Abstract
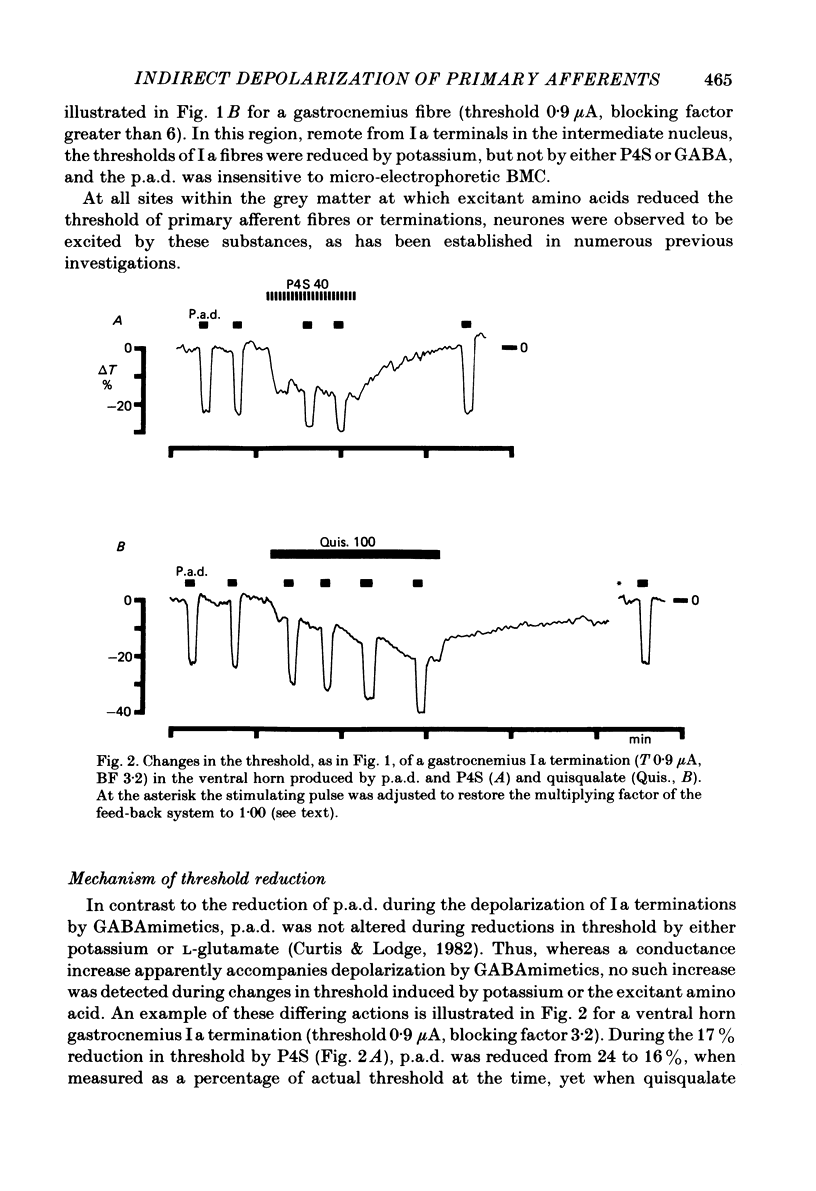
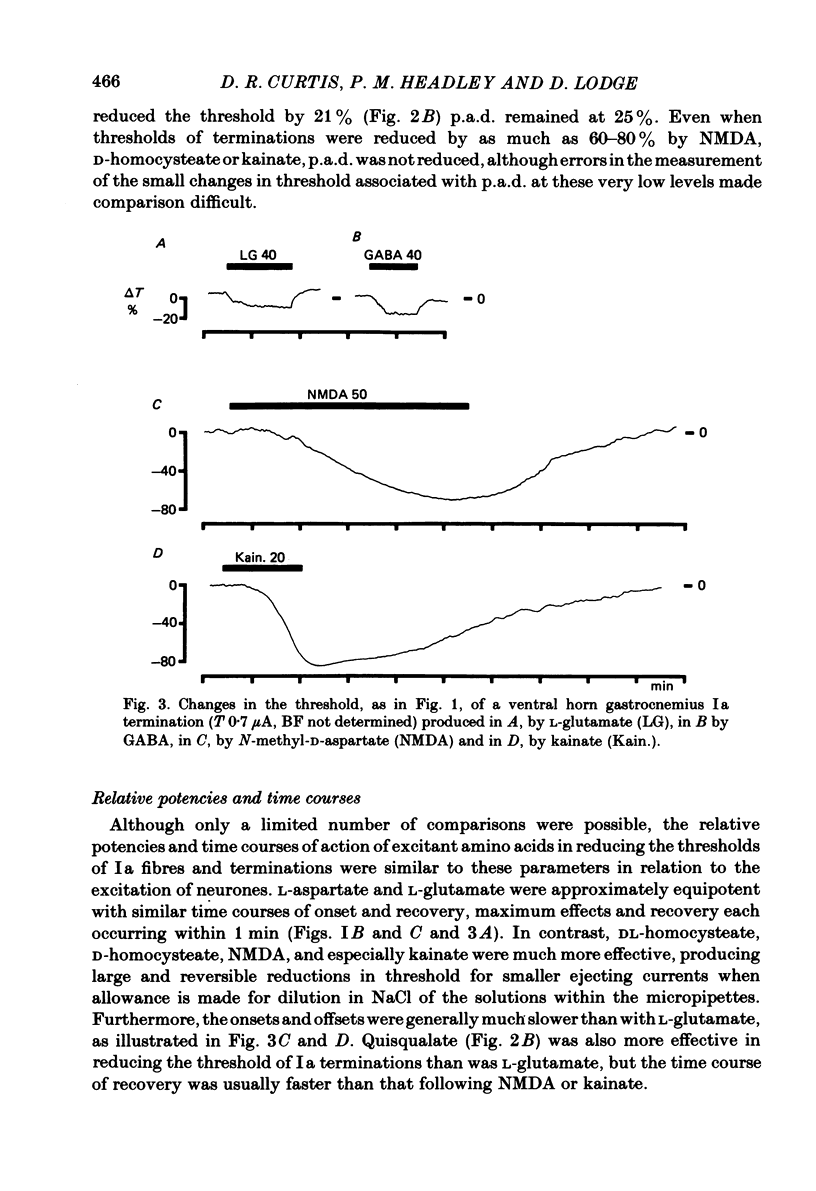
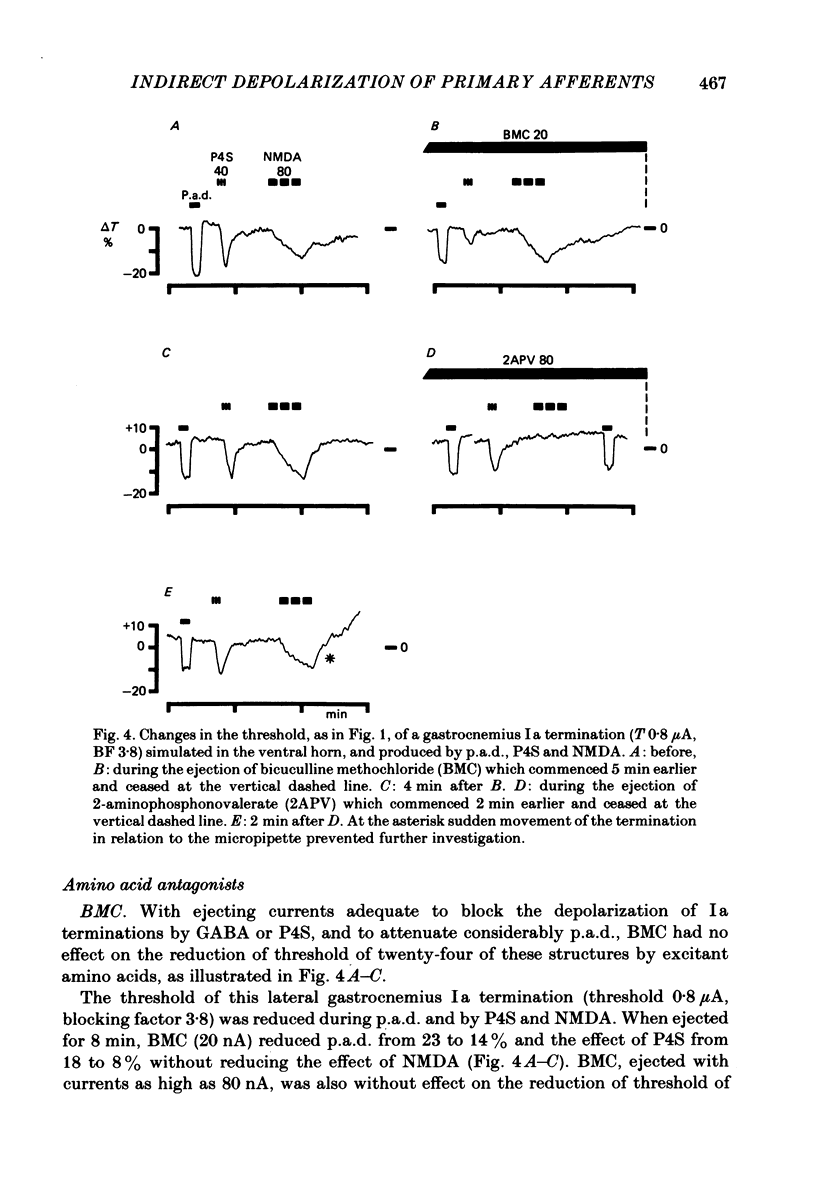
When administered micro-electrophoretically into the spinal grey matter of cats anaesthetized with pentobarbitone, acidic amino acids known to be neuronal excitants lower the threshold of electrically stimulated muscle and cutaneous primary afferent fibres and terminations. This depolarizing effect was not observed with fibres stimulated in the white matter. Depolarization by micro-electrophoretic potassium and excitant amino acids appeared not be be associated with an alteration in terminal membrane conductance since there was no change in synaptically evoked primary afferent depolarization. Excitant amino acid depolarization was not blocked by the gamma-aminobutyric acid antagonist bicuculline methochloride, but was reduced by selective excitant amino acid antagonists. The results are discussed in relation to the probable absence of specific excitant amino acid receptors on afferent terminals, the depolarizing effect of the amino acids on myelinated fibres and non-myelinated terminals being more likely a consequence of changes in the extracellular medium associated with the depolarization and firing of neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Evans R. H., Francis A. A., Martin M. R., Watkins J. C., Davies J., Dray A. D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature. 1977 Dec 22;270(5639):743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- Bührle C. P., Sonnhof U. The ionic mechanism of the excitatory action of glutamate upon the membranes of motoneurones of the frog. Pflugers Arch. 1983 Feb;396(2):154–162. doi: 10.1007/BF00615520. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PERRIN D. D., WATKINS J. C. The excitation of spinal neurones by the ionophoretic application of agents which chelate calcium. J Neurochem. 1960 Aug;6:1–20. doi: 10.1111/j.1471-4159.1960.tb13443.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R. A method for continuously monitoring the electrical threshold of single intraspinal nerve fibers. Electroencephalogr Clin Neurophysiol. 1979 Oct;47(4):503–506. doi: 10.1016/0013-4694(79)90167-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J., Leah J. D. The dual effects of GABA and related amino acids on the electrical threshold of ventral horn group Ia afferent terminations in the cat. Exp Brain Res. 1982;48(3):387–400. doi: 10.1007/BF00238615. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Brand S. J. GABA and spinal afferent terminal excitability in the cat. Brain Res. 1977 Jul 15;130(2):360–363. doi: 10.1016/0006-8993(77)90283-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D. The depolarization of feline ventral horn group Ia spinal afferent terminations by GABA. Exp Brain Res. 1982;46(2):215–233. doi: 10.1007/BF00237180. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. Pharmacological studies upon spinal presynaptic fibres. Exp Brain Res. 1966;1(2):195–204. doi: 10.1007/BF00236871. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Evans R. H. Evidence supporting the indirect depolarization of primary afferent terminals in the frog by excitatory amino acids. J Physiol. 1980 Jan;298:25–35. doi: 10.1113/jphysiol.1980.sp013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H. The effects of amino acids and antagonists on the isolated hemisected spinal cord of the immature rat. Br J Pharmacol. 1978 Feb;62(2):171–176. doi: 10.1111/j.1476-5381.1978.tb08442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U., Pumain R. Extracellular calcium activity changes in cat sensorimotor cortex induced by iontophoretic application of aminoacids. Exp Brain Res. 1980;40(3):247–250. doi: 10.1007/BF00237788. [DOI] [PubMed] [Google Scholar]
- Hösli L., Hösli E., Landolt H., Zehntner C. Efflux of potassium from neurones excited by glutamate and aspartate causes a depolarization of cultured glial cells. Neurosci Lett. 1981 Jan 1;21(1):83–86. doi: 10.1016/0304-3940(81)90062-8. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Rudomín P., Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol. 1981 Sep;46(3):506–516. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Fukuda H. Alteration of extracellular K+-activity induced by amino acids in the frog spinal cord. Jpn J Pharmacol. 1976 Jun;26(3):385–387. doi: 10.1254/jjp.26.385. [DOI] [PubMed] [Google Scholar]
- Lodge D., Headley P. M., Curtis D. R. Selective antagonism by D-alpha-aminoadipate of amino acid and synaptic excitation of cat spinal neurons. Brain Res. 1978 Sep 8;152(3):603–608. doi: 10.1016/0006-8993(78)91117-4. [DOI] [PubMed] [Google Scholar]
- Lux H. D. Fast recording ion specific microelectrodes: their use in pharmacological studies in the CNS. Neuropharmacology. 1974 Jun;13(6):509–517. doi: 10.1016/0028-3908(74)90140-3. [DOI] [PubMed] [Google Scholar]
- Padjen A. L., Smith P. A. The role of the electrogenic sodium pump in the glutamate afterhyperpolarization of frog spinal cord. J Physiol. 1983 Mar;336:433–451. doi: 10.1113/jphysiol.1983.sp014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M. J., Leah J. D., Curtis D. R. Antagonists of synaptic and amino acid excitation of neurones in the cat spinal cord. Brain Res. 1983 Apr 25;266(1):83–95. doi: 10.1016/0006-8993(83)91311-2. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977 Sep;40(5):1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- Sastry B. R. Gamma-Aminobutyric acid and primary afferent depolarization in feline spinal cord. Can J Physiol Pharmacol. 1979 Oct;57(10):1157–1167. doi: 10.1139/y79-171. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Tamarova Z. A. Differential sensitivity of individual primary afferents to glutamic and gamma-aminobutyric acids in the amphibian spinal cord in vitro. Exp Brain Res. 1983;49(1):140–142. doi: 10.1007/BF00235549. [DOI] [PubMed] [Google Scholar]
- Shefner S. A., Levy R. A. The contribution of increases in extracellular potassium to primary afferent depolarization in the bullfrog spinal cord. Brain Res. 1981 Feb 2;205(2):321–335. doi: 10.1016/0006-8993(81)90343-7. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Sonnhof U., Bührle C. P. On the postsynaptic action of glutamate in frog spinal motoneurons. Pflugers Arch. 1980 Nov;388(2):101–109. doi: 10.1007/BF00584115. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Vyskocil F., Kolaj M., Jastreboff P. Primary afferent depolarization and changes in extracellular potassium concentration induced by L-glutamate and L-proline in the isolated spinal cord of the frog. Neurosci Lett. 1982 Oct 8;32(2):159–164. doi: 10.1016/0304-3940(82)90267-1. [DOI] [PubMed] [Google Scholar]


