Abstract
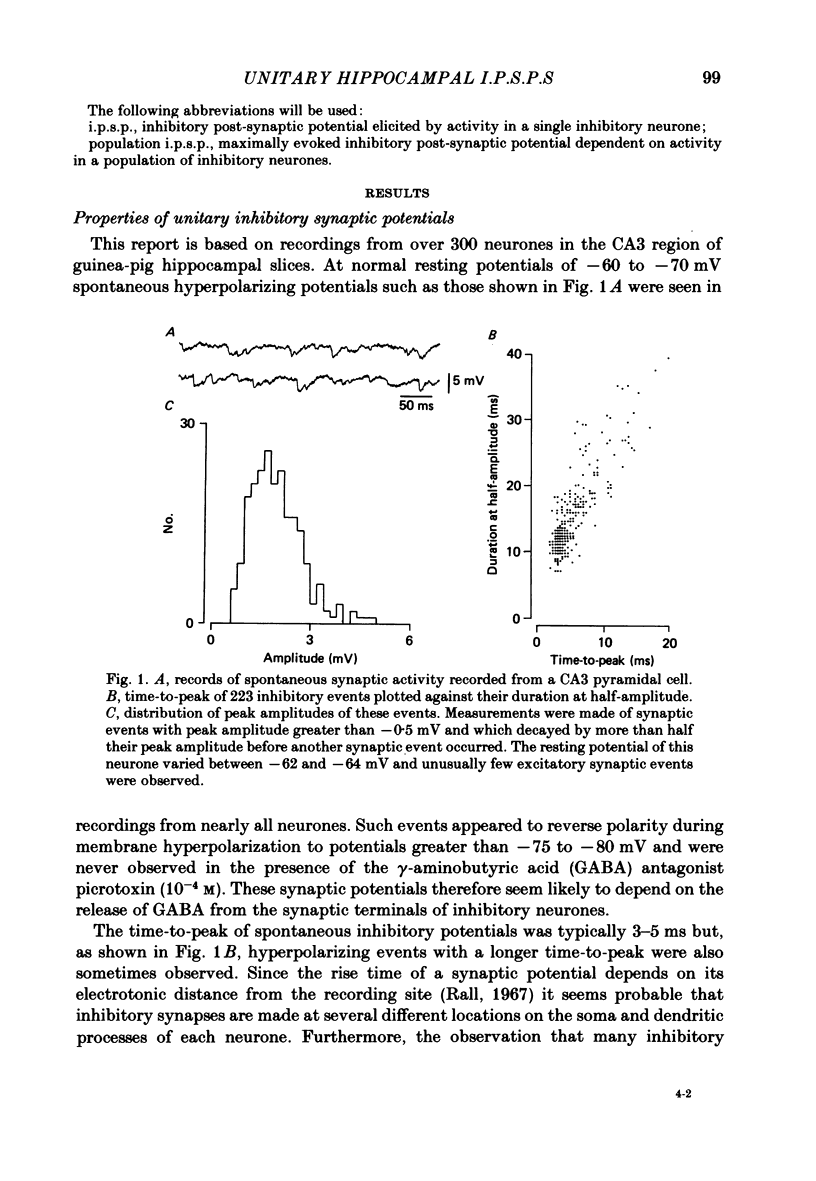
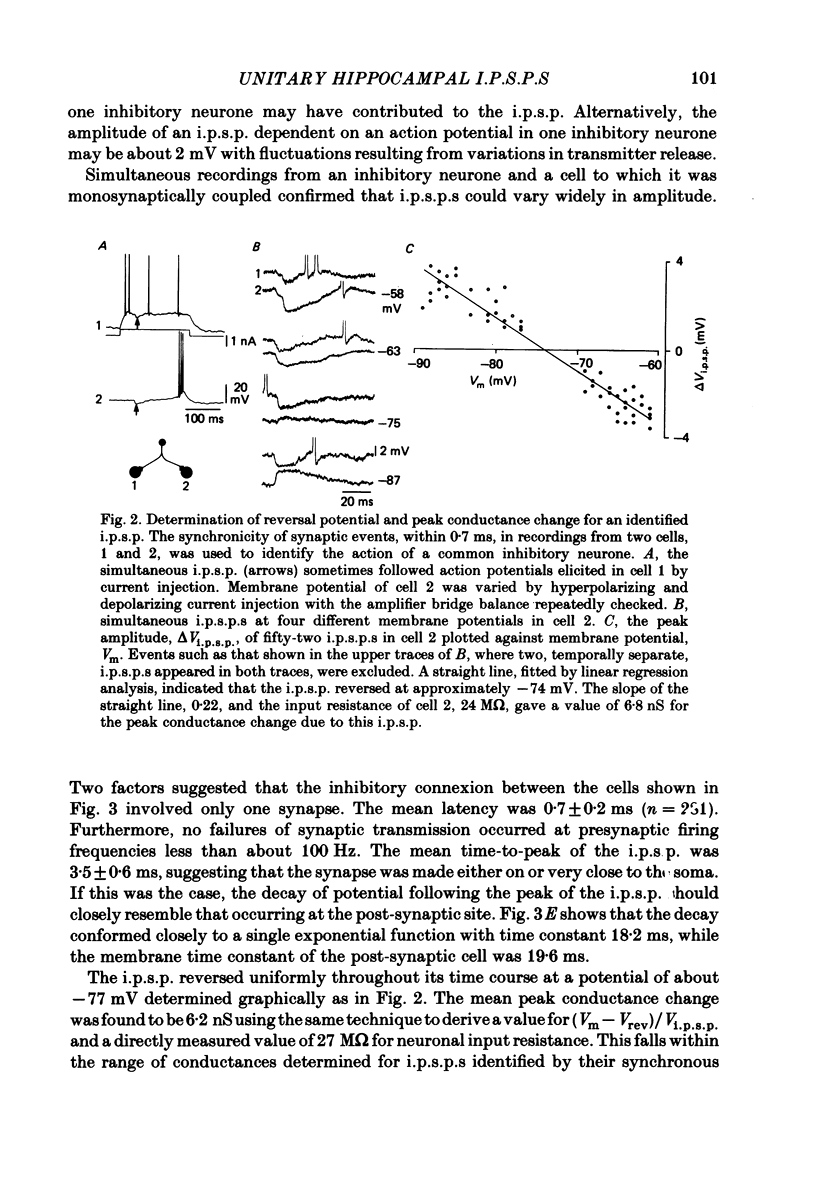
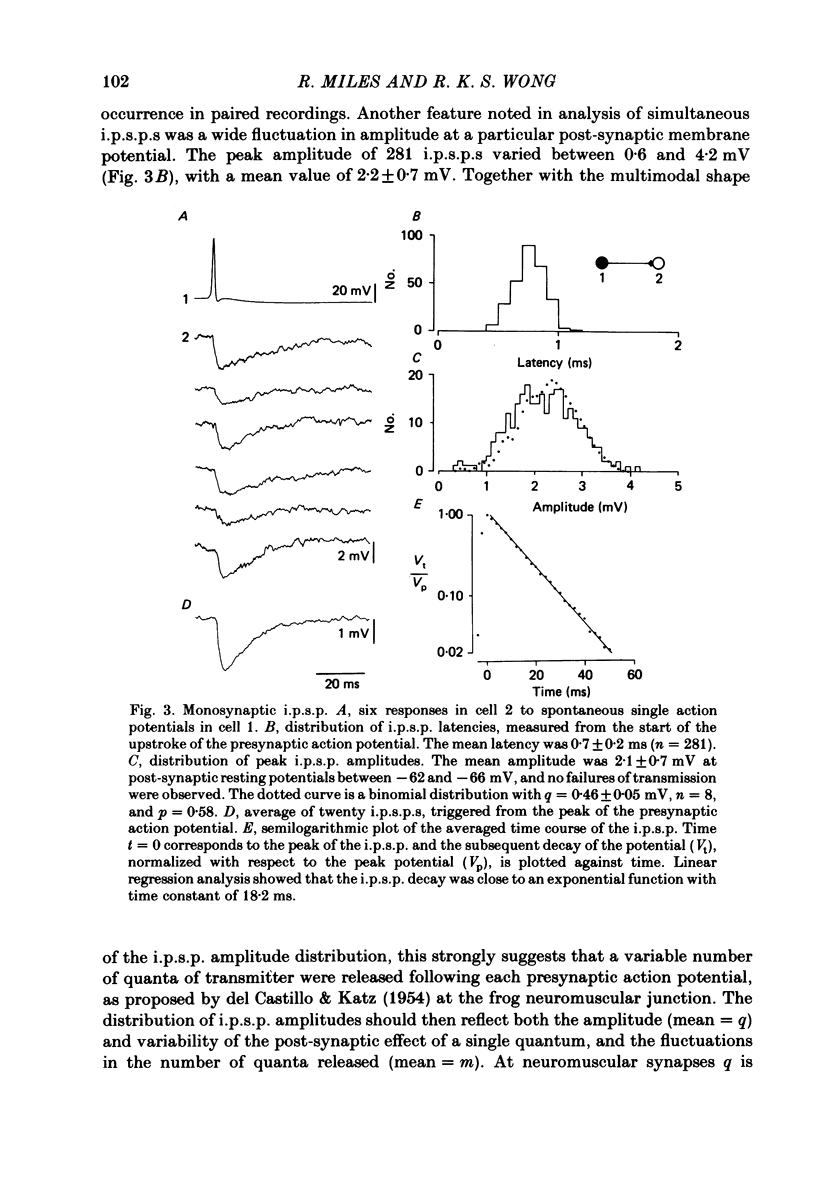
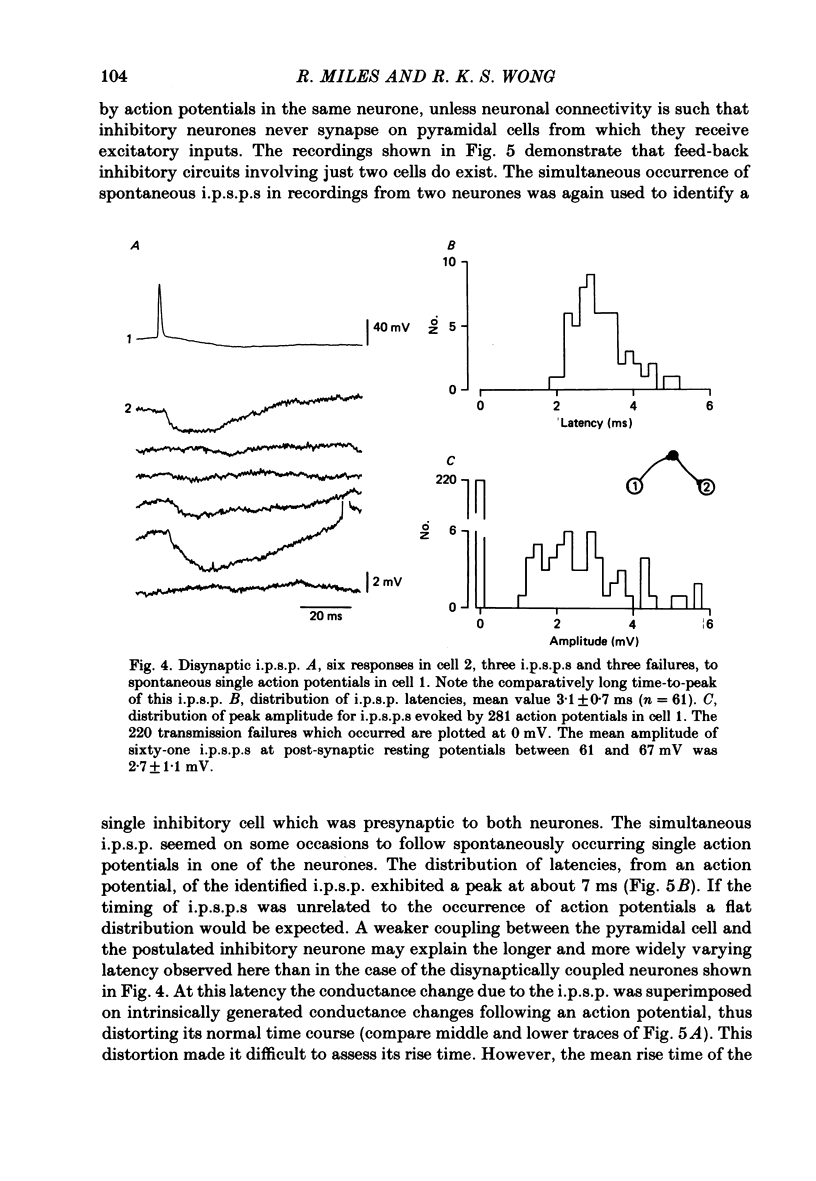
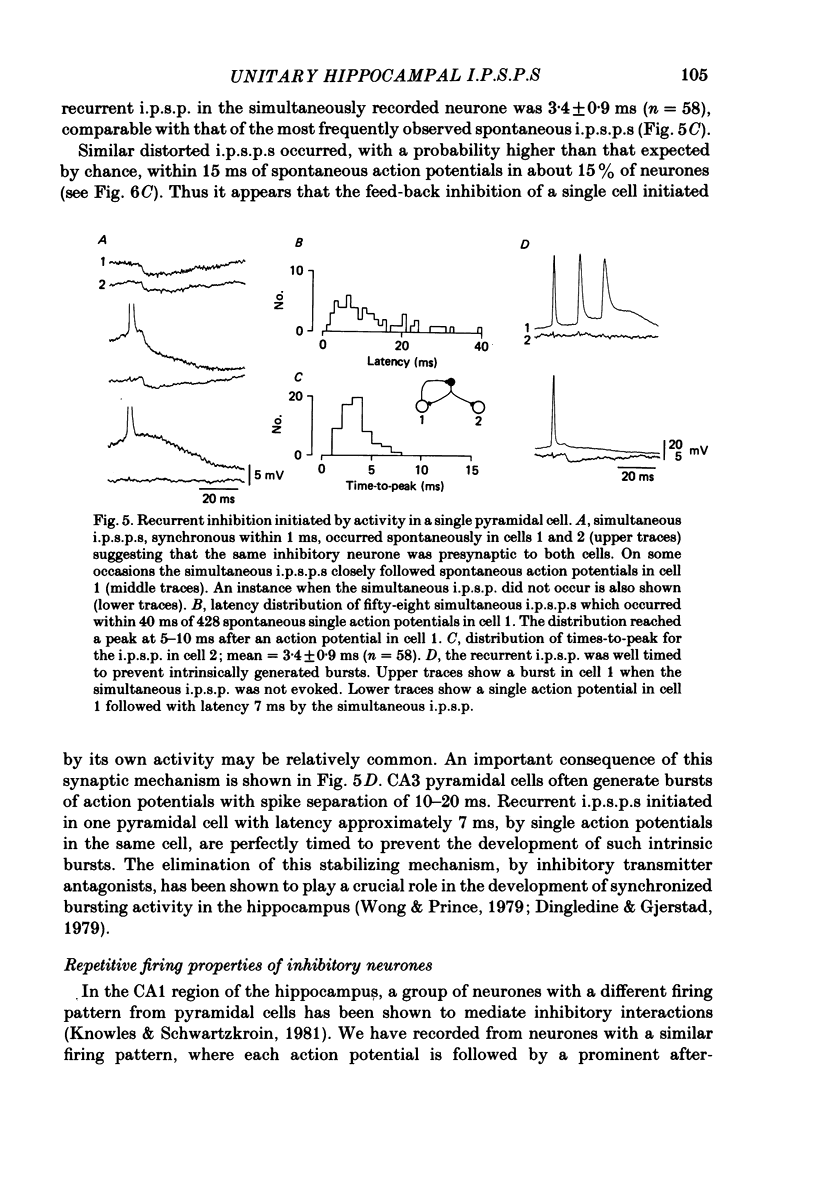
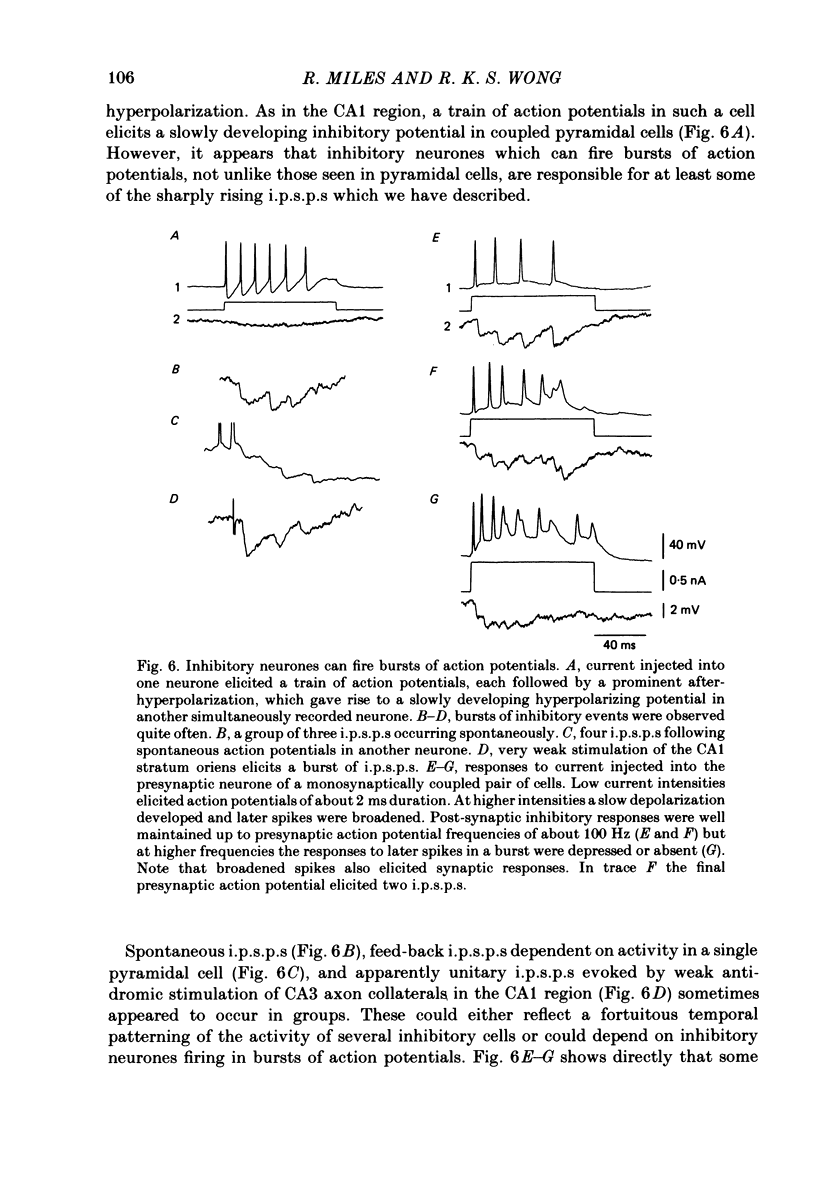
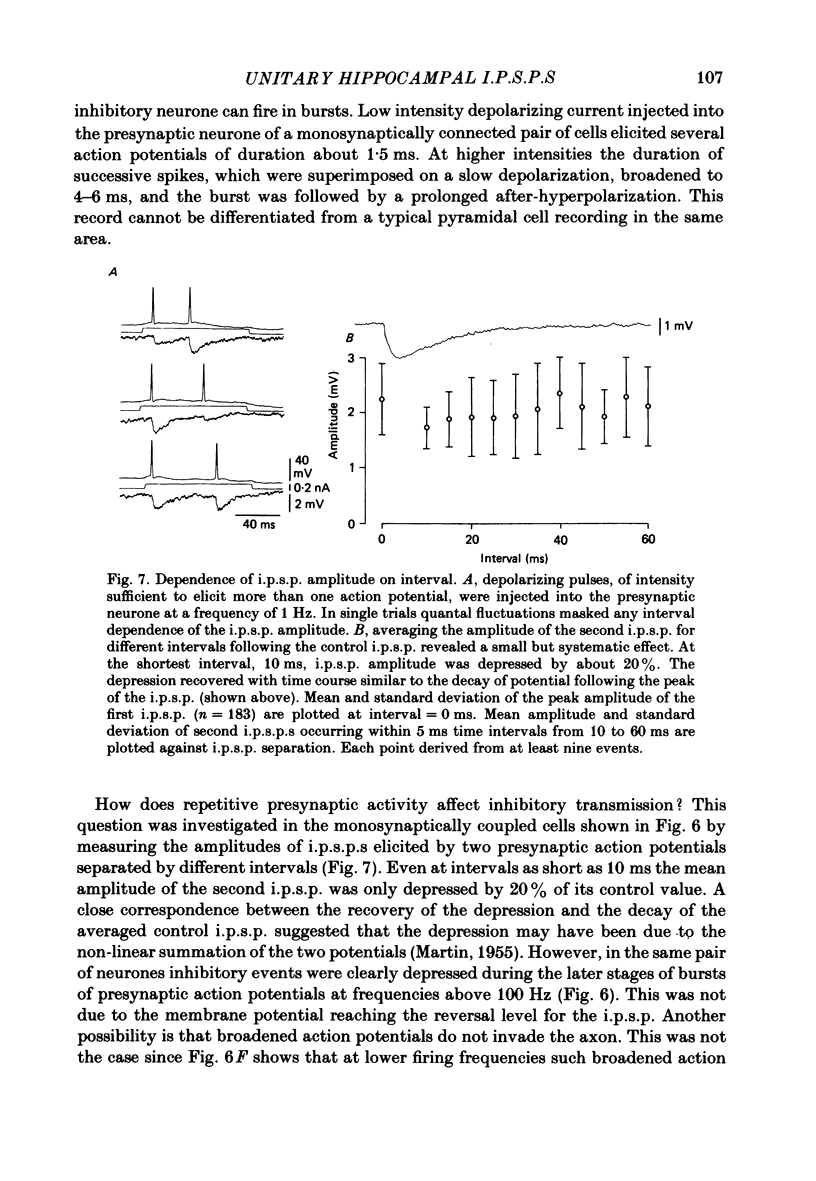
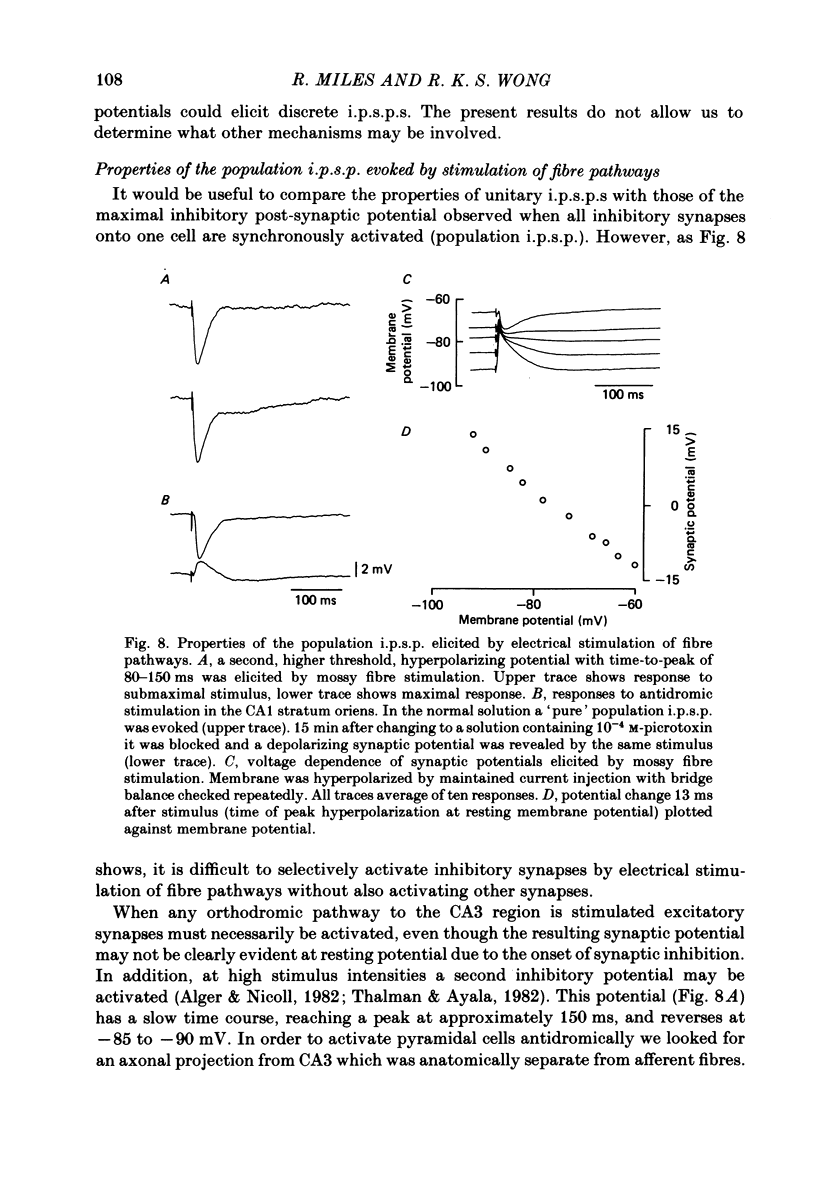
Mechanisms involved in the generation of synaptic inhibition have been investigated by making simultaneous intracellular recordings from pairs of neurones in the CA3 pyramidal cell field of guinea-pig hippocampal slices. Inhibitory post-synaptic potentials dependent on single presynaptic action potentials (i.p.s.p.s) and mediated through monosynaptic and disynaptic connexions have been identified. The recurrent nature of some hippocampal inhibition has been demonstrated by showing that activity in a single cell may initiate feed-back i.p.s.p.s onto itself. The observation of synchronous i.p.s.p.s in recordings from two cells illustrates the divergence of synaptic contacts made by inhibitory neurones. The peak conductance change associated with an i.p.s.p. was in the range 5-9 nS and it reversed uniformly throughout its time course at membrane potentials between -73 and -80 mV. The shortest time-to-peak of synaptic potentials was approximately 3 ms and in this case the i.p.s.p. decayed with a time constant comparable to the passive membrane time constant of the post-synaptic neurone. The peak amplitude of i.p.s.p.s fluctuated in a way consistent with the quantal release of inhibitory neurotransmitter. Inhibitory neurones could fire bursts of action potentials not unlike those generated by pyramidal cells in this area. A comparison of the conductance change associated with identified i.p.s.p.s with that associated with the maximal inhibitory post-synaptic potential resulting from electrical stimulation of fibre pathways suggested that, in the slice, a pyramidal cell is innervated by up to fifteen inhibitory neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963 May 11;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Krnjević K., Reiffenstein R. J., Reinhardt W. Inhibitory conductance changes and action of gamma-aminobutyrate in rat hippocampus. Neuroscience. 1981;6(12):2445–2463. doi: 10.1016/0306-4522(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980 Aug;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Langmoen I. A. Conductance changes and inhibitory actions of hippocampal recurrent IPSPs. Brain Res. 1980 Mar 10;185(2):277–287. doi: 10.1016/0006-8993(80)91068-9. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982 Sep;48(3):654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Finch D. M., Babb T. L. Response decrement in a hippocampal basket cell. Brain Res. 1977 Jul 15;130(2):354–359. doi: 10.1016/0006-8993(77)90282-7. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. Evidence for the existence of inhibitory postsynaptic potentials in dendrites and their functional significance in hippocampal pyramidal cells of adult rabbits. Brain Res. 1979 Oct 12;175(1):59–69. doi: 10.1016/0006-8993(79)90514-6. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Cowan W. M. On the distribution of axonal terminals containing spheroidal and flattened synaptic vesicles in the hippocampus and dentate gyrus of the rat and cat. Z Zellforsch Mikrosk Anat. 1972;129(3):413–429. doi: 10.1007/BF00307297. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Kuno M., Weakly J. N. Quantal components of the inhibitory synaptic potential in spinal mononeurones of the cat. J Physiol. 1972 Jul;224(2):287–303. doi: 10.1113/jphysiol.1972.sp009895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980 Feb 17;184(1):220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978 Jan 27;140(2):315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H., Ayala G. F. A late increase in potassium conductance follows synaptic stimulation of granule neurons of the dentate gyrus. Neurosci Lett. 1982 Apr 26;29(3):243–248. doi: 10.1016/0304-3940(82)90324-x. [DOI] [PubMed] [Google Scholar]
- Van Keulen L. Autogenetic recurrent inhibition of individual spinal motoneurones of the cat. Neurosci Lett. 1981 Feb 6;21(3):297–300. doi: 10.1016/0304-3940(81)90220-2. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Dendritic mechanisms underlying penicillin-induced epileptiform activity. Science. 1979 Jun 15;204(4398):1228–1231. doi: 10.1126/science.451569. [DOI] [PubMed] [Google Scholar]



