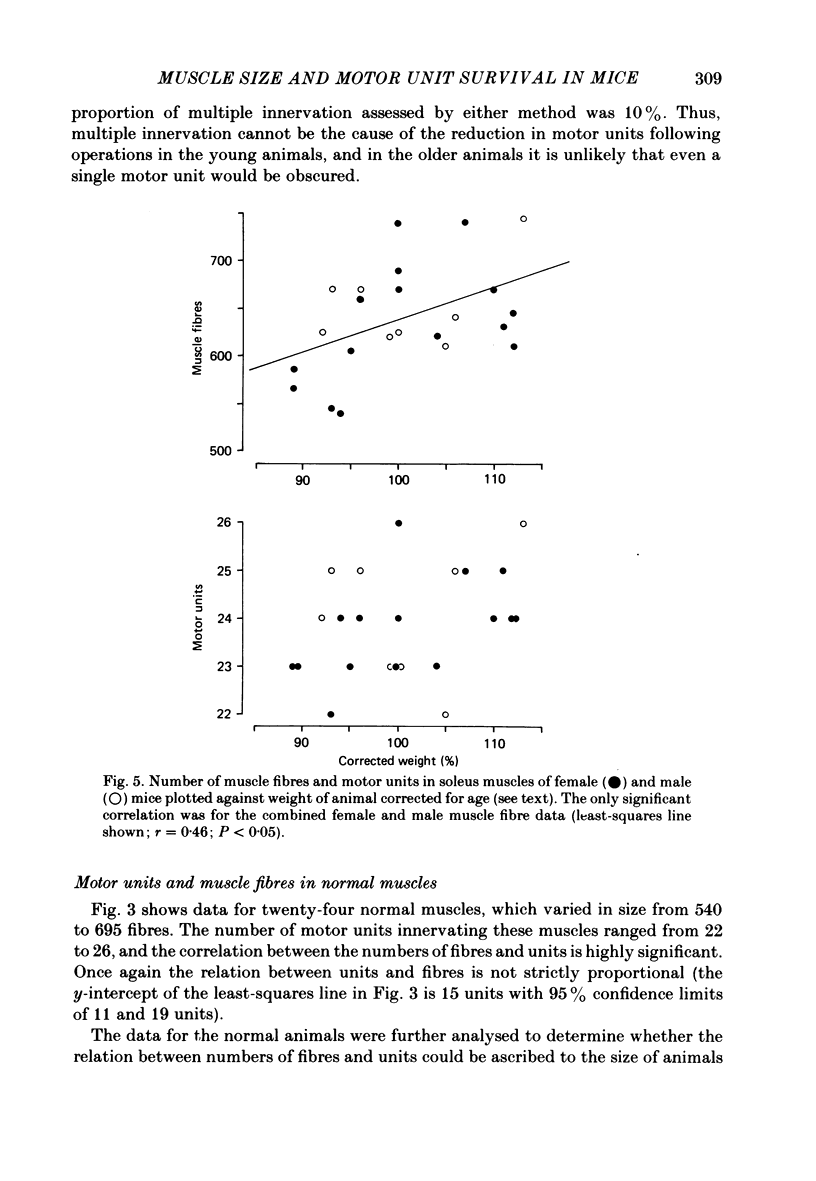
Abstract
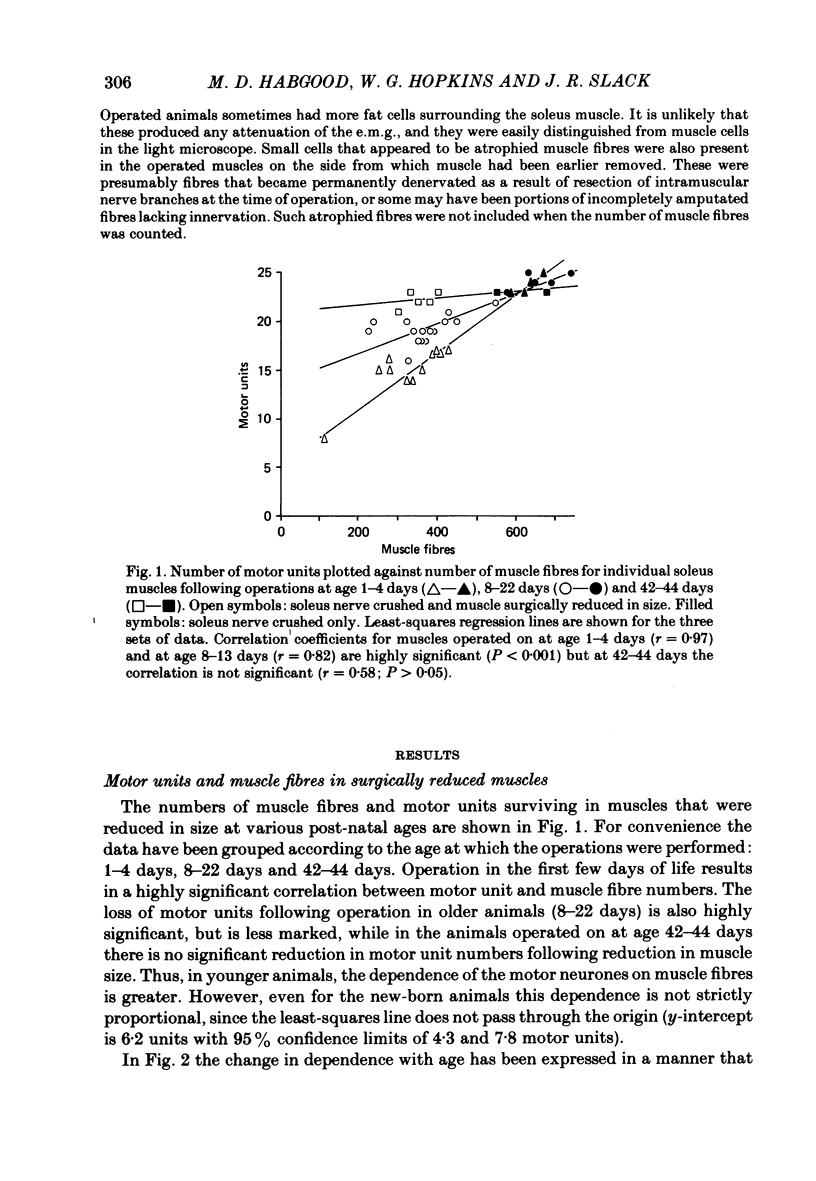
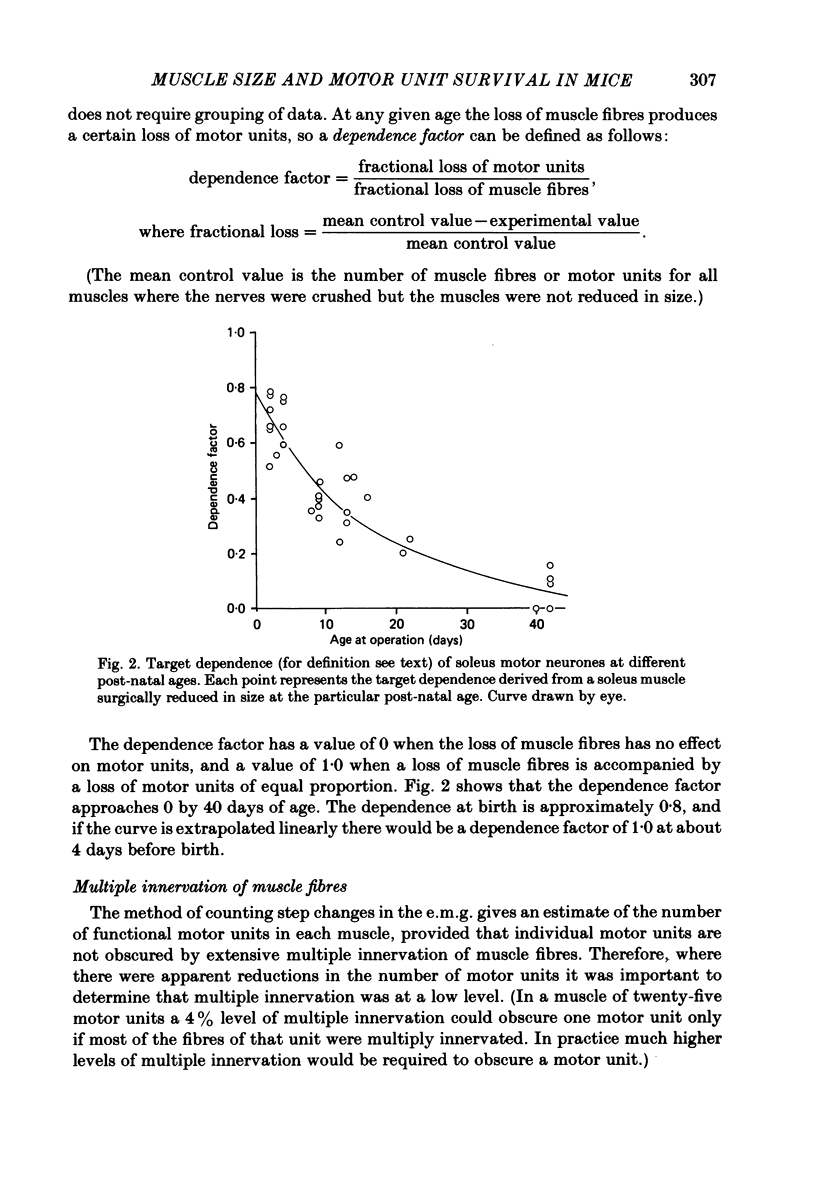
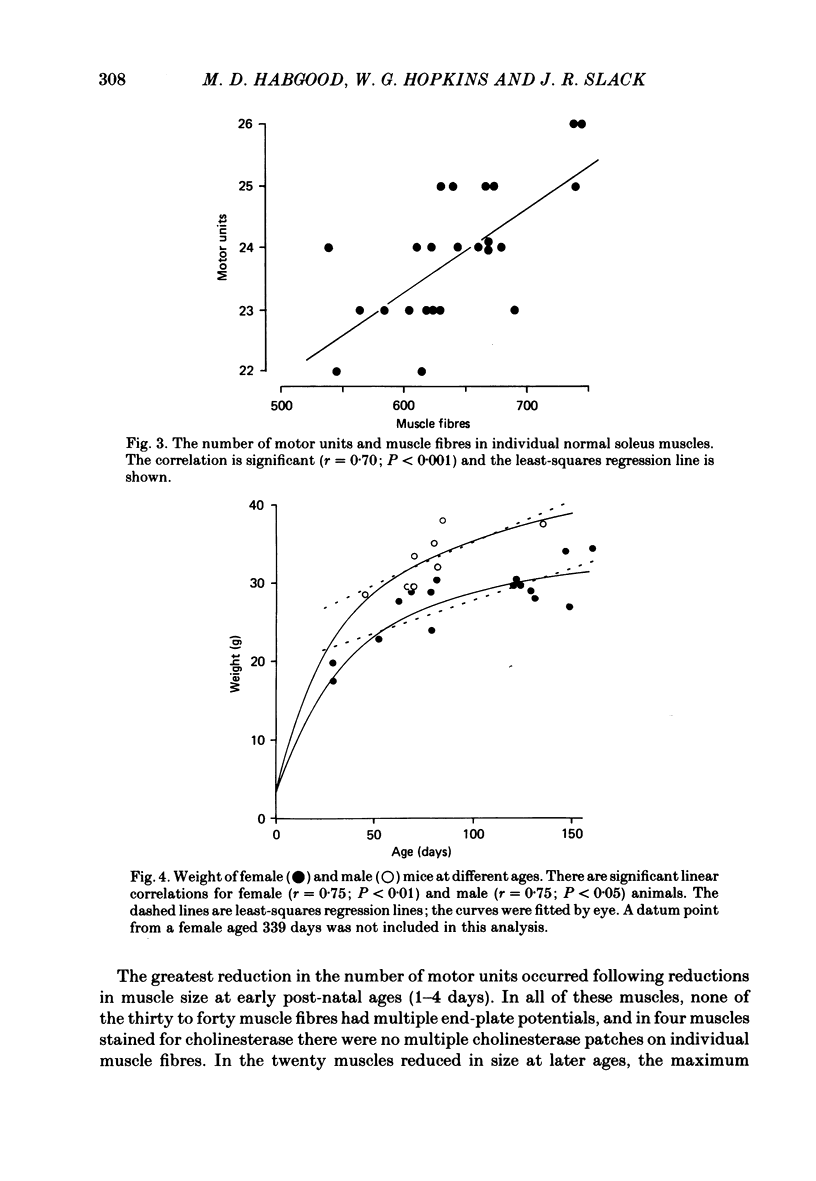
The soleus muscles in neonatal and adult mice were surgically reduced in size on one side of the animal. The experimental and control muscles were excised 6-48 weeks later and the number of motor units in each muscle was estimated by stimulating the muscle nerve and counting step increments in the electromyogram recorded in vitro. Multiple innervation in individual muscle fibres was then assessed by intracellular recording and by visualization of end-plates in the light microscope with cholinesterase stain. Muscle fibres were counted in cross-sections of each muscle in the light microscope. Surgical reductions in the size of the muscle during the first 3 weeks of life produced correlated reductions in the number of motor units in the muscle. This could not be attributed to masking of motor units by multiple innervation, which was always less than 10% in these muscles. The loss of motor units was greatest following reduction in muscle size in newborn mice, whereas in 6-week-old mice there was no significant loss of motor units following the operation. Thus, survival of neonatal motor units shows an age-related dependence on the number of muscle fibres available for innervation. In control muscles there was a highly significant correlation between motor unit and muscle fibre numbers, which is consistent with the hypothesis that motor neurone survival during the embryonic period of cell death is dependent upon the number of muscle fibres available for innervation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McGrath P. A., Davey D. F., Hutchinson I. Death of motorneurons during the postnatal loss of polyneuronal innervation of rat muscles. J Comp Neurol. 1983 Aug 10;218(3):351–363. doi: 10.1002/cne.902180311. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Booth C. M. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983 Aug 25;304(5928):741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- HAMBURGER V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958 May;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975 Apr 15;160(4):535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Harris A. J. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos Trans R Soc Lond B Biol Sci. 1981 Jul 16;293(1065):257–277. doi: 10.1098/rstb.1981.0076. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Wilson P. Mechanical properties of dystrophic mouse muscle. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):512–520. doi: 10.1136/jnnp.34.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyday M., Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976 Dec 1;170(3):311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Laing N. G. Motor projection patterns to the hind limb of normal and paralysed chick embryos. J Embryol Exp Morphol. 1982 Dec;72:269–286. [PubMed] [Google Scholar]
- Lamb A. H. Motoneurone counts in Xenopus frogs reared with one bilaterally-innervated hindlimb. Nature. 1980 Mar 27;284(5754):347–350. doi: 10.1038/284347a0. [DOI] [PubMed] [Google Scholar]
- Lamb A. H. Target dependency of developing motoneurons in Xenopus laevis. J Comp Neurol. 1981 Dec 1;203(2):157–171. doi: 10.1002/cne.902030202. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Brain Res. 1982 Aug;256(4):473–479. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Landmesser L. T. The generation of neuromuscular specificity. Annu Rev Neurosci. 1980;3:279–302. doi: 10.1146/annurev.ne.03.030180.001431. [DOI] [PubMed] [Google Scholar]
- Lewis J., Chevallier A., Kieny M., Wolpert L. Muscle nerve branches do not develop in chick wings devoid of muscle. J Embryol Exp Morphol. 1981 Aug;64:211–232. [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. Neural dependence and independence of myotube production in chicken hindlimb muscles. Dev Biol. 1983 Aug;98(2):287–294. doi: 10.1016/0012-1606(83)90359-7. [DOI] [PubMed] [Google Scholar]
- Morris J. H., Hudson A. R., Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. IV. Changes in fascicular microtopography, perineurium and endoneurial fibroblasts. Z Zellforsch Mikrosk Anat. 1972;124(2):165–203. doi: 10.1007/BF00335678. [DOI] [PubMed] [Google Scholar]
- Purves D. Neuronal competition. Nature. 1980 Oct 16;287(5783):585–586. doi: 10.1038/287585a0. [DOI] [PubMed] [Google Scholar]
- Romanes G. J. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946 Jul;80(Pt 3):117–131. [PMC free article] [PubMed] [Google Scholar]
- Rootman D. S., Tatton W. G., Hay M. Postnatal histogenetic death of rat forelimb motoneurons. J Comp Neurol. 1981 Jun 10;199(1):17–27. doi: 10.1002/cne.901990103. [DOI] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969 May;104(Pt 3):519–530. [PMC free article] [PubMed] [Google Scholar]
- Slack J. R., Hopkins W. G. Neuromuscular transmission at terminals of sprouted mammalian motor neurones. Brain Res. 1982 Apr 8;237(1):121–135. doi: 10.1016/0006-8993(82)90561-3. [DOI] [PubMed] [Google Scholar]
- Wetts R., Herrup K. Direct correlation between Purkinje and granule cell number in the cerebella of lurcher chimeras and wild-type mice. Brain Res. 1983 Oct;312(1):41–47. doi: 10.1016/0165-3806(83)90119-0. [DOI] [PubMed] [Google Scholar]