Abstract
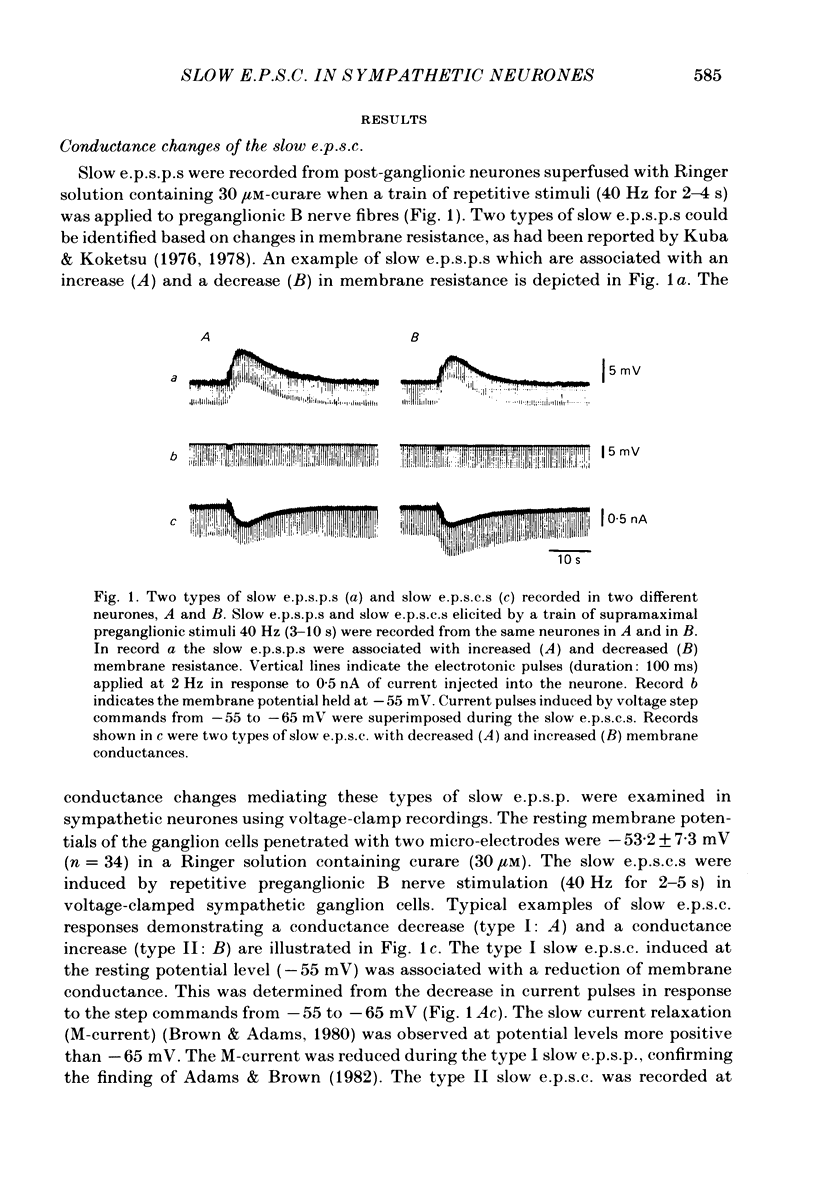
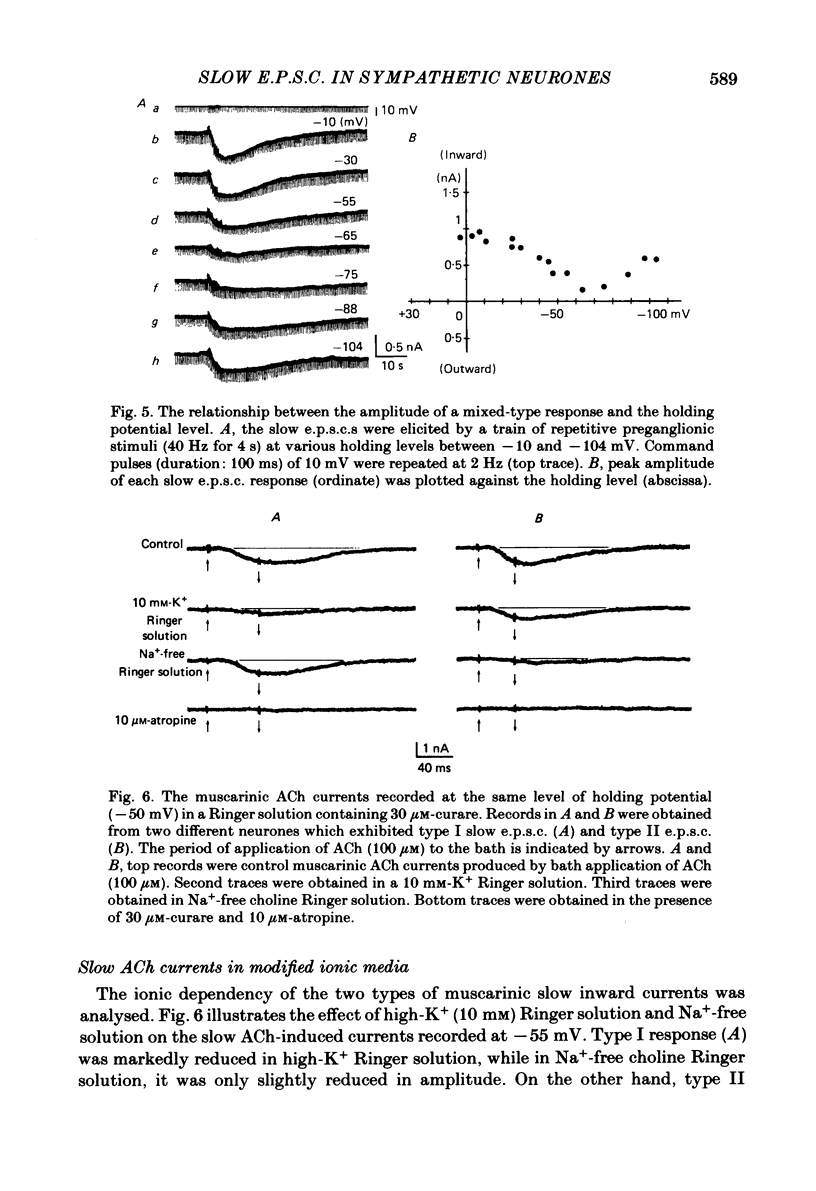
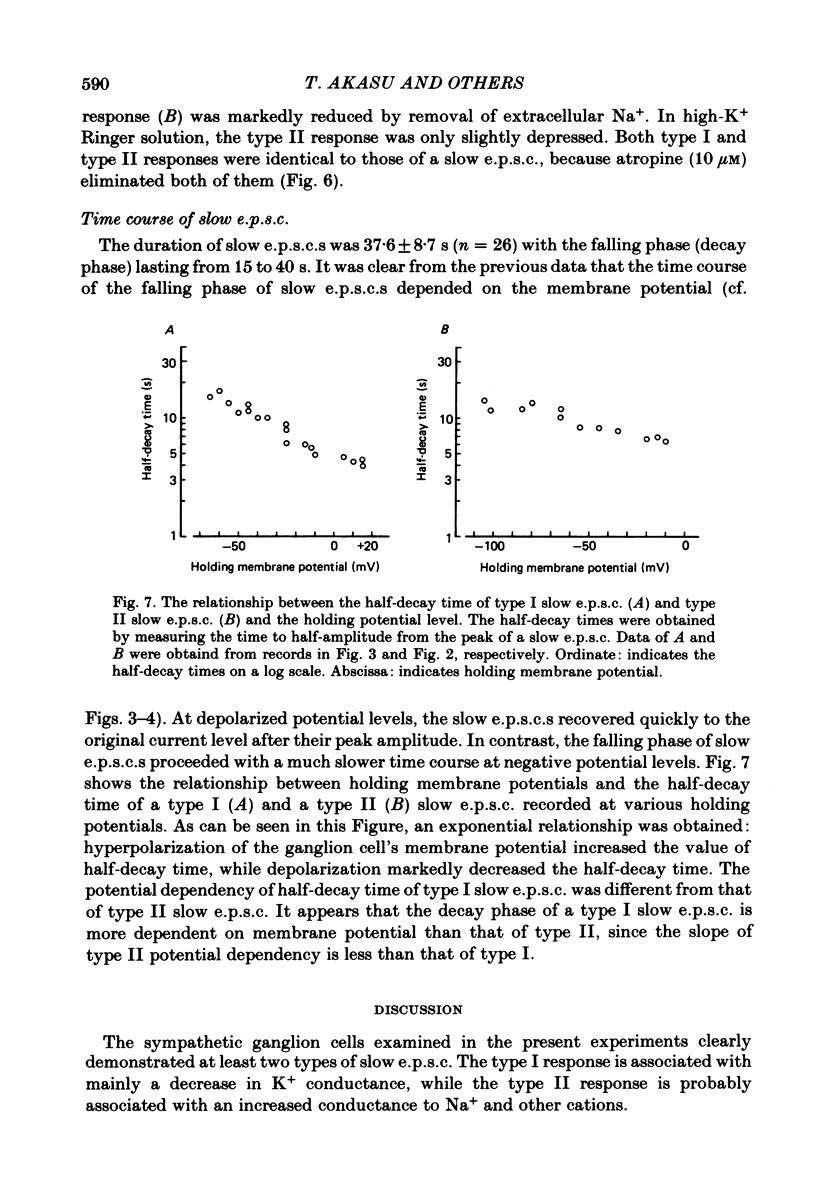
Electrogenesis of the slow excitatory post-synaptic current (slow e.p.s.c.) was analysed with voltage-clamp methods in curarized sympathetic ganglion cells of bull-frogs. Three types of slow e.p.s.c. were observed from B neurones of sympathetic ganglia. The type I slow e.p.s.c. was associated with a decrease in membrane conductance, was depressed by membrane hyperpolarization and nullified at -60 to -70 mV. It was observed in 65% of the sympathetic neurones studied. The type II slow e.p.s.c. was associated with an increase in membrane conductance, was depressed by membrane depolarization and nullified at around +5 mV. It was observed in 14% of the neurones studied. A third type of slow e.p.s.c. was recorded from 21% of the sympathetic neurones in this study. This slow e.p.s.c. was a mixed type having characteristics of both type I and type II slow e.p.s.c.s. Activation of muscarinic cholinergic receptors by application of acetylcholine (ACh) also produced two types of inward currents. The nature of each type of muscarinic slow ACh current was similar to that of each type of slow e.p.s.c. The time course of the falling phase of type I and type II slow e.p.s.c.s was dependent on the membrane potential. The type I slow e.p.s.c. was primarily dependent on extracellular K+ and appeared to be produced by a suppression of the M-current (Brown & Adams, 1980). The type II slow e.p.s.c. was due to an increased conductance, probably to Na+, and other cations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol. 1982 Nov;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Voltage-clamp studies of a slow inward current in bullfrog sympathetic ganglion cells. Neurosci Lett. 1981 Nov 4;26(3):259–262. doi: 10.1016/0304-3940(81)90142-7. [DOI] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Nishi S. Voltage-clamp analysis of peptidergic slow depolarizations in bullfrog sympathetic ganglion cells. J Physiol. 1982 Dec;333:305–313. doi: 10.1113/jphysiol.1982.sp014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Is inactivation of potassium conductance involved in slow postsynaptic excitation of sympathetic ganglion cells? Effects of nicotine. Life Sci. 1974 May 16;14(10):1871–1883. doi: 10.1016/0024-3205(74)90404-4. [DOI] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Ionic mechanism of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Brain Res. 1974 Dec 6;81(2):338–342. doi: 10.1016/0006-8993(74)90949-4. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Libet B., Weight F. F., Votava J. Inactivation of potassium conductance in slow postsynaptic excitation. Science. 1971 Apr 30;172(3982):503–504. doi: 10.1126/science.172.3982.503-a. [DOI] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Unusual nature of ganglionic slow EPSP studied by a voltage-clamp method. Life Sci. 1969 Jan 1;8(1):33–42. doi: 10.1016/0024-3205(69)90290-2. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Tasaka J., Miyazaki T., Libet B. Hyperpolarization following activation of K+ channels by excitatory postsynaptic potentials. Nature. 1983 Sep 8;305(5930):148–150. doi: 10.1038/305148a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]



