Abstract
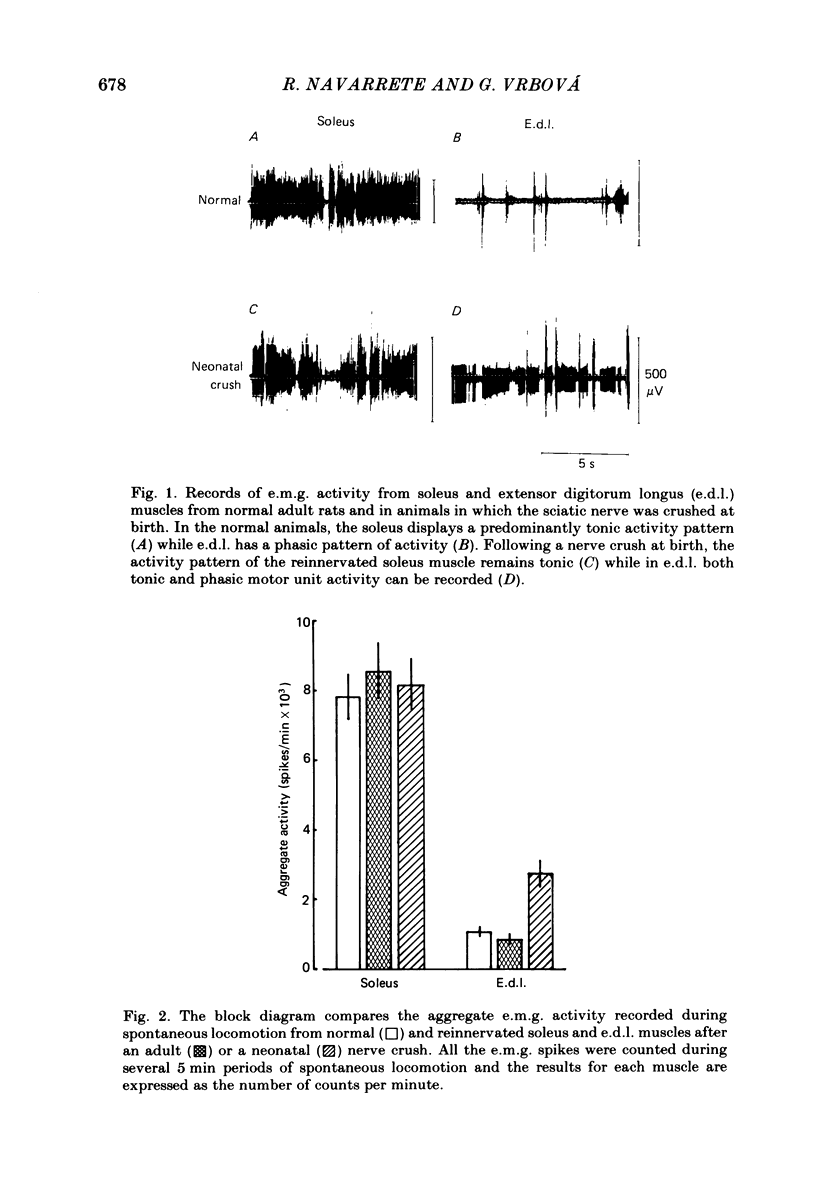
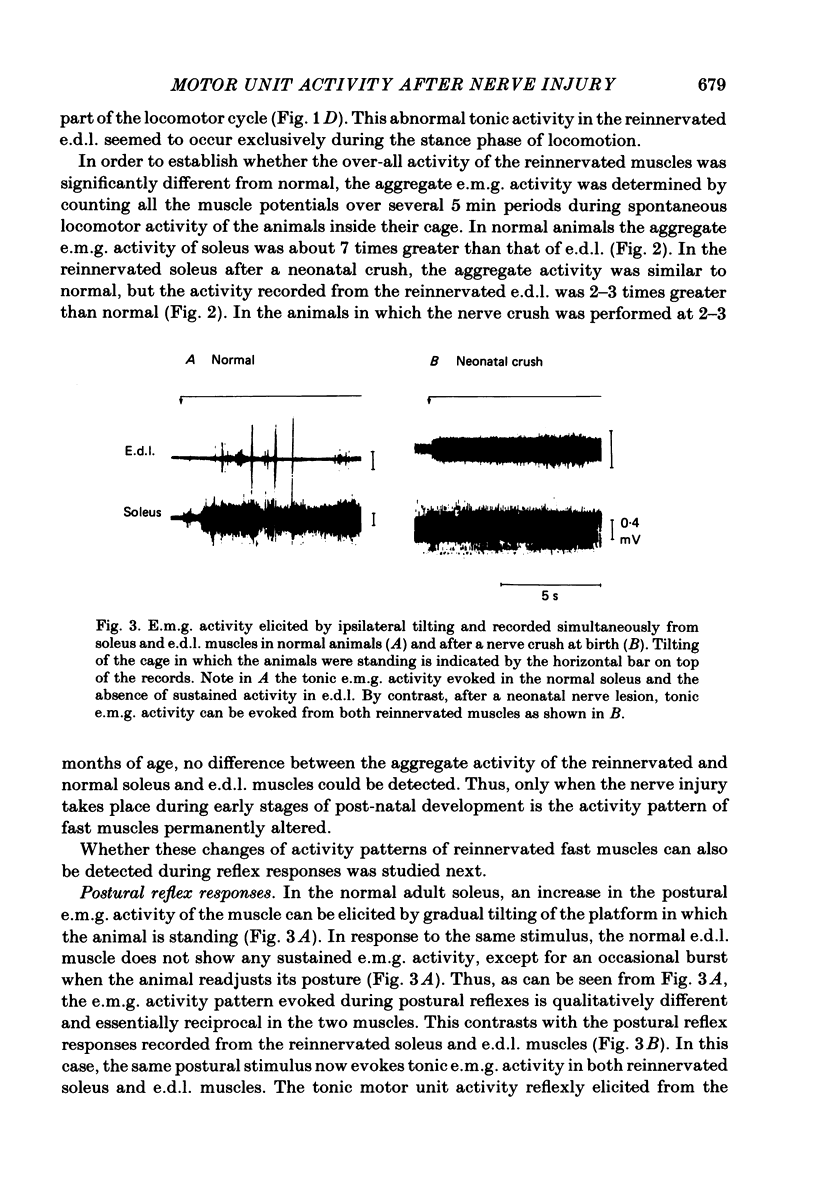
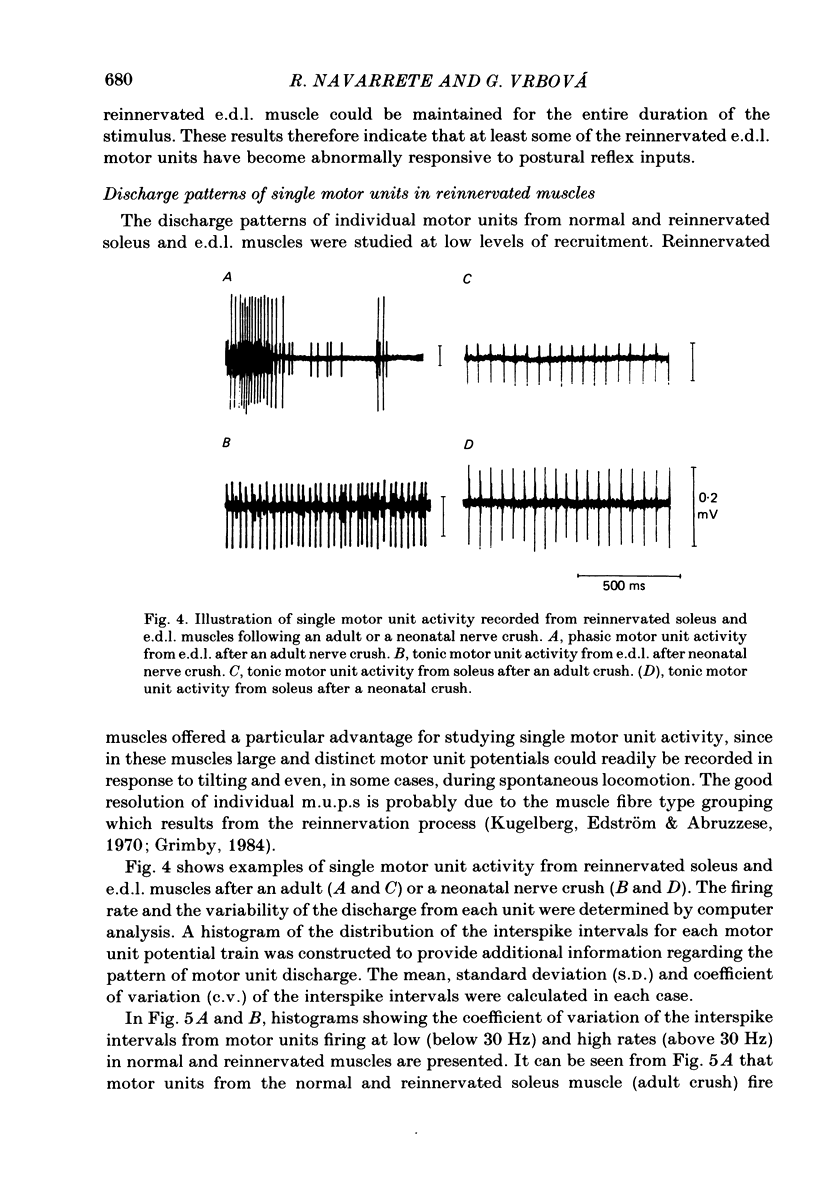
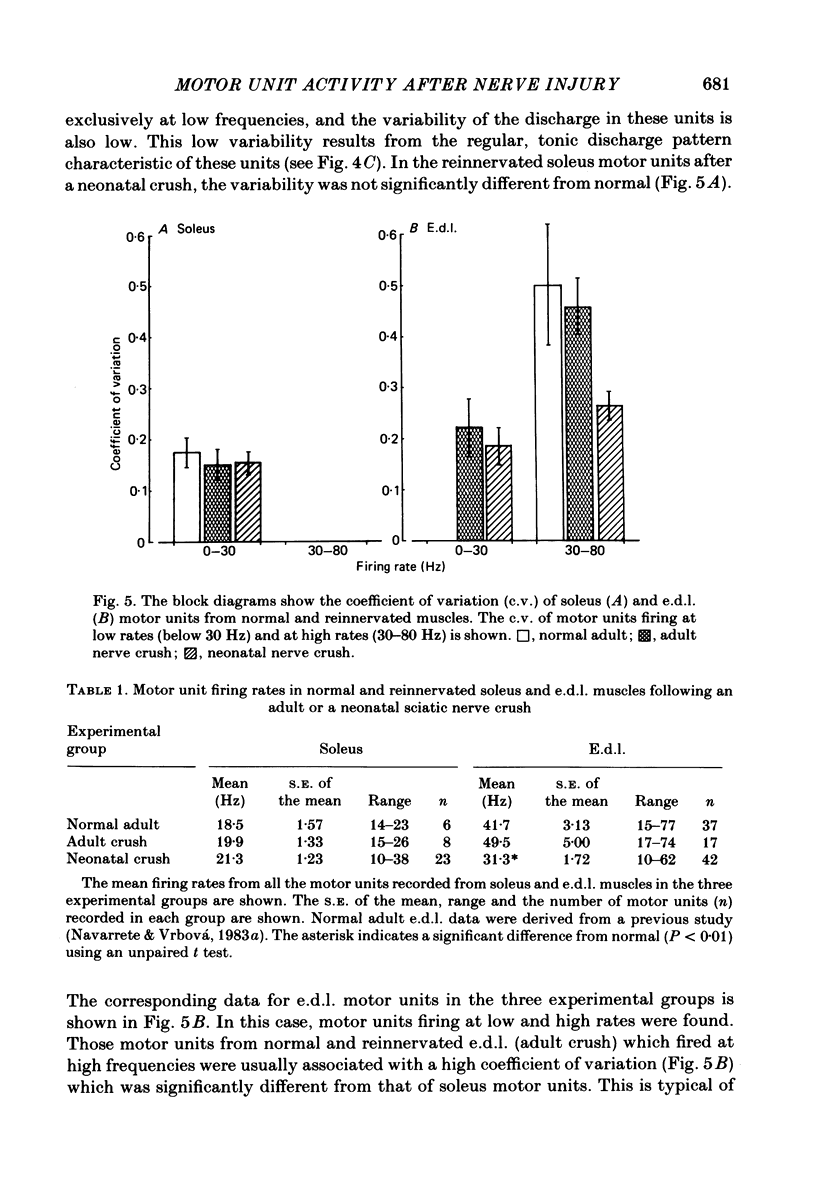
The activity patterns of the reinnervated slow soleus and fast extensor digitorum longus (e.d.l.) muscles were studied in rats during the first 6 months after sciatic nerve crush at birth, using chronic electromyography. When the nerve lesion was inflicted shortly after birth, the recovery of the muscle weight and size was always much less than if the same lesion was inflicted on adult animals. As previously demonstrated, this effect is due to motoneurone and muscle fibre loss. Following reinnervation after a neonatal crush, the soleus muscle recovered its normal tonic activity pattern during postural and spontaneous locomotor activity. By contrast, in the reinnervated e.d.l. muscle, abnormal tonic motor unit activity was recorded during locomotion, in addition to the phasic activity characteristic of the normal muscle. In response to postural reflexes elicited by tilting the animal, tonic motor unit activity was recorded from the reinnervated e.d.l. muscle, whereas the normal muscle was not activated by these stimuli. The aggregate activity recorded from the reinnervated e.d.l. during spontaneous locomotion was about 2-3 times greater than normal, whereas in the reinnervated soleus no significant change took place. In animals which had their nerves crushed as adults, the activity pattern and aggregate activity of both muscles was similar to normal. The firing pattern of individual motor units from normal and reinnervated muscles was compared. After a neonatal crush, the mean frequency of firing of e.d.l. motor units was significantly lower compared to normal or to that after an adult crush, whereas in soleus no significant change was found. These results indicate that peripheral nerve lesions during early development affect predominantly the development of motoneurones with a phasic, high-frequency discharge pattern resulting in a shift towards tonic, lower-frequency motor unit activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchthal F., Olsen P. Z. Electromyography and muscle biopsy in infantile spinal muscular atrophy. Brain. 1970;93(1):15–30. doi: 10.1093/brain/93.1.15. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. H., Cohan G. Changes in weight and volume of rat spinal cord motor neurons with increasing age. Acta Anat (Basel) 1968;71(2):311–319. doi: 10.1159/000143192. [DOI] [PubMed] [Google Scholar]
- Freund H. J. Motor unit and muscle activity in voluntary motor control. Physiol Rev. 1983 Apr;63(2):387–436. doi: 10.1152/physrev.1983.63.2.387. [DOI] [PubMed] [Google Scholar]
- Gordon T., Hoffer J. A., Jhamandas J., Stein R. B. Long-term effects of axotomy on neural activity during cat locomotion. J Physiol. 1980 Jun;303:243–263. doi: 10.1113/jphysiol.1980.sp013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L. Firing properties of single human motor units during locomotion. J Physiol. 1984 Jan;346:195–202. doi: 10.1113/jphysiol.1984.sp015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie M. B., Krishnan S., Vrbová G. Recovery of slow and fast muscles following nerve injury during early post-natal development in the rat. J Physiol. 1982 Oct;331:51–66. doi: 10.1113/jphysiol.1982.sp014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Sansone F. M. Re-innervation of fast and slow twitch muscle following nerve crush at birth. J Physiol. 1977 Oct;271(3):567–586. doi: 10.1113/jphysiol.1977.sp012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Munson J. B., Scott J. G. Connectivity changes of Ia afferents on axotomized motoneurons. Brain Res. 1974 Jun 20;73(2):338–342. doi: 10.1016/0006-8993(74)91054-3. [DOI] [PubMed] [Google Scholar]
- Romanes G. J. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946 Jul;80(Pt 3):117–131. [PMC free article] [PubMed] [Google Scholar]
- Sato M., Mizuno N., Konishi A. Postnatal differentiation of cell body volumes of spinal motoneurons innervating slow-twitch and fast-twitch muscles. J Comp Neurol. 1977 Sep 1;175(1):27–36. doi: 10.1002/cne.901750104. [DOI] [PubMed] [Google Scholar]
- Sumner B. E. A quantitative analysis of the response of presynaptic boutons to postsynaptic motor neuron axotomy. Exp Neurol. 1975 Mar;46(3):605–615. doi: 10.1016/0014-4886(75)90129-6. [DOI] [PubMed] [Google Scholar]


