Abstract
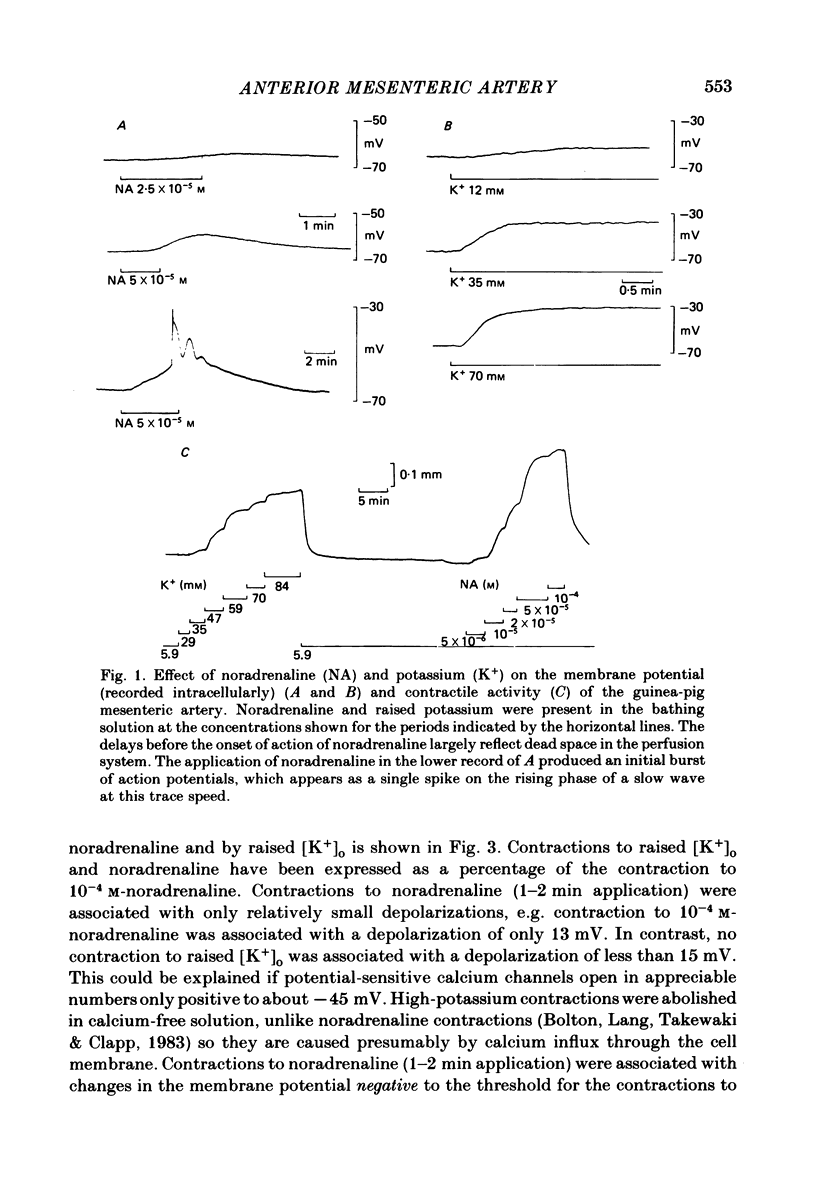
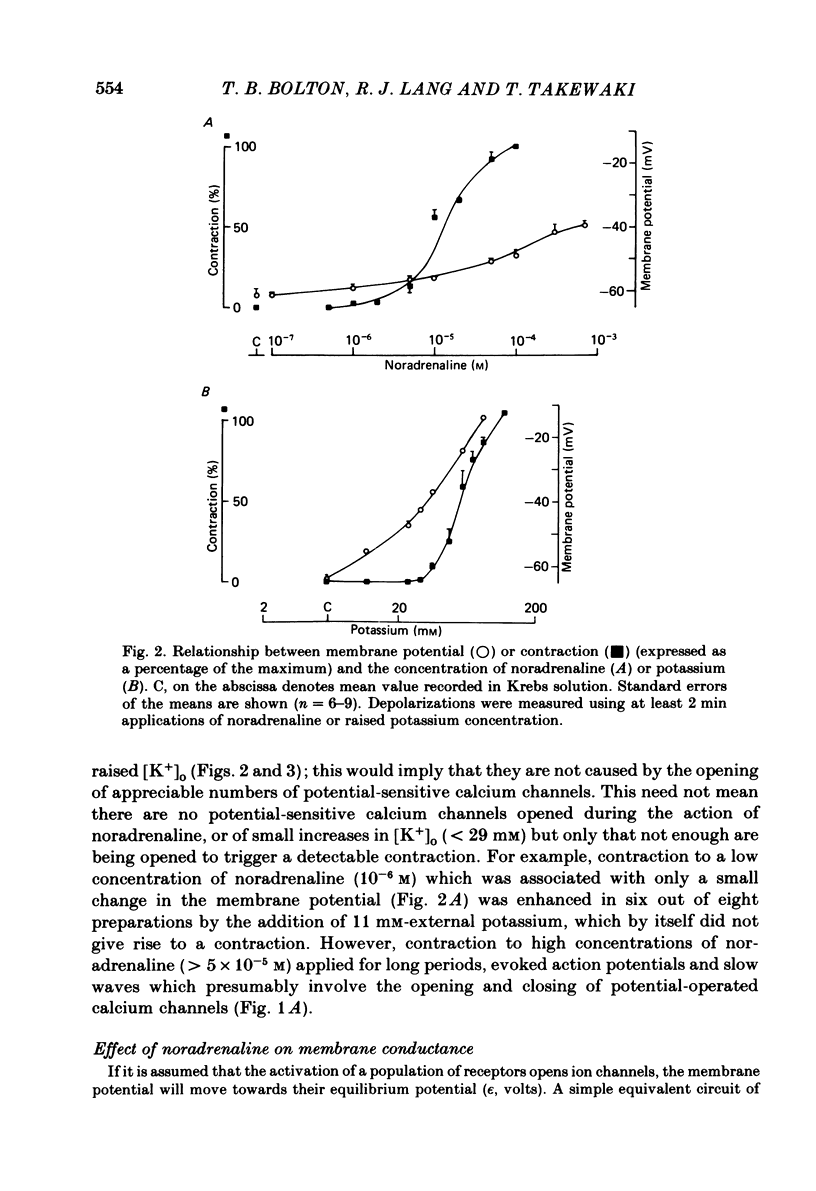
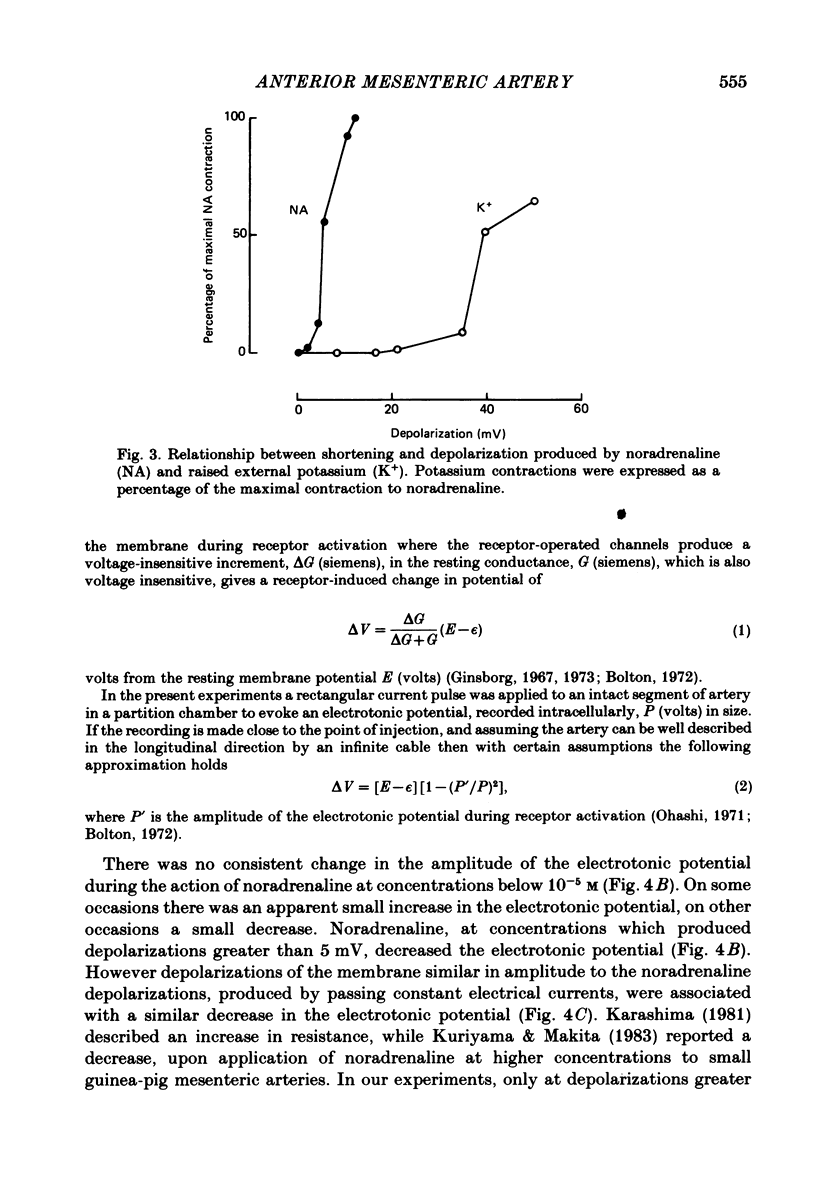
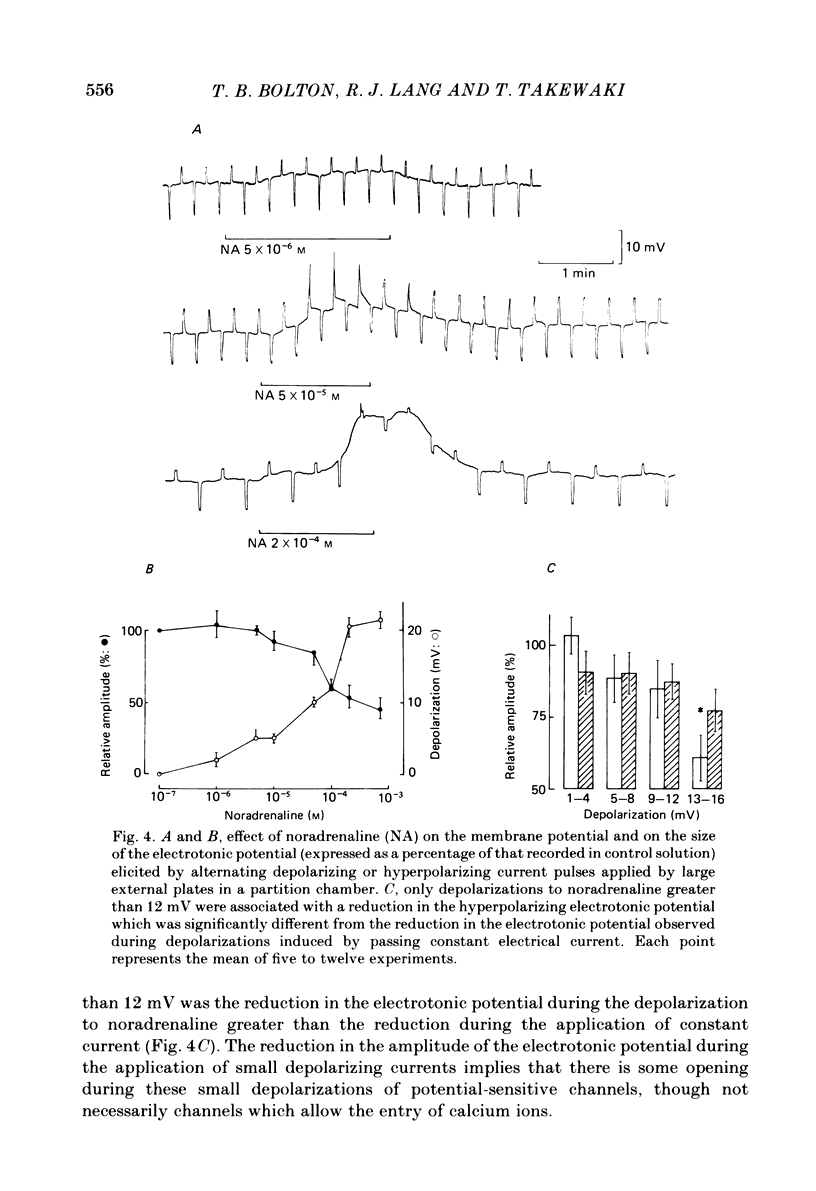
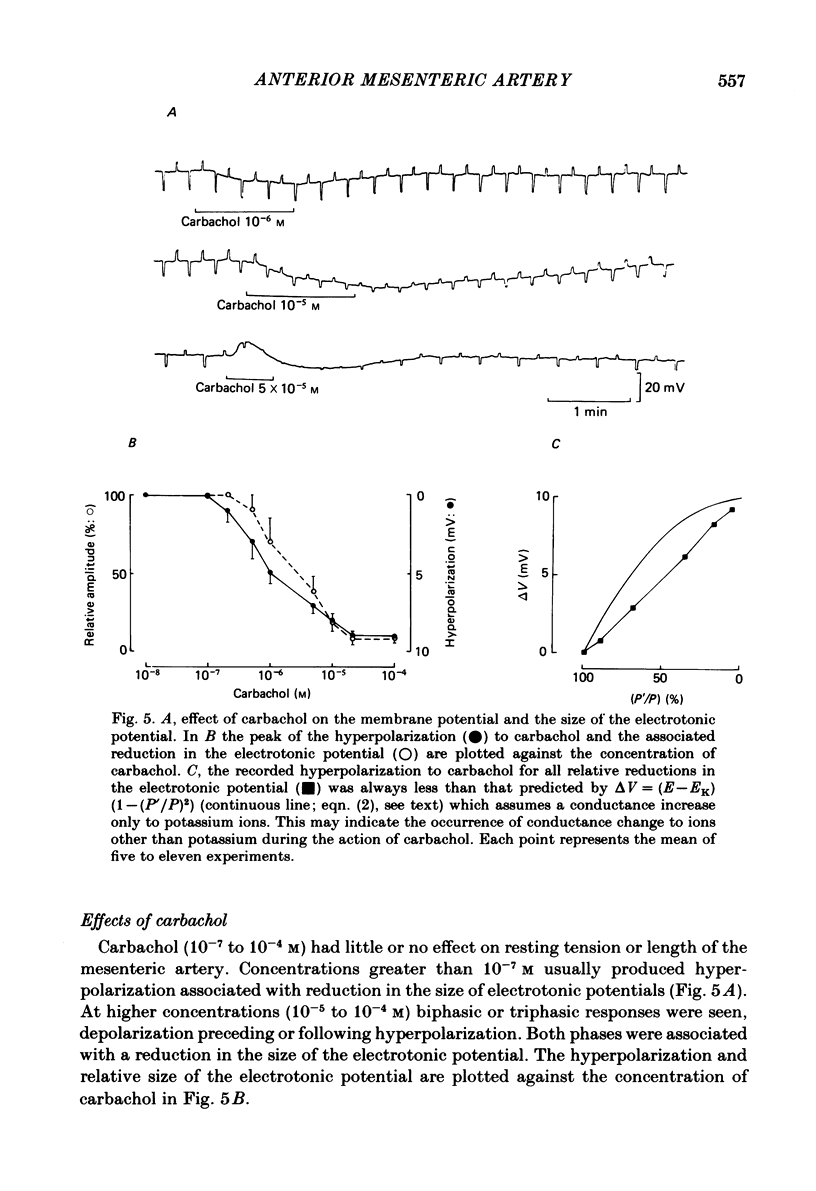
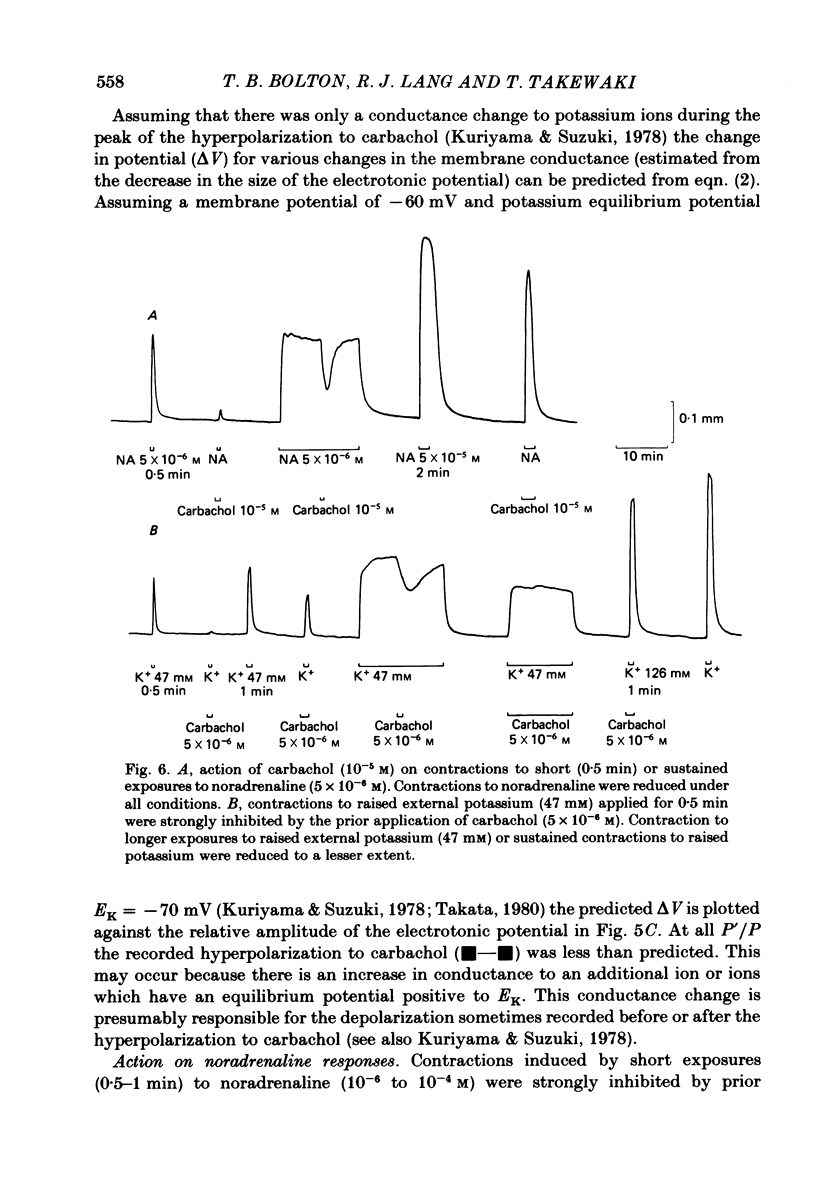
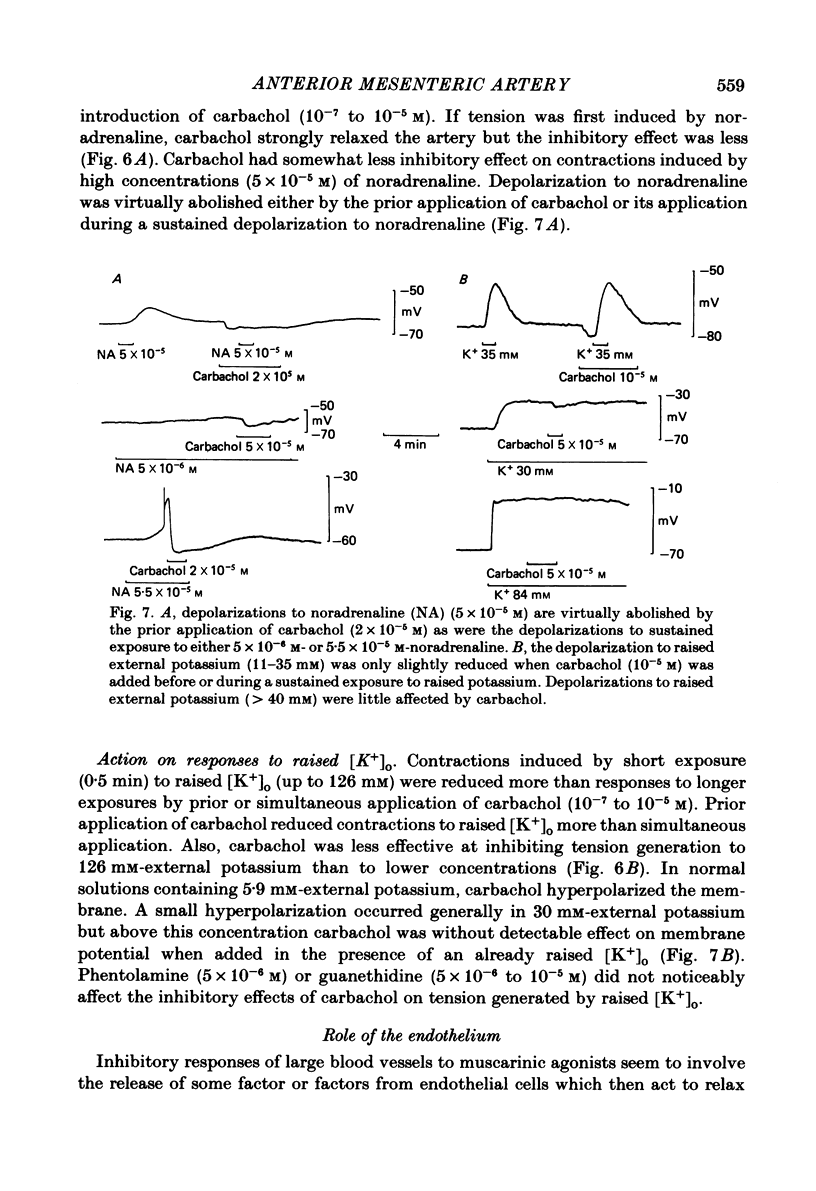
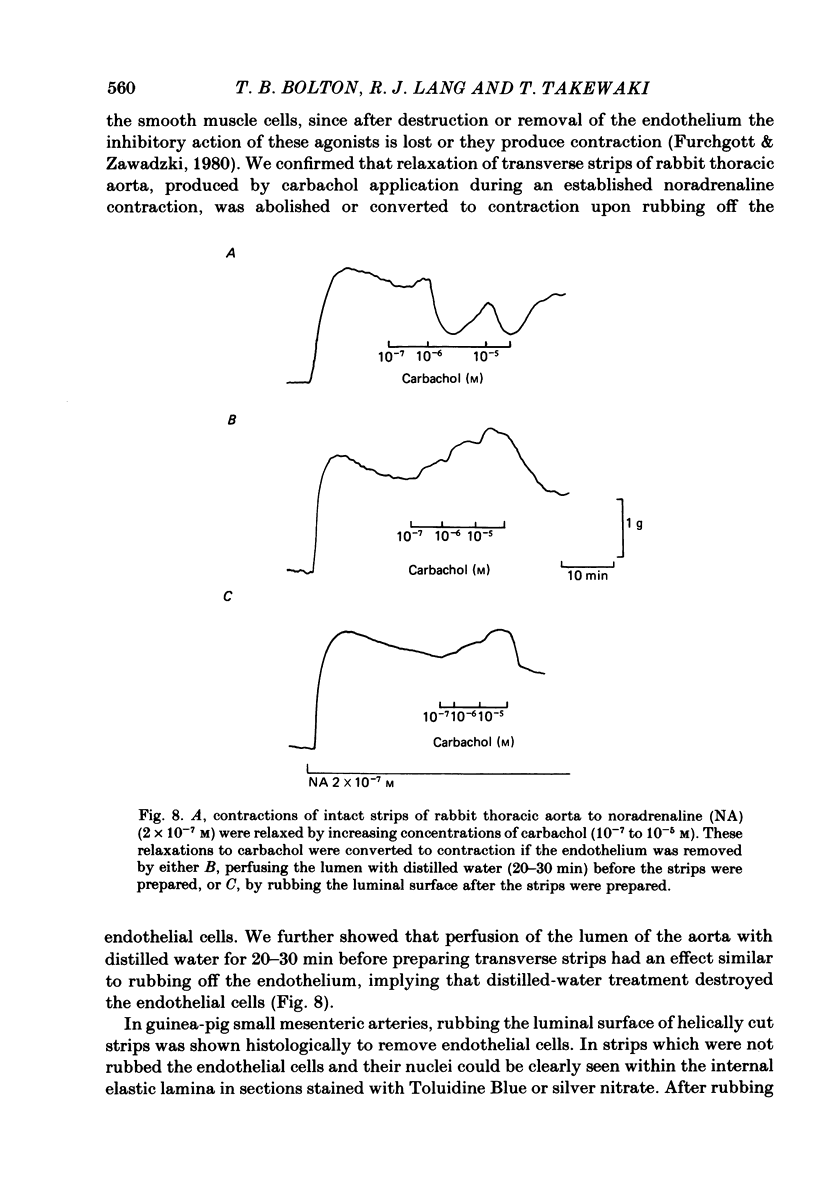
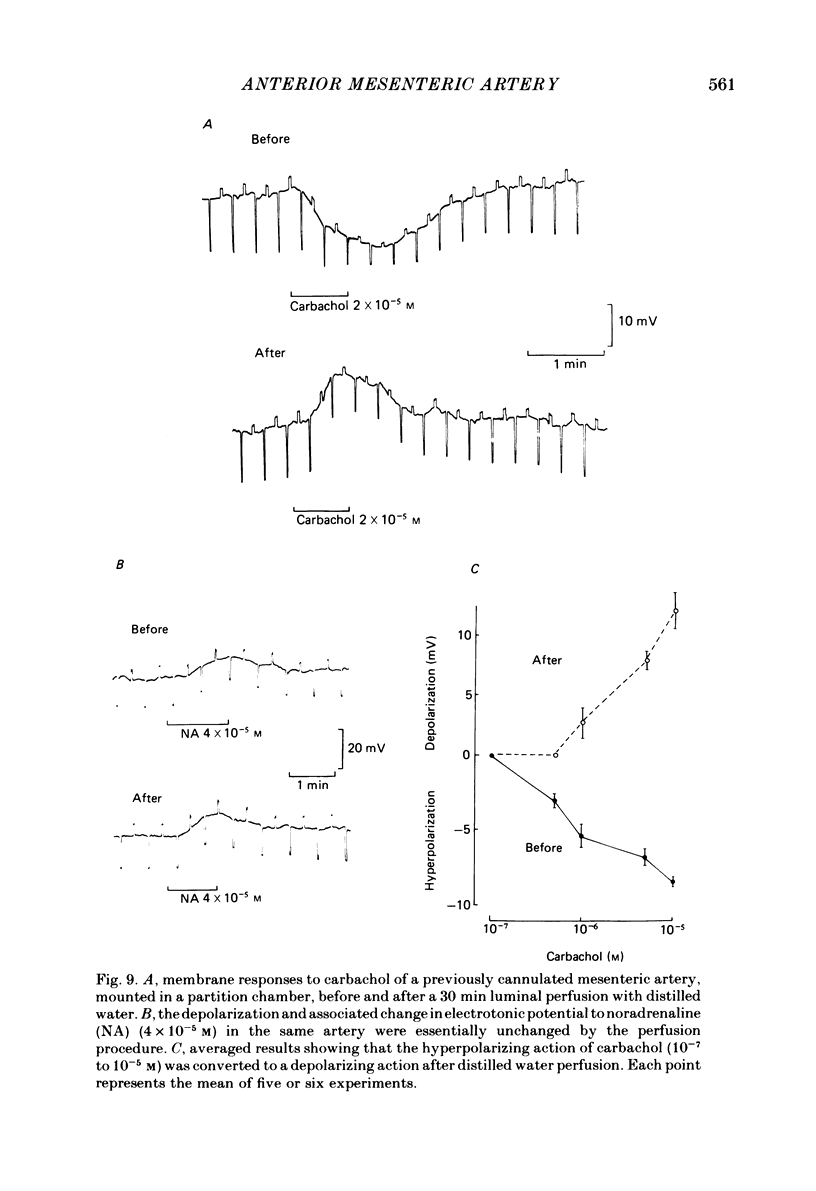
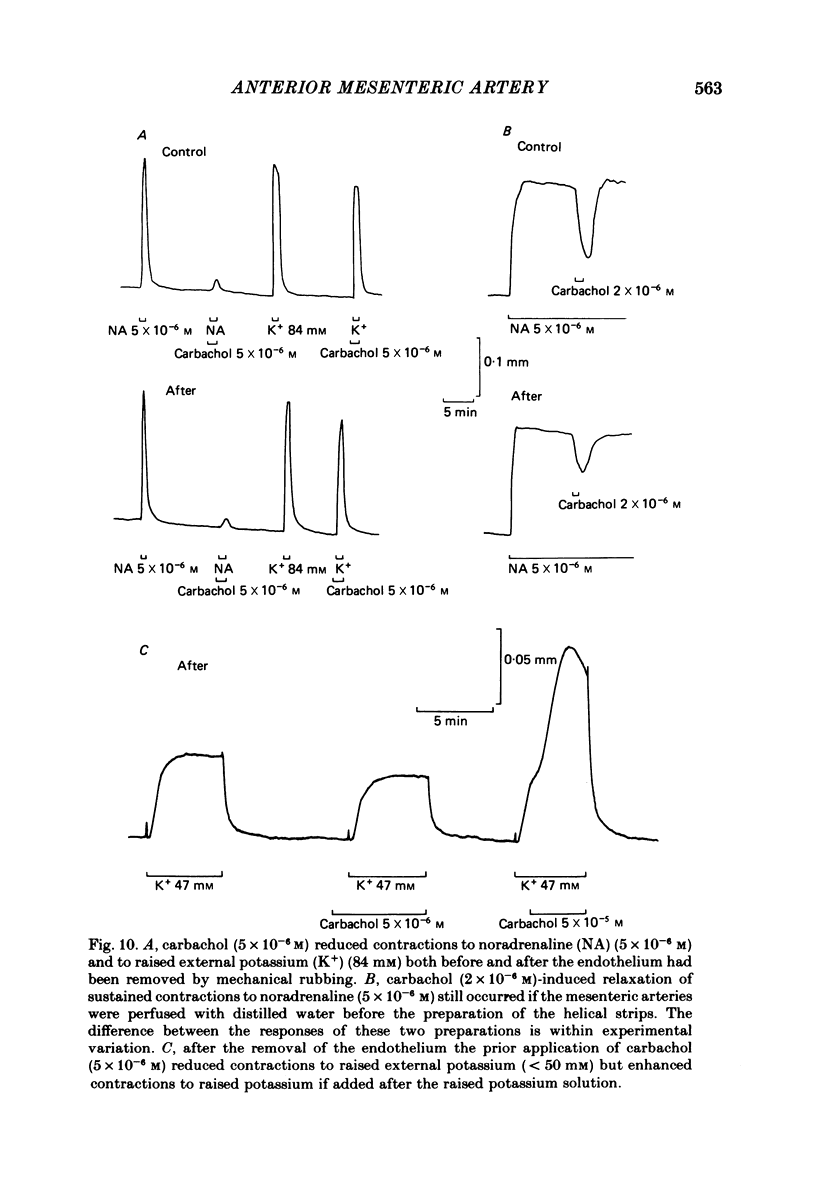
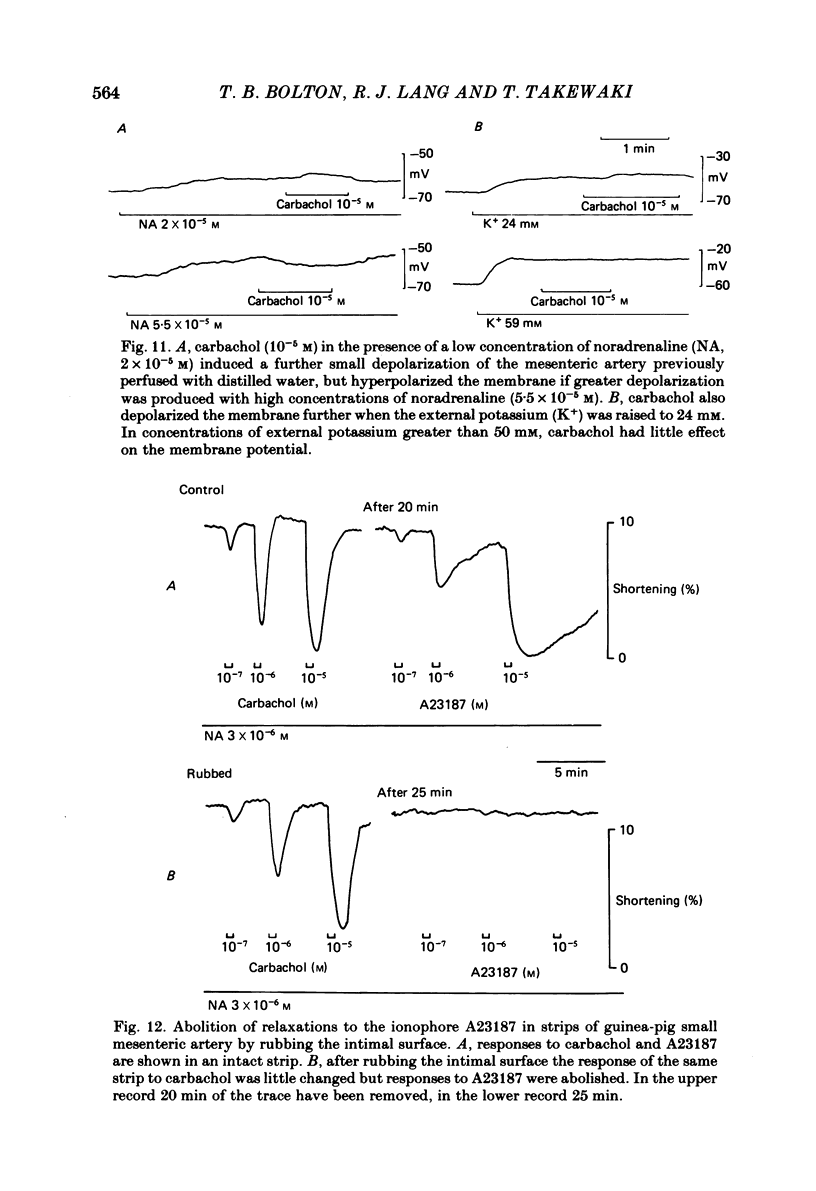
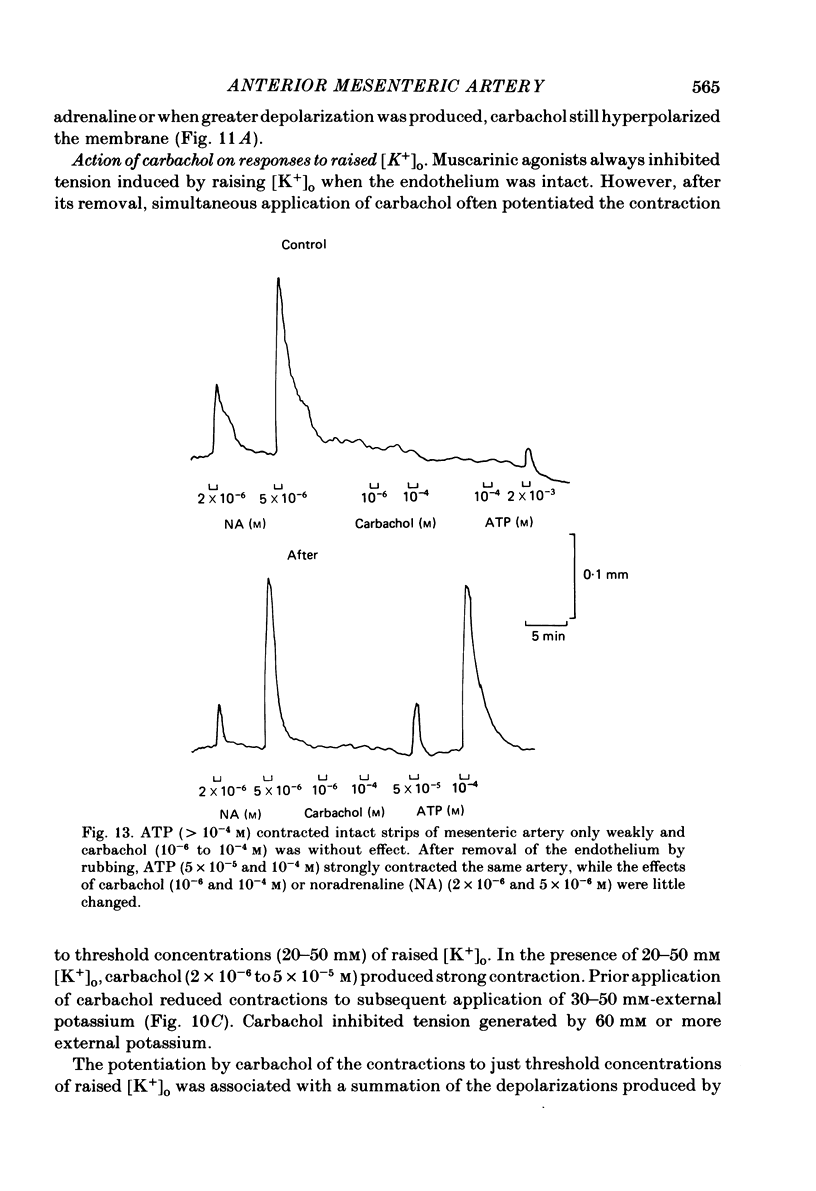
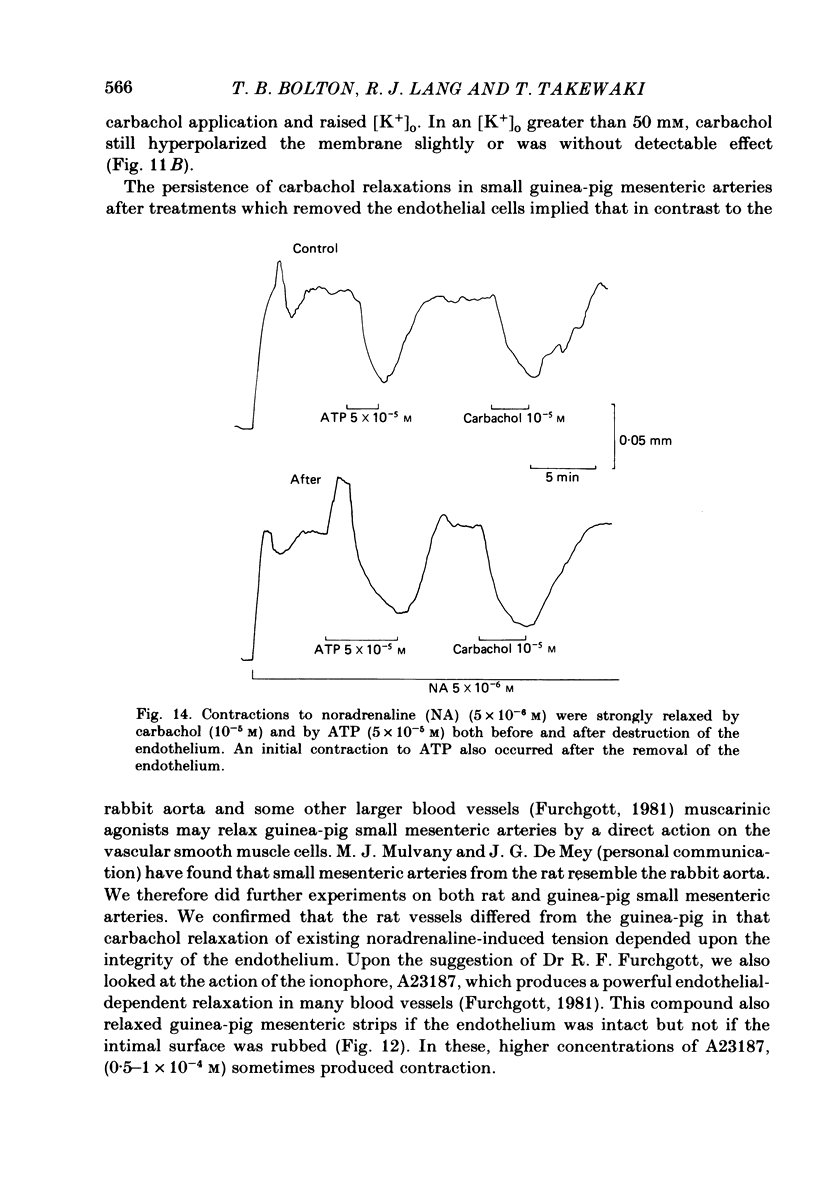
Membrane potential was recorded by micro-electrode in segments of small (200-500 microns o.d.) mesenteric arteries of guinea-pig. Isotonic shortening was recorded in helical strips cut from these arteries. Raising the external potassium concentration, [K+]o, caused shortening and substantial depolarization. The threshold for contraction was about 30 mM which corresponded to a membrane potential of about -45 mV. Since high-potassium contractions were abolished in calcium-free solution it was suggested that they occur due to potential-sensitive calcium channels opening positive to about -45 mV. Noradrenaline weakly depolarized the muscle and produced contractions resistant to calcium-free conditions. It was suggested that noradrenaline contractions are mainly caused by mechanisms other than the opening of potential-sensitive calcium channels, namely entry of calcium via other channels and release of stored calcium. Carbachol had no effect on basal tension but inhibited shortening by noradrenaline or by raising [K+]o. The inhibitory effect of carbachol on tension under various conditions was associated with hyperpolarization or depolarization in a range negative to -45 mV, or no effect on potential, so that modulation of the number of open potential-sensitive calcium channels could not be evoked to explain its relaxant action. Removal or destruction of the endothelium by rubbing or by distilled water perfusion left tension responses to noradrenaline or raised [K+]o essentially unchanged. However, the inhibitory effect of carbachol on tension was attenuated and hyperpolarization of the resting artery was converted to a depolarization. It was concluded that carbachol has both a strong inhibitory and a weak excitatory effect on these vascular smooth muscle cells. Membrane potential changes are not essential to its inhibitory action but may, by closing potential-sensitive calcium channels, sometimes reinforce it. Hyperpolarization by carbachol may be caused by a factor released by the action of carbachol on endothelial cells: in its absence carbachol may weakly depolarize but this alone is normally insufficient to generate tension.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. ACETYLCHOLINE IN ADRENERGIC TRANSMISSION. Annu Rev Pharmacol. 1965;5:163–182. doi: 10.1146/annurev.pa.05.040165.001115. [DOI] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Ottesen B. Mechanism of action of vasoactive intestinal polypeptide on myometrial smooth muscle of rabbit and guinea-pig. J Physiol. 1981 Sep;318:41–55. doi: 10.1113/jphysiol.1981.sp013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F. The pharmacology of vascular smooth muscle. Pharmacol Rev. 1955 Jun;7(2):183–265. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Gokhale S. D., Gulati O. D., Kelkar L. V., Kelkar V. V. Effect of some drugs on human umbilical artery in vitro. Br J Pharmacol Chemother. 1966 Aug;27(2):332–346. doi: 10.1111/j.1476-5381.1966.tb01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Abel P. W., Hermsmeyer K. Membrane electrical mechanism of basilar artery constriction and pial artery dilation by norepinephrine. Circ Res. 1981 Dec;49(6):1237–1242. doi: 10.1161/01.res.49.6.1237. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. Effects of tetraethylammonium chloride on sympathetic neuromuscular transmission in saphenous artery of young rabbits. J Physiol. 1980 Aug;305:451–465. doi: 10.1113/jphysiol.1980.sp013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Nitroglycerine and catecholamine actions on smooth muscle cells of the canine coronary artery. J Physiol. 1980 Dec;309:171–183. doi: 10.1113/jphysiol.1980.sp013502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M., Kitamura K., Kuriyama H. Neuromuscular transmission and smooth muscle membrane properties in the guinea-pig ear artery. J Physiol. 1981 Jun;315:283–302. doi: 10.1113/jphysiol.1981.sp013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T. Effects of vasopressin on smooth muscle cells of guinea-pig mesenteric vessels. Br J Pharmacol. 1981 Apr;72(4):673–684. doi: 10.1111/j.1476-5381.1981.tb09148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. Modulation of noradrenergic transmission in the guinea-pig mesenteric artery: an electrophysiological study. J Physiol. 1983 Feb;335:609–627. doi: 10.1113/jphysiol.1983.sp014554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. U., Ling G. M. Modification by acetylcholine of the response of rat mesenteric arteries to sympathetic stimulation. Circ Res. 1969 Jul;25(1):1–9. doi: 10.1161/01.res.25.1.1. [DOI] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Studies of the electrical excitability of aorta smooth muscle of rabbit. J Physiol. 1979 Aug;293:11–21. doi: 10.1113/jphysiol.1979.sp012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H. The relative contribution of K and Cl to the total increase of membrane conductance produced by adrenaline on the smooth muscle of guinea-pig Taenia coli. J Physiol. 1971 Jan;212(2):561–575. doi: 10.1113/jphysiol.1971.sp009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Varma B. The effects of cholinomimetic drugs on responses to sympathetic nerve stimulation and noradrenaline in the rabbit ear artery. Br J Pharmacol. 1970 Apr;38(4):758–770. doi: 10.1111/j.1476-5381.1970.tb09885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Steinsland O. S., Furchgott R. F., Kirpekar S. M. Inhibition of adrenergic neurotransmission by parasympathomimetics in the rabbit ear artery. J Pharmacol Exp Ther. 1973 Feb;184(2):346–356. [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Takata Y. Regional differences in electrical and mechanical properties of guinea-pig mesenteric vessels. Jpn J Physiol. 1980;30(5):709–728. doi: 10.2170/jjphysiol.30.709. [DOI] [PubMed] [Google Scholar]
- Trapani A., Matsuki N., Abel P. W., Hermsmeyer K. Norepinephrine produces tension through electromechanical coupling in rabbit ear artery. Eur J Pharmacol. 1981 Jun 10;72(1):87–91. doi: 10.1016/0014-2999(81)90301-0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]


