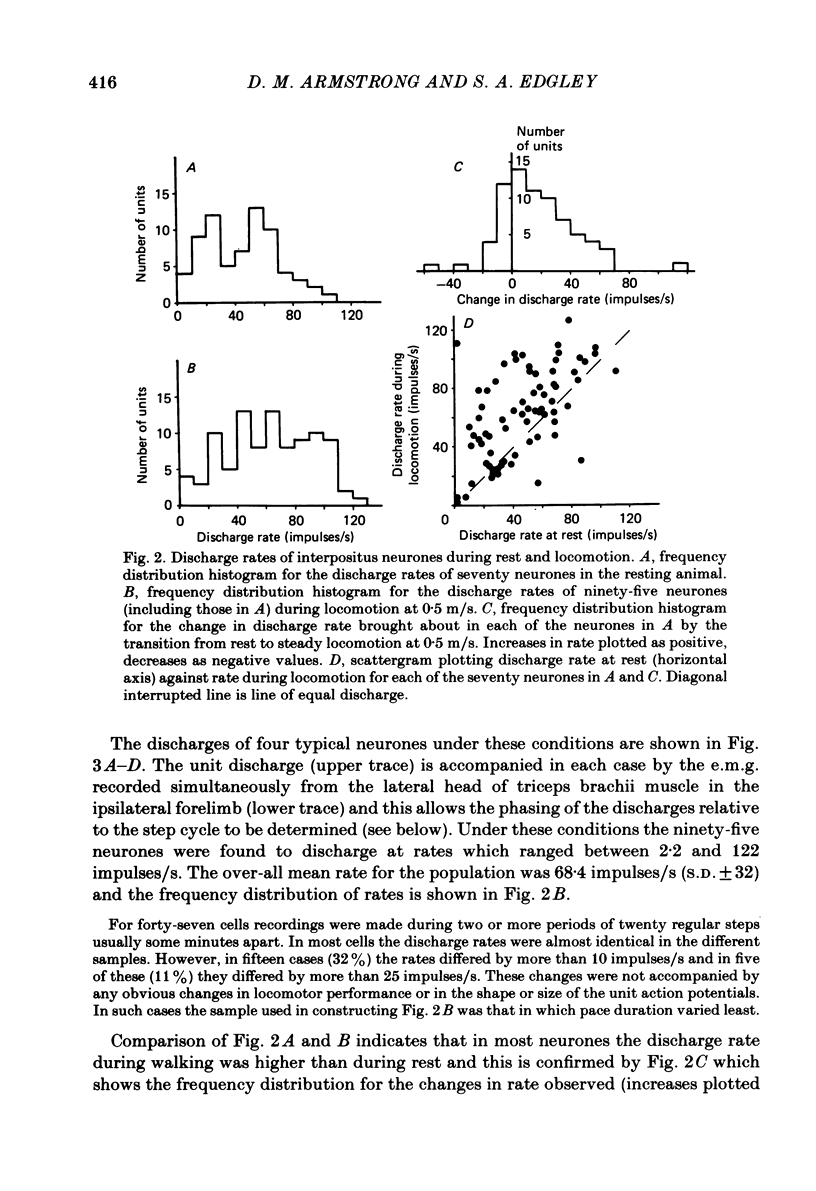
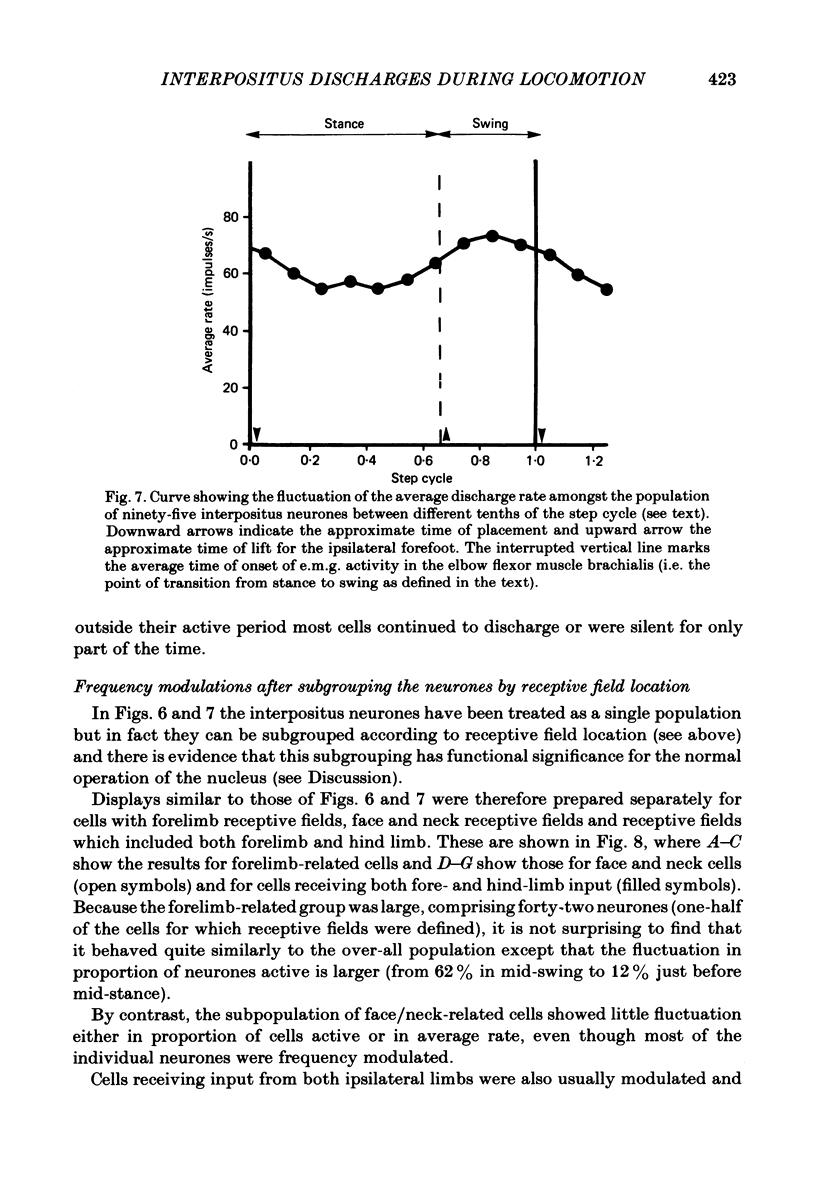
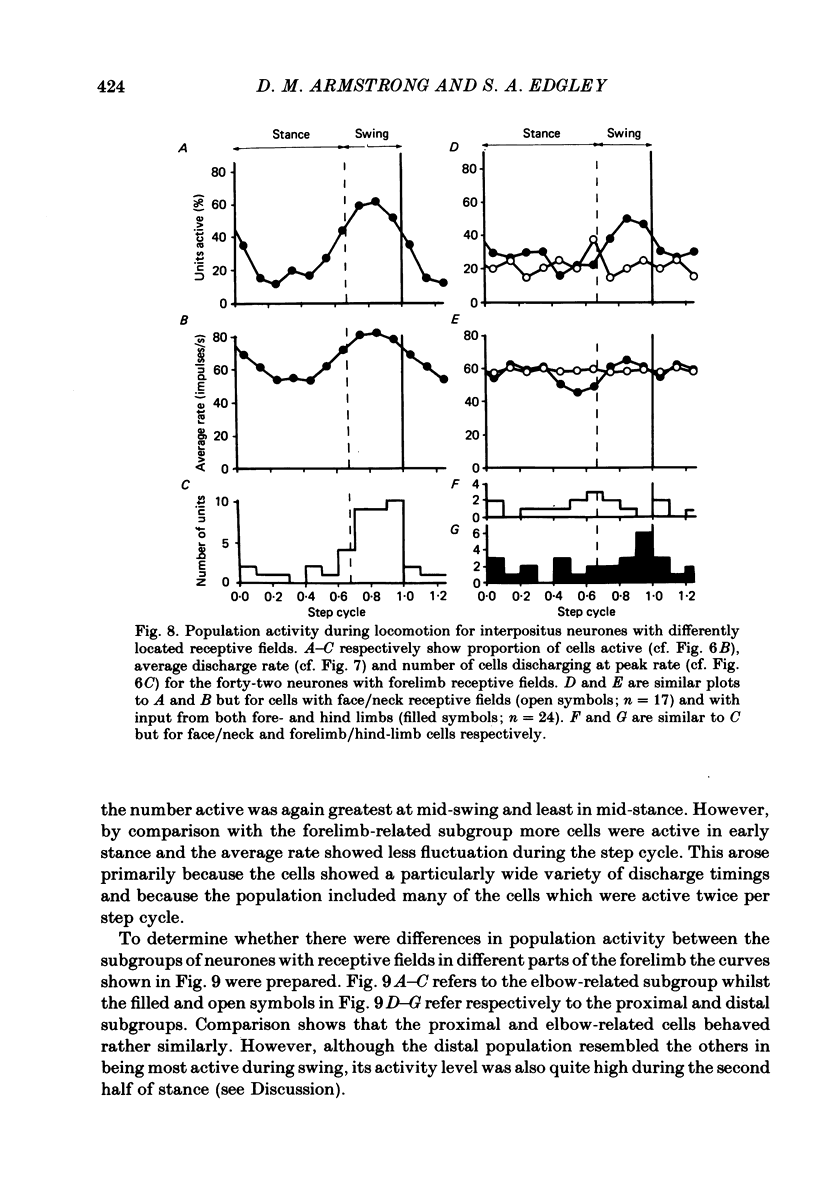
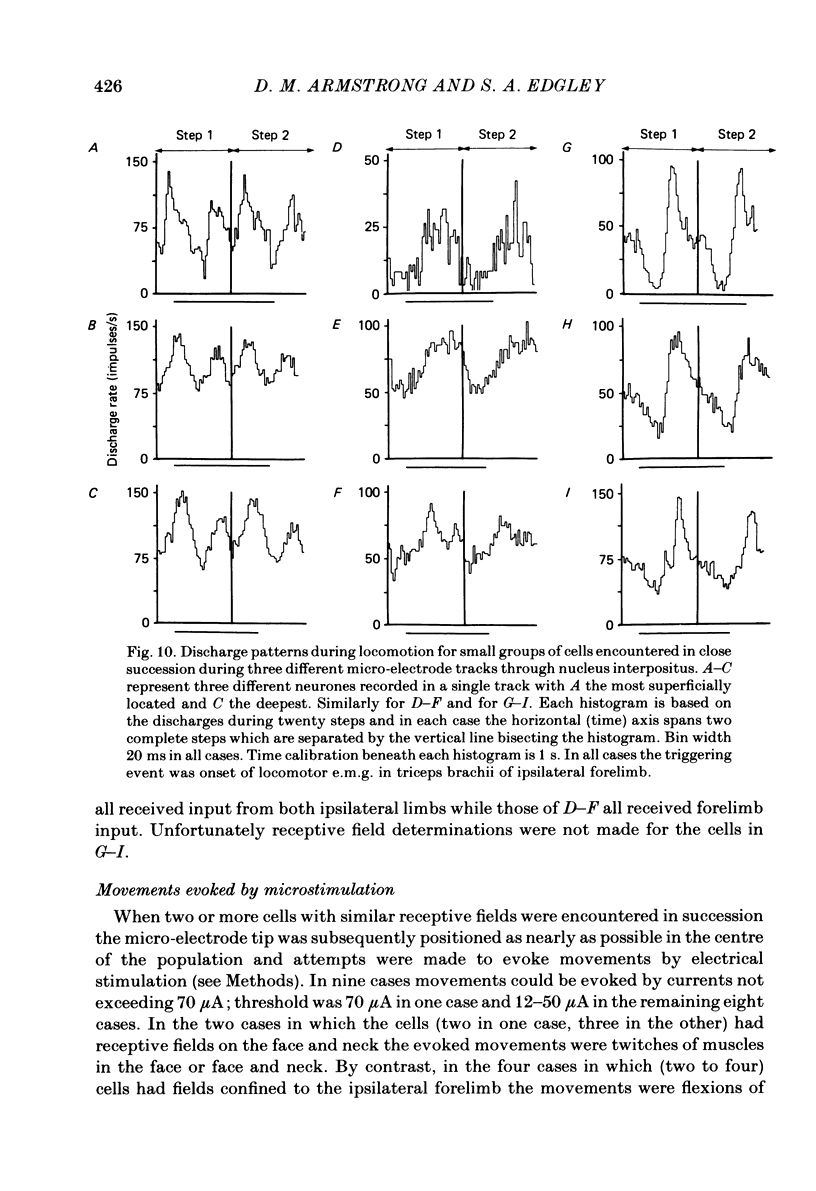
Extracellular recordings were made from ninety-five cerebellar nuclear neurones in the cat. All were studied during periods of steady walking at 0.5 m/s and most were also studied in the resting animal. Most neurones were in nucleus interpositus anterior; forty-four cells were shown by antidromic invasion to project to the mid-brain. Most neurones discharged tonically in the absence of overt movements and the mean rate was 42 impulses/s (S.D. +/- 23). During locomotion the mean rate was 68 impulses/s (S.D. +/- 32). In all but seven neurones the discharge during locomotion was frequency modulated but in different neurones the depth of modulation varied from 5 to 161 impulses/s (mean 52 impulses/s; S.D. +/- 30) and the time of peak discharge relative to the step cycle in the ipsilateral forelimb also varied widely. Despite the individual differences the population as a whole was much more active during forelimb swing than during stance, both in numbers of neurones strongly active and in over-all average discharge rate (74 impulses/s as compared with 55). Most neurones had tactile receptive fields on the ipsilateral forelimb while others received input from head and neck or from both ipsilateral limbs. The tendency to discharge preferentially during early swing was greatest for the first group, especially the subpopulations with receptive fields around or proximal to the elbow. Cells encountered in close sequence during a micro-electrode track had similarly located receptive fields and usually showed similar patterns of discharge during locomotion. These findings are discussed in relation to the suggestion by Orlovsky (1972a, b, c) that nucleus interpositus assists in regulating locomotion by evoking rubrospinal discharges which facilitate the flexor muscle activities produced by the spinal mechanisms responsible for generating the swing phase of the step cycle.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol. 1979 Apr;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky Y. I., Orlovsky G. N., Pavlova G. A., Perret C. Activity of neurons of cerebellar nuclei during fictitious scratch reflex in the cat. II. Interpositus and lateral nuclei. Brain Res. 1980 Nov 3;200(2):249–258. doi: 10.1016/0006-8993(80)90917-8. [DOI] [PubMed] [Google Scholar]
- Arshavsky Y. I., Orlovsky G. N., Pavlova G. A., Perret C. Messages conveyed by descending tracts during scratching in the cat. II. Activity of rubrospinal neurons. Brain Res. 1978 Dec 22;159(1):111–123. doi: 10.1016/0006-8993(78)90113-0. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Hunsperger R. W. Functional significance of projection from the cerebellar nuclei to the motor cortex in the cat. Brain Res. 1975 Nov 7;98(1):73–92. doi: 10.1016/0006-8993(75)90510-7. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Dependence of the activity of interpositus and red nucleus neurons on sensory input data generated by movement. Brain Res. 1978 Aug 18;152(1):41–63. doi: 10.1016/0006-8993(78)90133-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Moore R. B., Richardson H. C. Patterns of activity evoked in cerebellar interpositus nuclear neurones by natural somatosensory stimuli in awake cats. J Physiol. 1981 Aug;317:1–20. doi: 10.1113/jphysiol.1981.sp013810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Rantucci T., Rosén I., Scheid P., Táboríková H. Somatotopic studies on cerebellar interpositus neurons. J Neurophysiol. 1974 Nov;37(6):1449–1459. doi: 10.1152/jn.1974.37.6.1449. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. Patterns of convergence onto interpositus neurons from peripheral afferents. J Neurophysiol. 1974 Nov;37(6):1438–1448. doi: 10.1152/jn.1974.37.6.1438. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. Temporal patterns of responses of interpositus neurons to peripheral afferent stimulation. J Neurophysiol. 1974 Nov;37(6):1424–1437. doi: 10.1152/jn.1974.37.6.1424. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. The cerebellum as a computer: patterns in space and time. J Physiol. 1973 Feb;229(1):1–32. doi: 10.1113/jphysiol.1973.sp010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. W. An electromyographic analysis of forelimb muscles during overground stepping in the cat. J Exp Biol. 1978 Oct;76:105–122. doi: 10.1242/jeb.76.1.105. [DOI] [PubMed] [Google Scholar]
- Ghez C. Input-output relations of the red nucleus in the cat. Brain Res. 1975 Nov 7;98(1):93–308. doi: 10.1016/0006-8993(75)90511-9. [DOI] [PubMed] [Google Scholar]
- Giuffrida R., Li Volsi G., Pantò M. R., Perciavalle V., Sapienza S., Urbano A. Single muscle organization of interposito-rubral projections. Exp Brain Res. 1980;39(3):261–267. doi: 10.1007/BF00237115. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979 Dec;297(0):559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Larsen K. D., Yumiya H. Motor cortical modulation of feline red nucleus output: cortico-rubral and cerebellar-mediated responses. Exp Brain Res. 1980 Feb;38(3):321–331. doi: 10.1007/BF00236652. [DOI] [PubMed] [Google Scholar]
- Larsen K. D., Yumiya H. Organization of the convergence in the intermediate cerebellar nuclei of somatosensory receptive fields with motor cortical-evoked responses. Exp Brain Res. 1979 Aug 1;36(3):477–489. doi: 10.1007/BF00238517. [DOI] [PubMed] [Google Scholar]
- Li Volsi G., Pacitti C., Perciavalle V., Sapienza S., Urbano A. Interpositus nucleus influences on pyramidal tract neurons in the cat. Neuroscience. 1982;7(8):1929–1936. doi: 10.1016/0306-4522(82)90007-0. [DOI] [PubMed] [Google Scholar]
- Nishioka S., Nakahama H. Peripheral somatic activation of neurons in the cat red nucleus. J Neurophysiol. 1973 Mar;36(2):296–307. doi: 10.1152/jn.1973.36.2.296. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res. 1972 May 26;40(2):359–371. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- Palmer C. Interpositus and fastigial unit activity during sleep and waking in the cat. Electroencephalogr Clin Neurophysiol. 1979 Apr;46(4):357–370. doi: 10.1016/0013-4694(79)90137-8. [DOI] [PubMed] [Google Scholar]
- Perciavalle V., Santangelo F., Sapienza S., Serapide M. F., Urbano A. Direct afferents to interpositus nucleus responsible for triggering movement. Brain Res. 1979 Nov 16;177(2):367–372. doi: 10.1016/0006-8993(79)90787-x. [DOI] [PubMed] [Google Scholar]
- Schultz W., Montgomery E. B., Jr, Marini R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain. 1979 Mar;102(1):127–146. doi: 10.1093/brain/102.1.127. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980 Jul;44(1):119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]
- Udo M., Oda Y., Tanaka K., Horikawa J. Cerebellar control of locomotion investigated in cats: discharges from Deiters' neurones, EMG and limb movements during local cooling of the cerebellar cortex. Prog Brain Res. 1976;44:445–459. doi: 10.1016/S0079-6123(08)60751-7. [DOI] [PubMed] [Google Scholar]


