Abstract
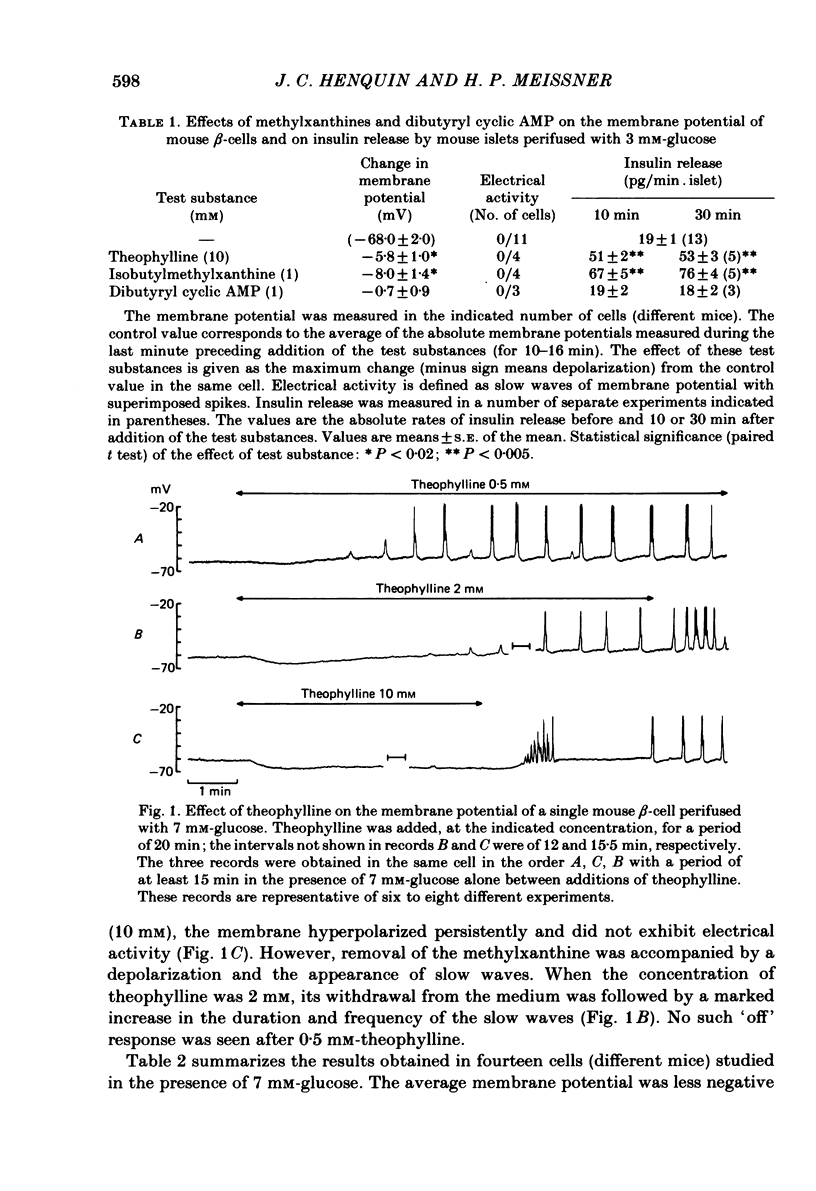
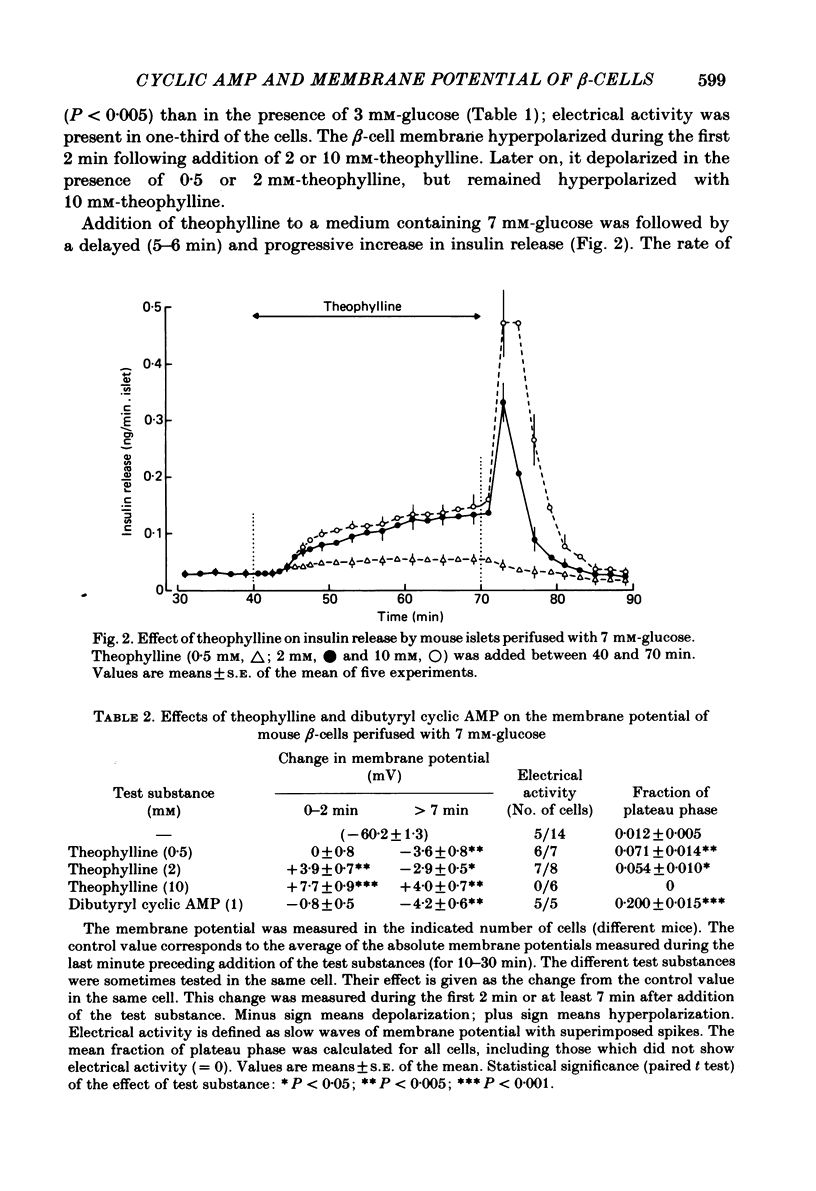
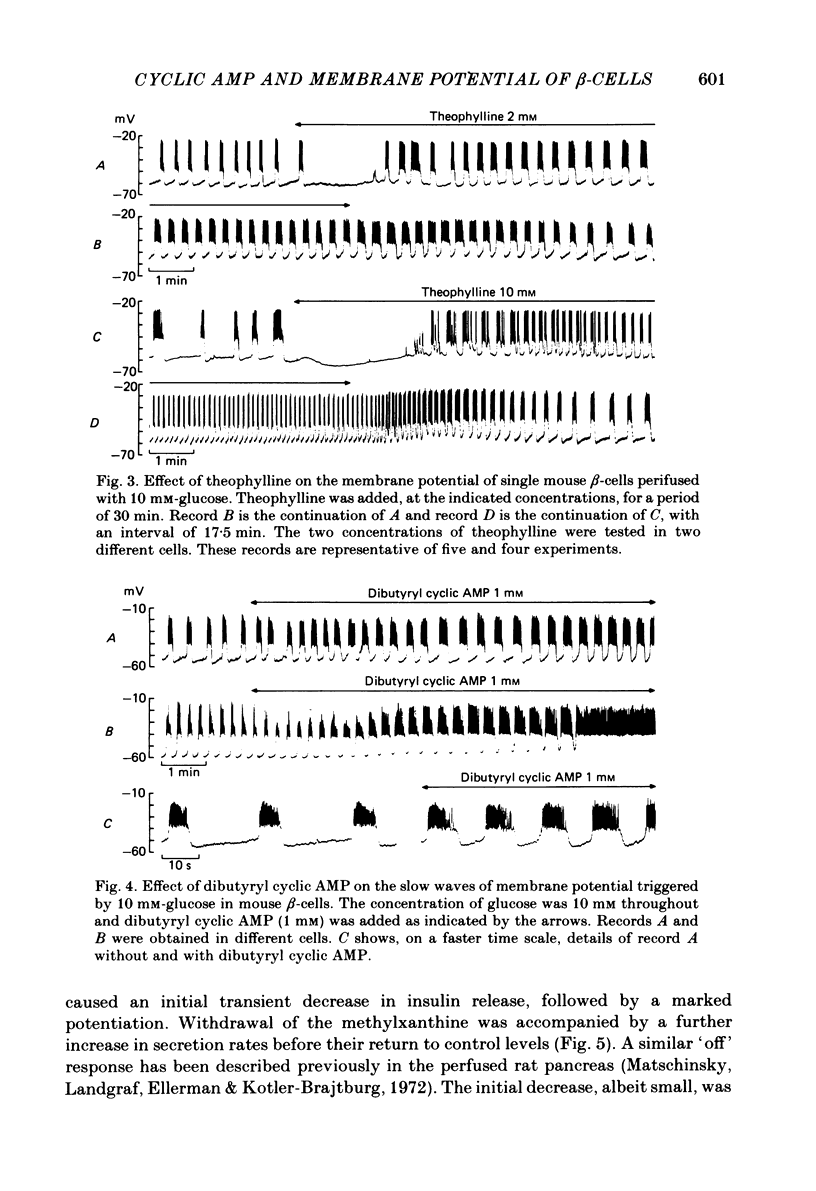
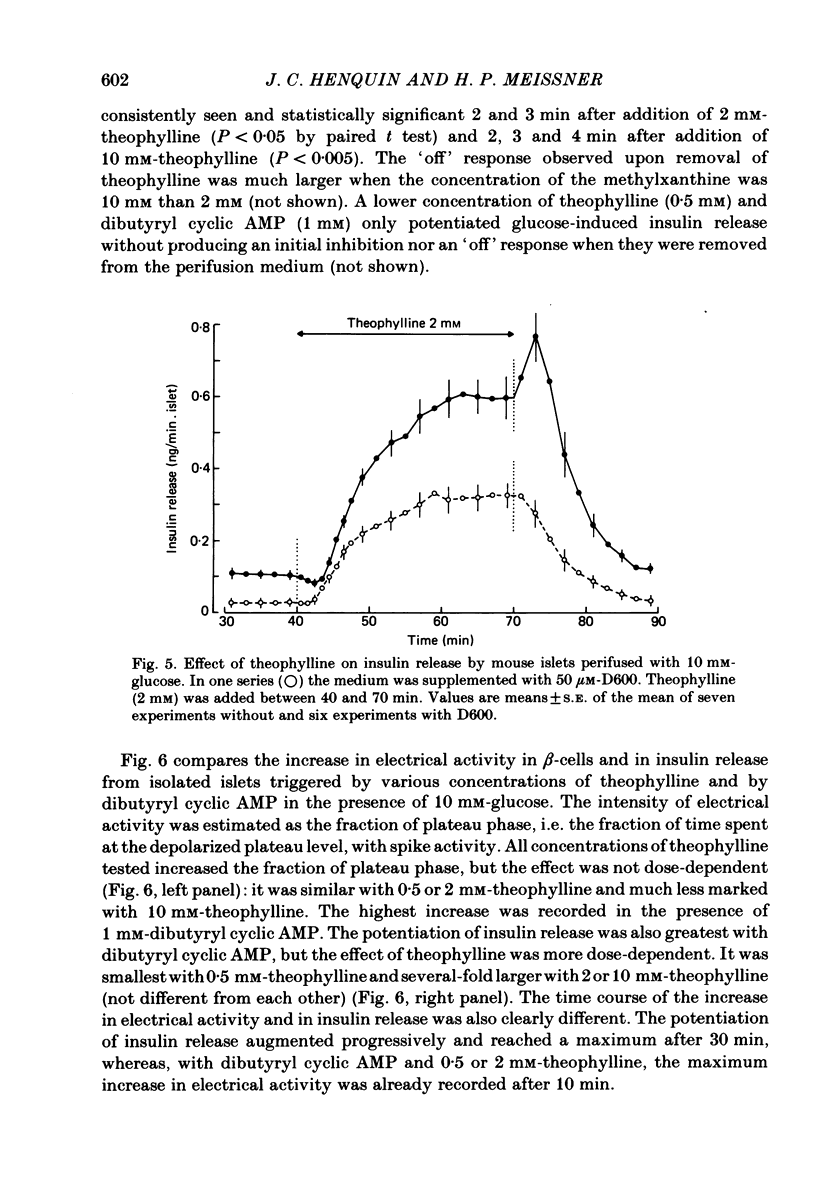
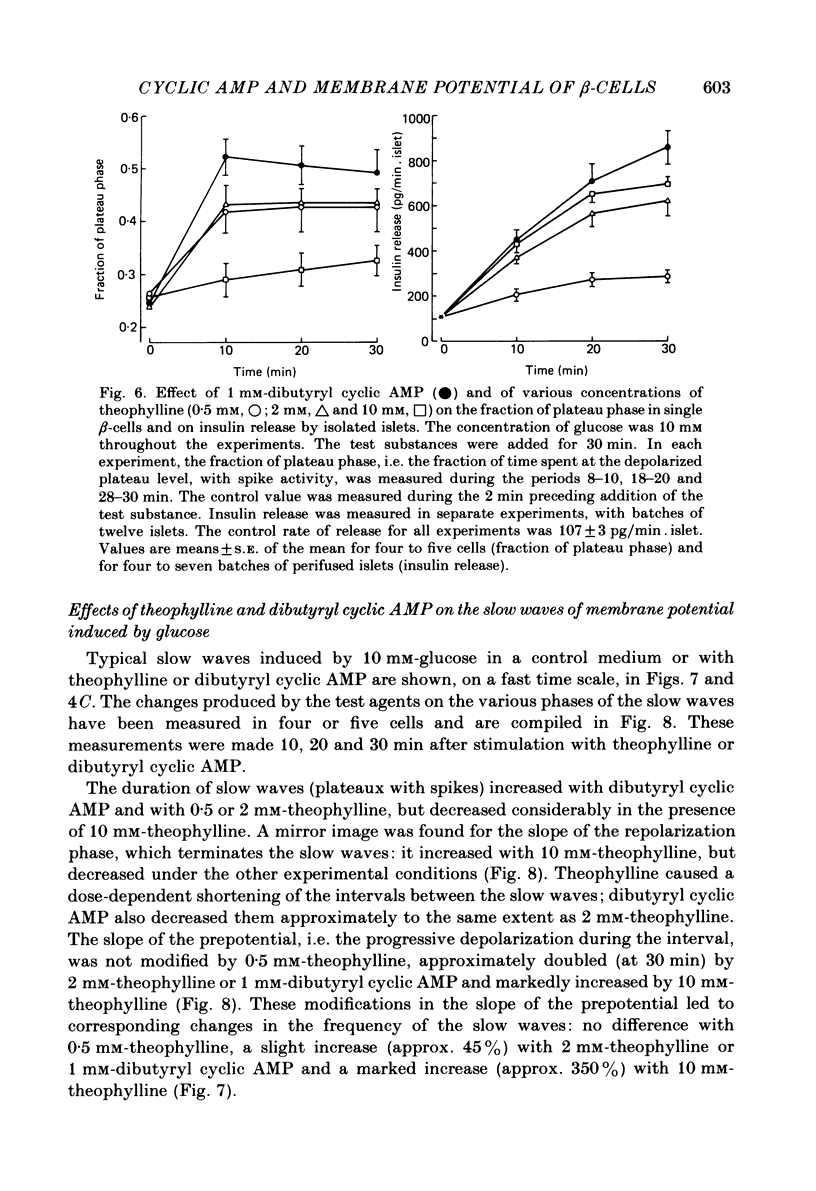
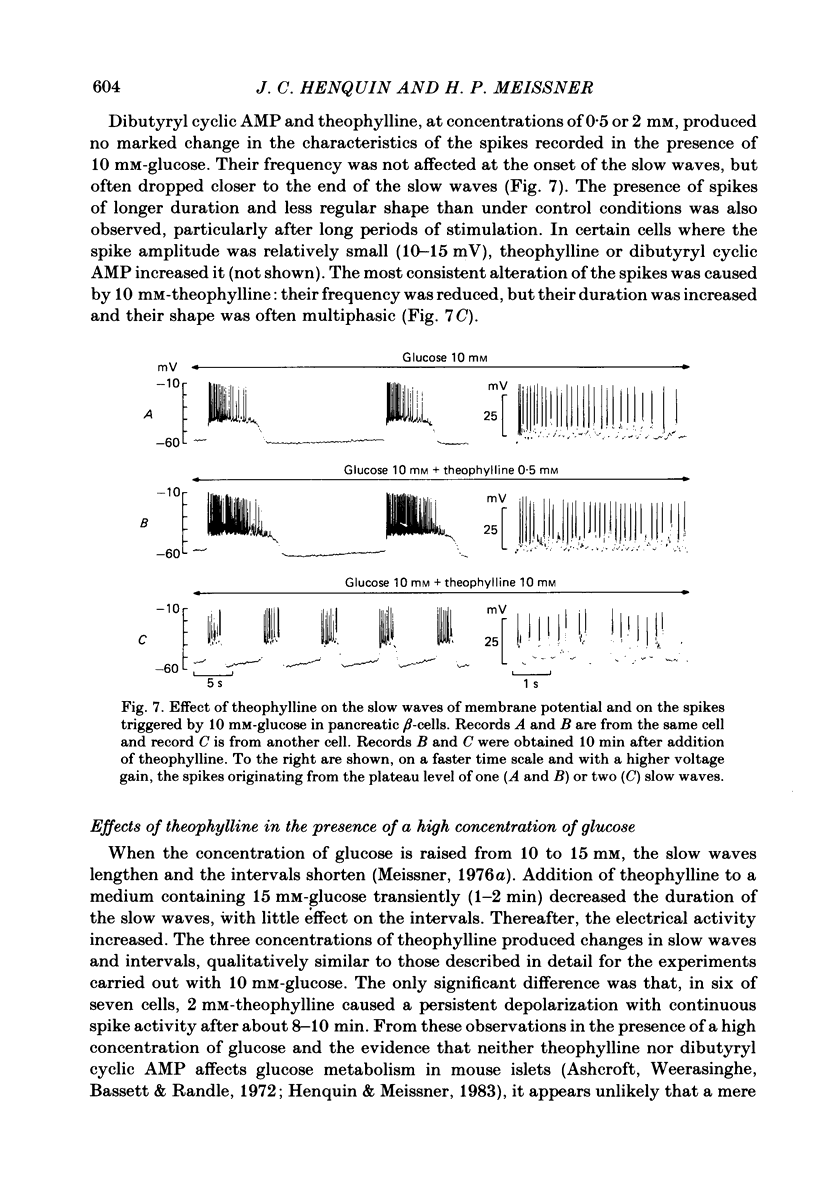
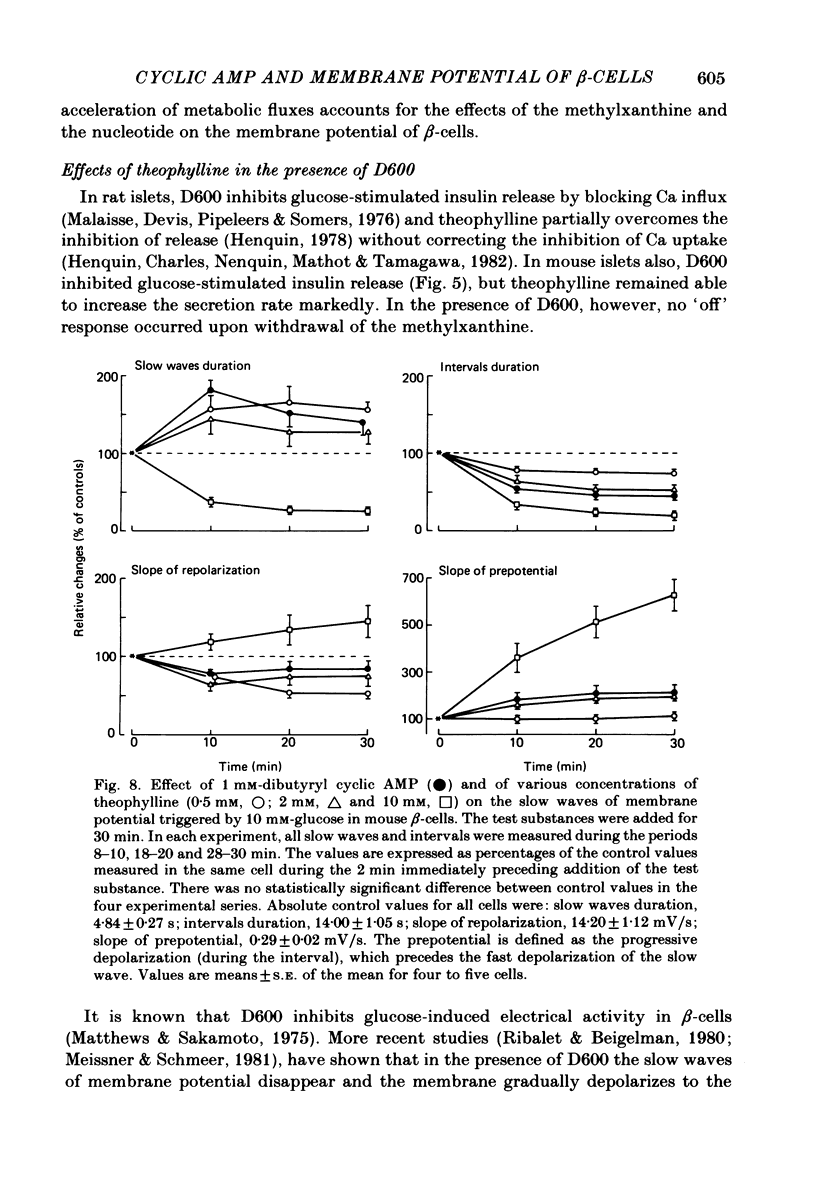
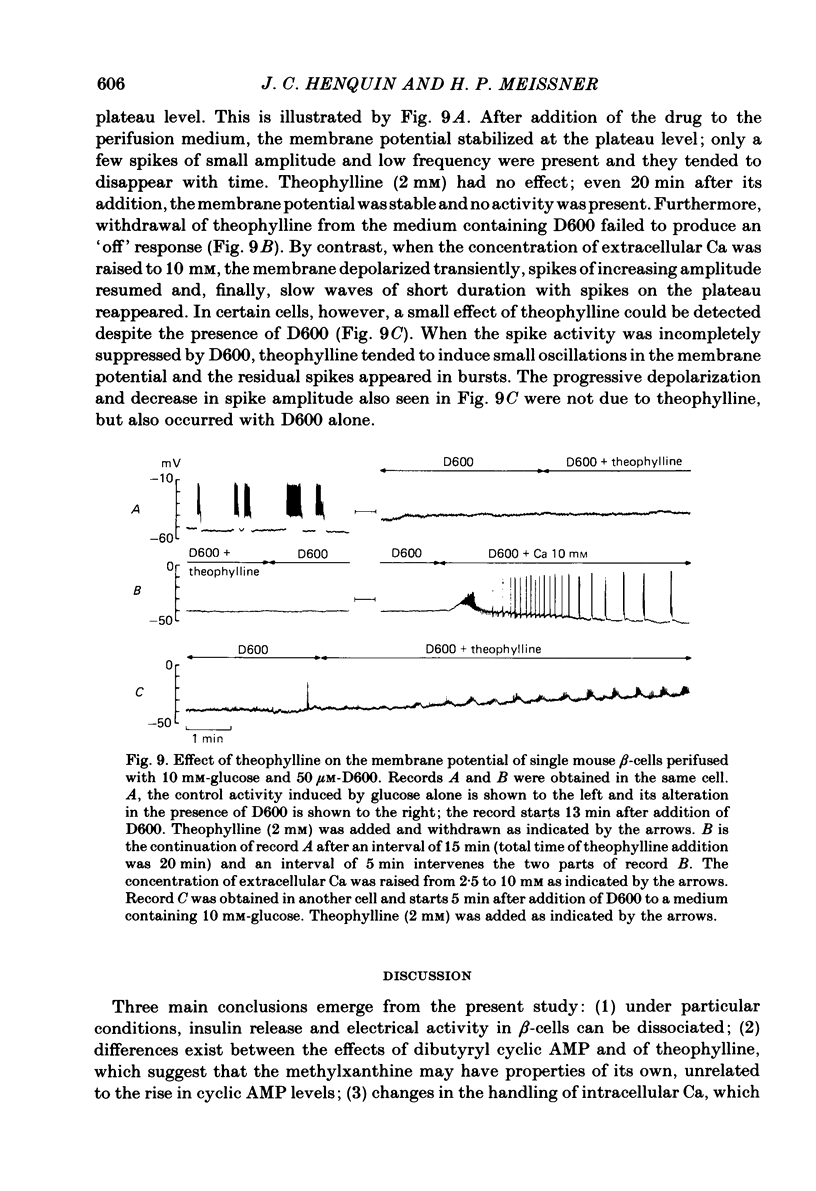
The effects of theophylline and dibutyryl cyclic AMP on the membrane potential of mouse beta-cells were studied with micro-electrodes. They were compared to their effects on insulin release by perifused mouse islets. In 3 mM-glucose, theophylline (10 mM) depolarized the beta-cell membrane and stimulated insulin release, but did not induce electrical activity. Dibutyryl cyclic AMP (1 mM) was without effect. In 7 mM-glucose, theophylline (0.5-2 mM) and dibutyryl cyclic AMP (1 mM) slightly depolarized the beta-cell membrane, induced electrical activity in otherwise silent cells and increased insulin release. A higher concentration of theophylline (10 mM) hyperpolarized the beta-cell membrane, did not induce electrical activity, but also stimulated insulin release. In 10 mM-glucose, the membrane potential of beta-cells exhibited repetitive slow waves with bursts of spikes on the plateau. Under steady state, these slow waves were differently affected by low or high concentrations of theophylline. At 0.5-2 mM, theophylline shortened the intervals, lengthened the slow waves and slightly increased their frequency. On the other hand, 10 mM-theophylline markedly decreased the duration of both intervals and slow waves, and increased their frequency. The effects of 1 mM-dibutyryl cyclic AMP were similar to those of 2 mM-theophylline. With 2-10 mM-theophylline, two other effects were also observed: a transient hyperpolarization with suppression of electrical activity immediately after addition of the methylxanthine and an increase in electrical activity upon its withdrawal. Theophylline and dibutyryl cyclic AMP markedly potentiated insulin release induced by 10 mM-glucose. The magnitude of these changes did not correlate well with the importance of the changes in electrical activity. However, with 2-10 mM-theophylline the increase in release was also preceded by an initial transient inhibition, whereas withdrawal of the methylxanthine was accompanied by a further increase. When Ca influx was inhibited by D600, the slow waves were suppressed, the membrane was depolarized to the plateau level and only few spikes were present. Although theophylline markedly increased insulin release under these conditions, it did not affect the membrane potential. Several conclusions can be drawn from this study. Insulin release and electrical activity in beta-cells can be dissociated when intracellular Ca is used to trigger exocytosis. High concentrations of theophylline produce effects unrelated to cyclic AMP.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Ribalet B. Theophylline inhibition and stimulation of glucose-induced electrical activity in mouse pancreatic beta-cells [proceedings]. J Physiol. 1979 Jun;291:55–50. [PubMed] [Google Scholar]
- Berridge M. J., Rapp P. E. A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol. 1979 Aug;81:217–279. doi: 10.1242/jeb.81.1.217. [DOI] [PubMed] [Google Scholar]
- Brisson G. R., Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. VII. A proposed site of action for adenosine-3',5'-cyclic monophosphate. J Clin Invest. 1972 Feb;51(2):232–241. doi: 10.1172/JCI106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capito K., Hedeskov C. J. The effect of starvation on phosphodiesterase activity and the content of adenosine 3' :5'-cyclic monophosphate in isolated mouse pancreatic islets. Biochem J. 1974 Sep;142(3):653–658. doi: 10.1042/bj1420653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinelli A. R., Malaisse W. J. Regulation of 86Rb outflow from pancreatic islets: the dual effect of nutrient secretagogues. J Physiol. 1981 Jun;315:143–156. doi: 10.1113/jphysiol.1981.sp013738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. Calcium and pancreatic beta-cell function: modification of 45Ca fluxes by methylxanthines and dibutyryl cyclic-AMP. Biochem Med. 1981 Dec;26(3):365–376. doi: 10.1016/0006-2944(81)90012-0. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Charles S., Nenquin M., Mathot F., Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982 Sep;31(9):776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Dibutyryl cyclic AMP triggers Ca2+ influx and Ca2+-dependent electrical activity in pancreatic B cells. Biochem Biophys Res Commun. 1983 Apr 29;112(2):614–620. doi: 10.1016/0006-291x(83)91508-5. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P., Preissler M. 9-Aminoacridine- and tetraethylammonium-induced reduction of the potassium permeability in pancreatic B-cells. Effects on insulin release and electrical properties. Biochim Biophys Acta. 1979 Nov 1;587(4):579–592. doi: 10.1016/0304-4165(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. The electrogenic sodium-potassium pump of mouse pancreatic B-cells. J Physiol. 1982 Nov;332:529–552. doi: 10.1113/jphysiol.1982.sp014429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. Metabolic control of potassium permeability in pancreatic islet cells. Biochem J. 1980 Feb 15;186(2):541–550. doi: 10.1042/bj1860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Relative importance of extracellular and intracellular calcium for the two phases of glucose-stimulated insulin release: studies with theophylline. Endocrinology. 1978 Mar;102(3):723–730. doi: 10.1210/endo-102-3-723. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Schmeer W., Meissner H. P. Forskolin, an activator of adenylate cyclase, increases CA2+-dependent electrical activity induced by glucose in mouse pancreatic B cells. Endocrinology. 1983 Jun;112(6):2218–2220. doi: 10.1210/endo-112-6-2218. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Adams W. B. Cyclic AMP modulation of a specific ion channel in an identified nerve cell: possible role for protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:647–653. [PubMed] [Google Scholar]
- Malaisse W. J., Devis G., Pipeleers D. G., Somers G. Calcium-antagonists and islet function. IV. Effect of D600. Diabetologia. 1976 Mar;12(1):77–81. doi: 10.1007/BF01221969. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Landgraf R., Ellerman J., Kotler-Brajtburg J. Glucoreceptor mechanisms in islets of Langerhans. Diabetes. 1972;21(2 Suppl):555–569. doi: 10.2337/diab.21.2.s555. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P., Atwater I. J. The kinetics of electrical activity of beta cells in response to a "square wave" stimulation with glucose or glibenclamide. Horm Metab Res. 1976 Jan;8(1):11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Meissner H. P. Electrical characteristics of the beta-cells in pancreatic islets. J Physiol (Paris) 1976 Nov;72(6):757–767. [PubMed] [Google Scholar]
- Meissner H. P., Preissler M. Glucose-induced changes of the membrane potential of pancreatic B-cells: their significance for the regulation of insulin release. Adv Exp Med Biol. 1979;119:97–107. doi: 10.1007/978-1-4615-9110-8_15. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Montague W., Howell S. L. Cyclic AMP and the physiology of the islets of Langerhans. Adv Cyclic Nucleotide Res. 1975;6:201–243. [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Ribalet B., Beigelman P. M. Calcium action potentials and potassium permeability activation in pancreatic beta-cells. Am J Physiol. 1980 Sep;239(3):C124–C133. doi: 10.1152/ajpcell.1980.239.3.C124. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Scott A. M., Atwater I., Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981 Nov;21(5):470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Sehilin J. Calcium uptake by subcellular fractions of pancreatic islets. Effects of nucleotides and theophylline. Biochem J. 1976 Apr 15;156(1):63–69. doi: 10.1042/bj1560063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Dependency of cyclic AMP-induced insulin release on intra- and extracellular calcium in rat islets of Langerhans. J Clin Invest. 1980 Feb;65(2):233–241. doi: 10.1172/JCI109665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumwasser F., Kaczmarek L. K., Jennings K. R. Intracellular modulation of membrane channels by cyclic AMP-mediated protein phosphorylation in peptidergic neurons of Aplysia. Fed Proc. 1982 Nov;41(13):2933–2939. [PubMed] [Google Scholar]
- Sugden M. C., Ashcroft S. J. Effects of phosphoenolpyruvate, other glycolytic intermediates and methylxanthines on calcium uptake by a mitochondrial fraction from rat pancreatic islets. Diabetologia. 1978 Sep;15(3):173–180. doi: 10.1007/BF00421235. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]


