Abstract
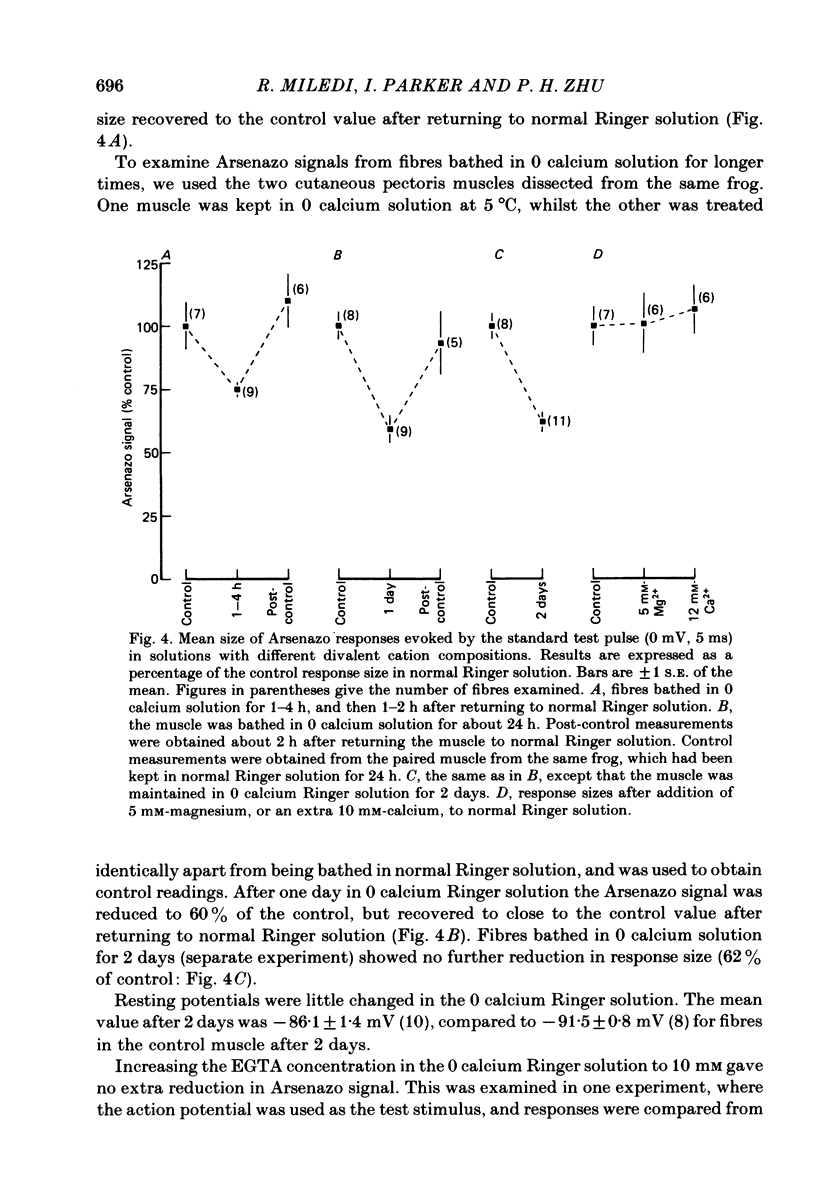
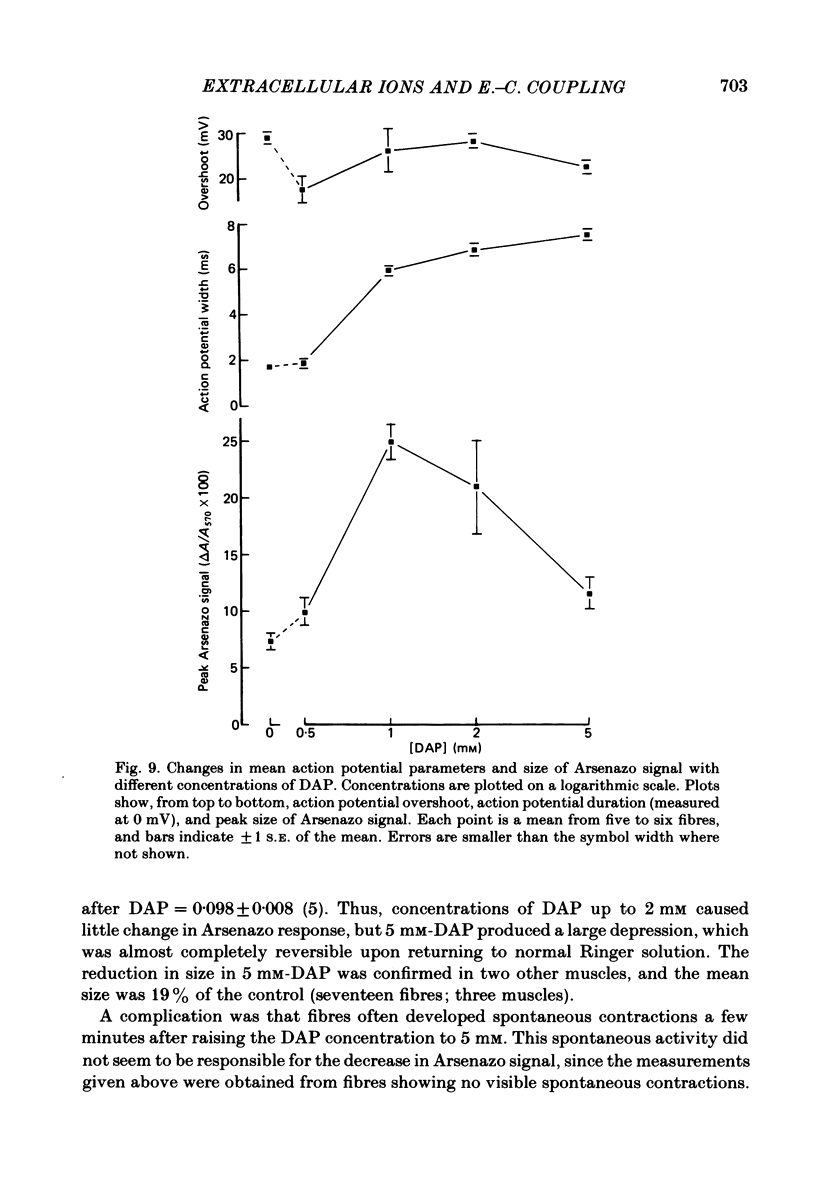
Intracellular calcium transients were recorded from voltage-clamped frog twitch muscle fibres using Arsenazo III. The possible role of extracellular ions in excitation-contraction (e.-c.) coupling was examined using ion substitutions and blocking drugs in the bathing medium. Parameters measured included the Arsenazo response size to a standard depolarizing pulse (5 ms, 0 mV) and the strength-duration curve for threshold Arsenazo signal. Addition of tetrodotoxin (TTX) decreased the response size to small (-30 mV, 5 ms), but not large (+30 mV, 10 ms) depolarizations, probably because of poor voltage clamp of the tubular membrane in the absence of TTX. Clamping TTX-treated fibres with the wave form of a recorded action potential gave an Arsenazo response similar to that elicited by the normal action potential (at 10 degrees C). Complete substitution of sodium (by choline, lithium or Tris) or chloride (by methyl sulphate or maleate) in the bathing solution gave no appreciable changes in the size of the Arsenazo response. Reduction of extracellular free [Ca2+] to low levels using EGTA caused a slight reduction in the calcium signal elicited by the standard depolarization (to 74% after a few hours, and to 62% after 2 days; temperature 5-10 degrees C). The strength-duration curve was unchanged. Arsenazo responses about 75% of the control size could be elicited in high potassium solution (42 mM-K2SO4) by strong (+80 mV, 20 ms) depolarizations, after re-polarizing the fibres to -90 mV for a few minutes. The voltage dependence of activation was shifted to more positive potentials in this solution. Tetraethylammonium (TEA) bromide at a concentration of 20 mM did not alter the Arsenazo signal, whilst 120 mM-TEA reduced the response by 25%. 3,4-diaminopyridine (DAP) reduced the size of the Arsenazo signal at a concentration of 5 mM, and caused spontaneous release of calcium from the sarcoplasmic reticulum (s.r.) in the absence of membrane potential changes. The Arsenazo signal elicited by an action potential was enhanced by 1 mM-DAP, because of prolongation of the action potential, but was depressed by higher concentrations. We conclude that e.-c. coupling does not involve the influx of any external ions into the muscle fibre. If a current flow between the T-tubules and the s.r. is involved in e.-c. coupling, then this is probably carried by an efflux of potassium ions.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. doi: 10.1146/annurev.bb.07.060178.000505. [DOI] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Barrett N., Barrett E. F. Excitation-contraction coupling in skeletal muscle: blockade by high extracellular concentrations of calcium buffers. Science. 1978 Jun 16;200(4347):1270–1272. doi: 10.1126/science.96524. [DOI] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P. D., Miledi R., Parker I. Blocking of frog endplate channels by the organic calcium antagonist D600. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):15–24. doi: 10.1098/rspb.1980.0155. [DOI] [PubMed] [Google Scholar]
- Caillé J., Ildefonse M., Rougier O. Existence of a sodium current in the tubular membrane of frog twitch muscle fibre; its possible role in the activation of contraction. Pflugers Arch. 1978 May 18;374(2):167–177. doi: 10.1007/BF00581298. [DOI] [PubMed] [Google Scholar]
- Caputo C. Excitation and contraction processes in muscle. Annu Rev Biophys Bioeng. 1978;7:63–83. doi: 10.1146/annurev.bb.07.060178.000431. [DOI] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in skeletal muscle. Prog Biophys Mol Biol. 1975;29(2):197–224. doi: 10.1016/0079-6107(76)90023-7. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Miledi R., Takahashi T. Aequorin-calcium transients in frog twitch muscle fibres. J Physiol. 1983 Jul;340:91–106. doi: 10.1113/jphysiol.1983.sp014751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G. B. The effects of reducing the extracellular calcium concentration on the twitch in isolated frog's skeletal muscle fibres. Jpn J Physiol. 1982;32(4):589–608. doi: 10.2170/jjphysiol.32.589. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D. A., Garcia M. C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982 Jul 15;298(5871):292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Marshall I. G. The facilitatory actions of aminopyridines and tetraethylammonium on neuromuscular transmission and muscle contractility in avian muscle. Naunyn Schmiedebergs Arch Pharmacol. 1977 Aug;299(1):53–60. doi: 10.1007/BF00508637. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P. P., Miller C. A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol. 1981;61(1):31–38. doi: 10.1007/BF01870750. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T., Levis R. A., Eisenberg R. S. Electrical models of excitation-contraction coupling and charge movement in skeletal muscle. J Gen Physiol. 1980 Jul;76(1):1–31. doi: 10.1085/jgp.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Miledi R., Nakajima S., Parker I., Takahashi T. Effects of membrane polarization on sarcoplasmic calcium release in skeletal muscle. Proc R Soc Lond B Biol Sci. 1981 Sep 17;213(1190):1–13. doi: 10.1098/rspb.1981.0049. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Calcium transients evoked by action potentials in frog twitch muscle fibres. J Physiol. 1982 Dec;333:655–679. doi: 10.1113/jphysiol.1982.sp014474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Calcium transients in frog skeletal muscle fibres following conditioning stimuli. J Physiol. 1983 Jun;339:223–242. doi: 10.1113/jphysiol.1983.sp014713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Calcium transients studied under voltage-clamp control in frog twitch muscle fibres. J Physiol. 1983 Jul;340:649–680. doi: 10.1113/jphysiol.1983.sp014785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Changes in threshold for calcium transients in frog skeletal muscle fibres owing to calcium depletion in the T-tubules. J Physiol. 1983 Nov;344:233–241. doi: 10.1113/jphysiol.1983.sp014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Potreau D., Raymond G. Calcium-dependent electrical activity and contraction of voltage-clamped frog single muscle fibres. J Physiol. 1980 Oct;307:9–22. doi: 10.1113/jphysiol.1980.sp013420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potreau D., Raymond G. Existence of a sodium-induced calcium release mechanism of frog skeletal muscle fibres. J Physiol. 1982 Dec;333:463–480. doi: 10.1113/jphysiol.1982.sp014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOW A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952 Dec;25(3):176–201. [PMC free article] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A., Krishna M., Pagala D., Sphicas E. C. Excitation-contraction coupling: effects of "zero"-Ca2+ medium. Biochim Biophys Acta. 1975 Sep 8;404(1):157–163. doi: 10.1016/0304-4165(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977 Aug 11;268(5620):556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Chiarandini D. J. Ionic channels in skeletal muscle. Annu Rev Physiol. 1982;44:357–372. doi: 10.1146/annurev.ph.44.030182.002041. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W. Activation of fast skeletal muscle: contributions of studies on skinned fibers. Am J Physiol. 1981 Jan;240(1):C1–19. doi: 10.1152/ajpcell.1981.240.1.C1. [DOI] [PubMed] [Google Scholar]



