Abstract
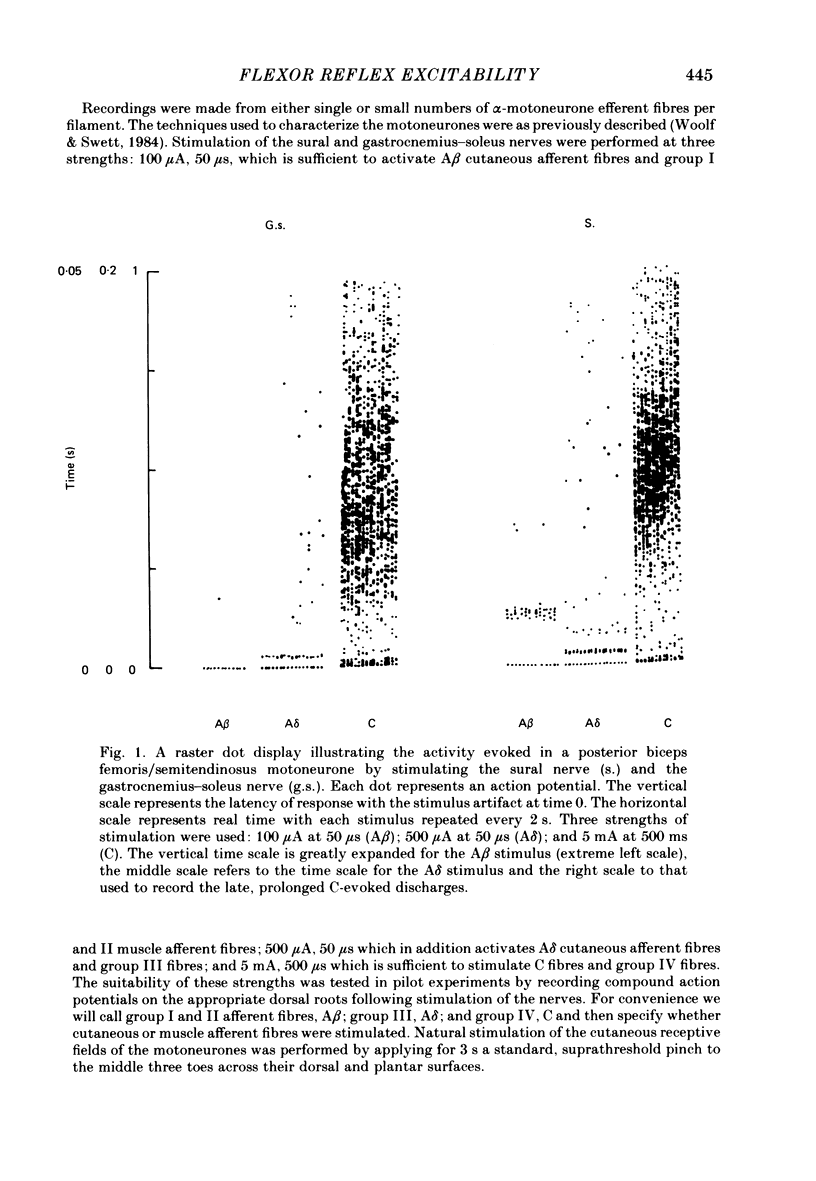
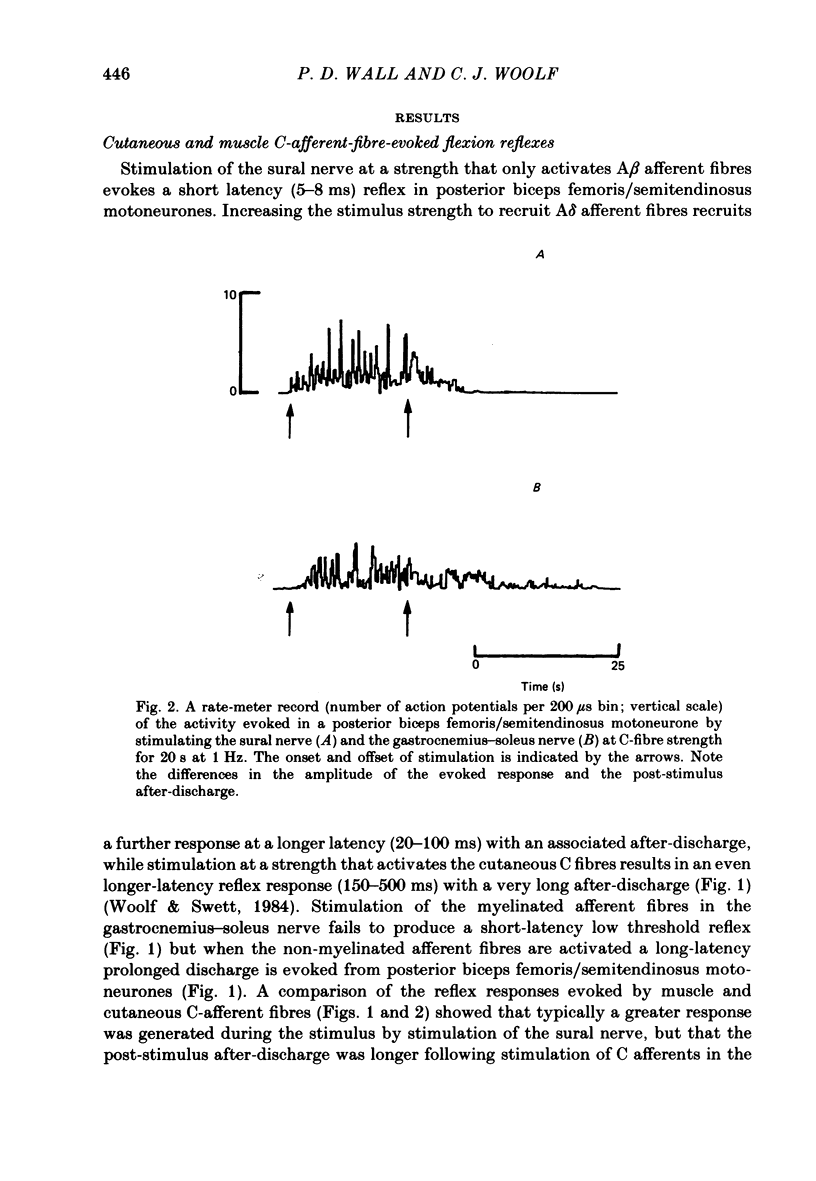
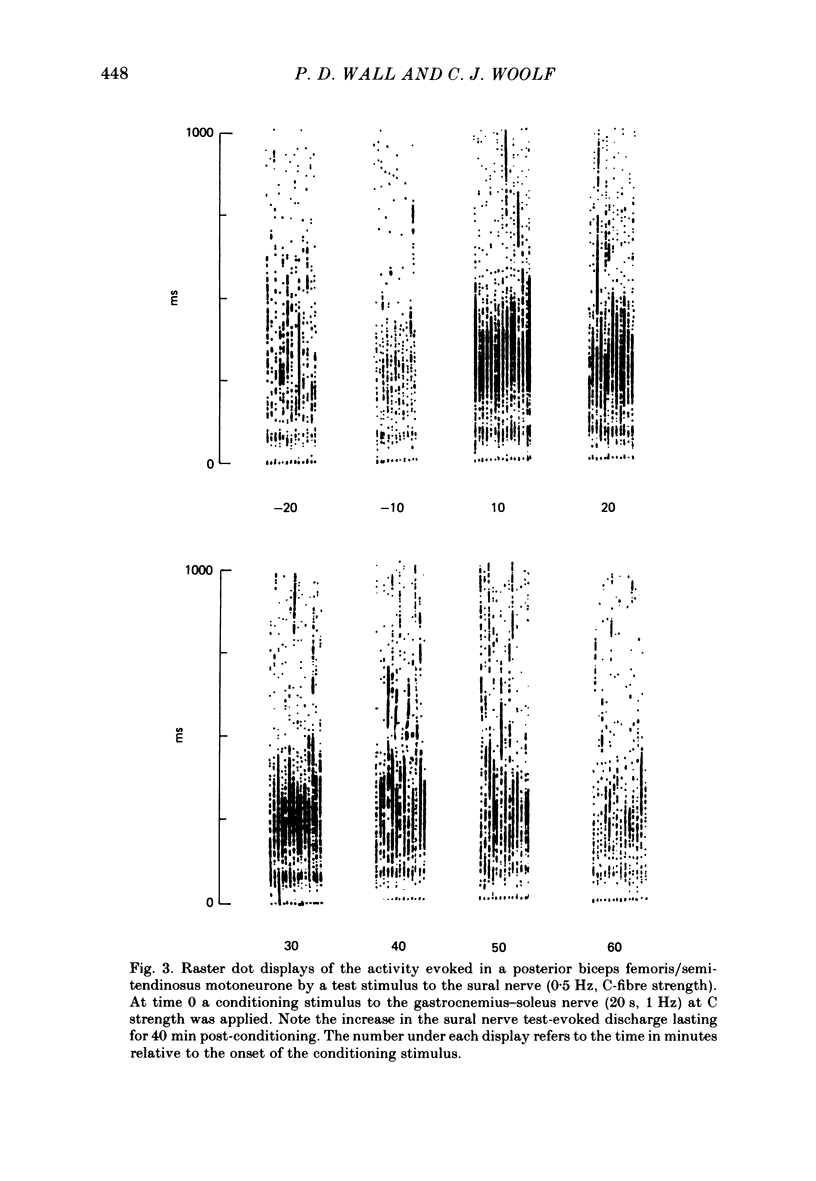
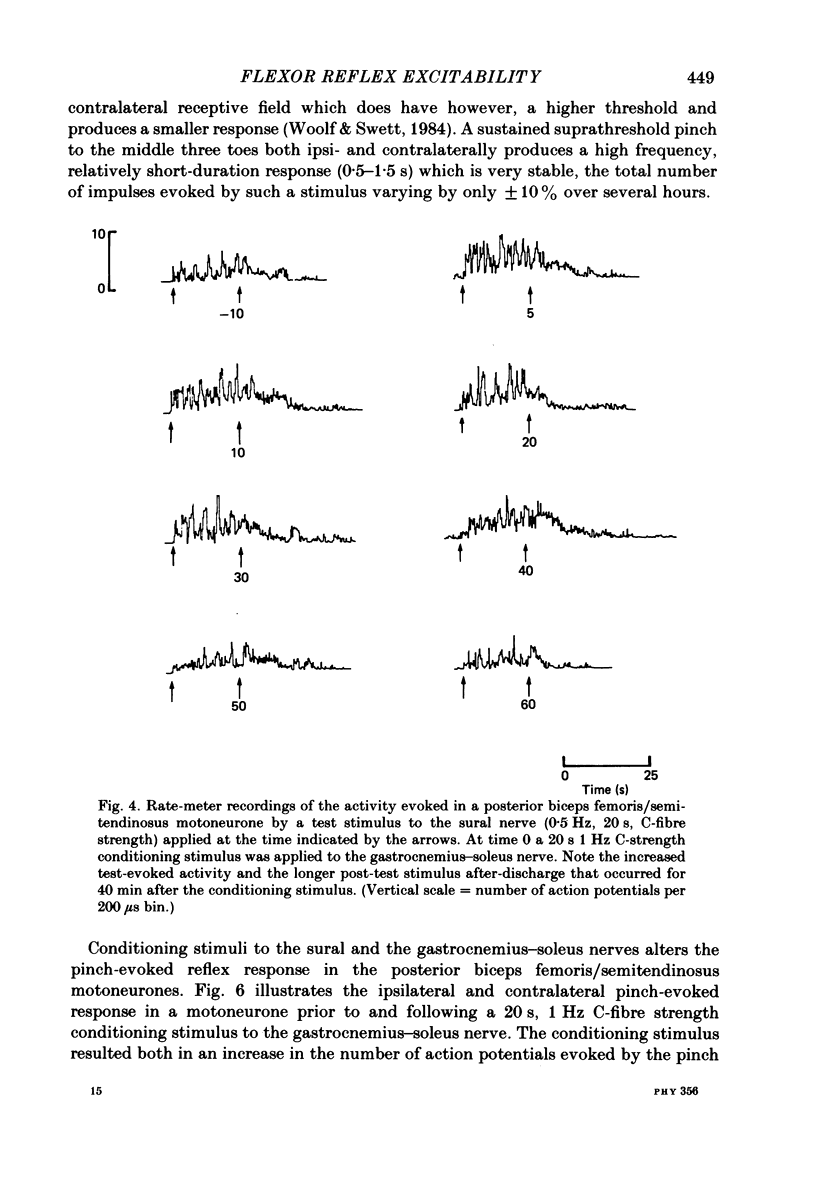
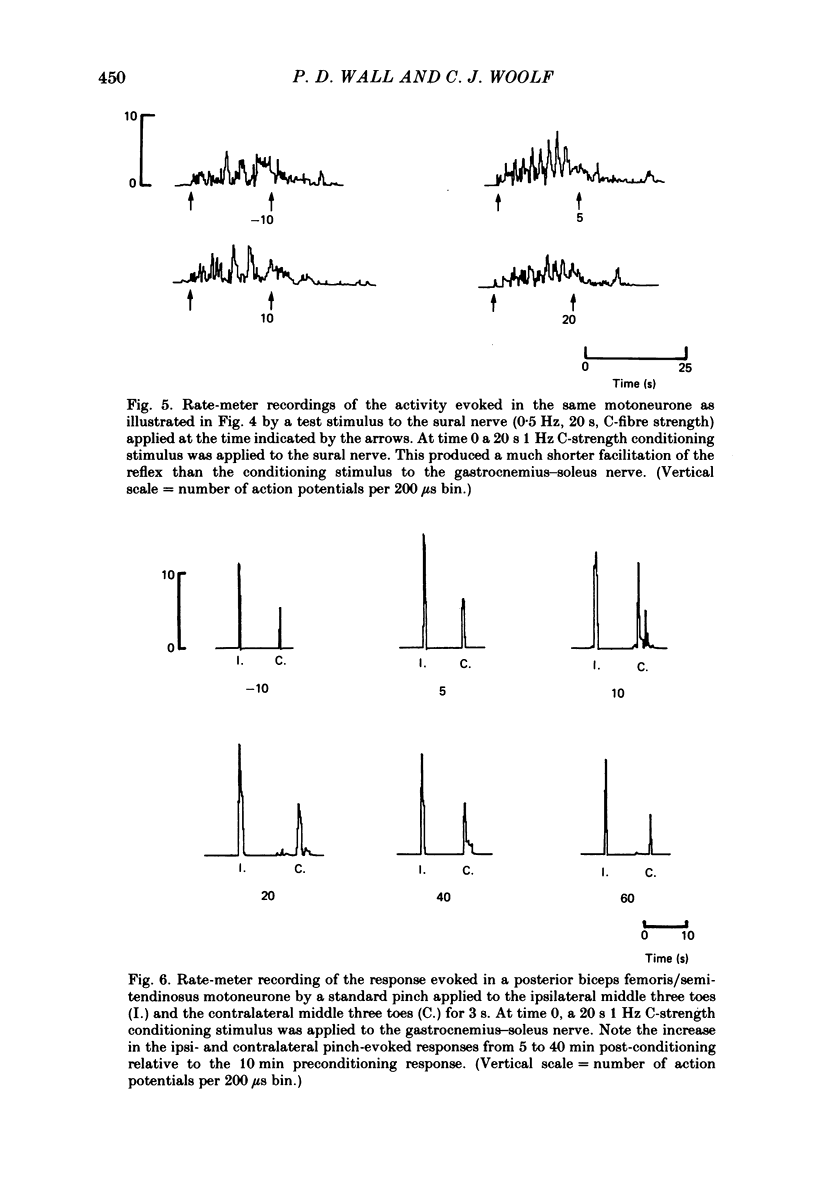
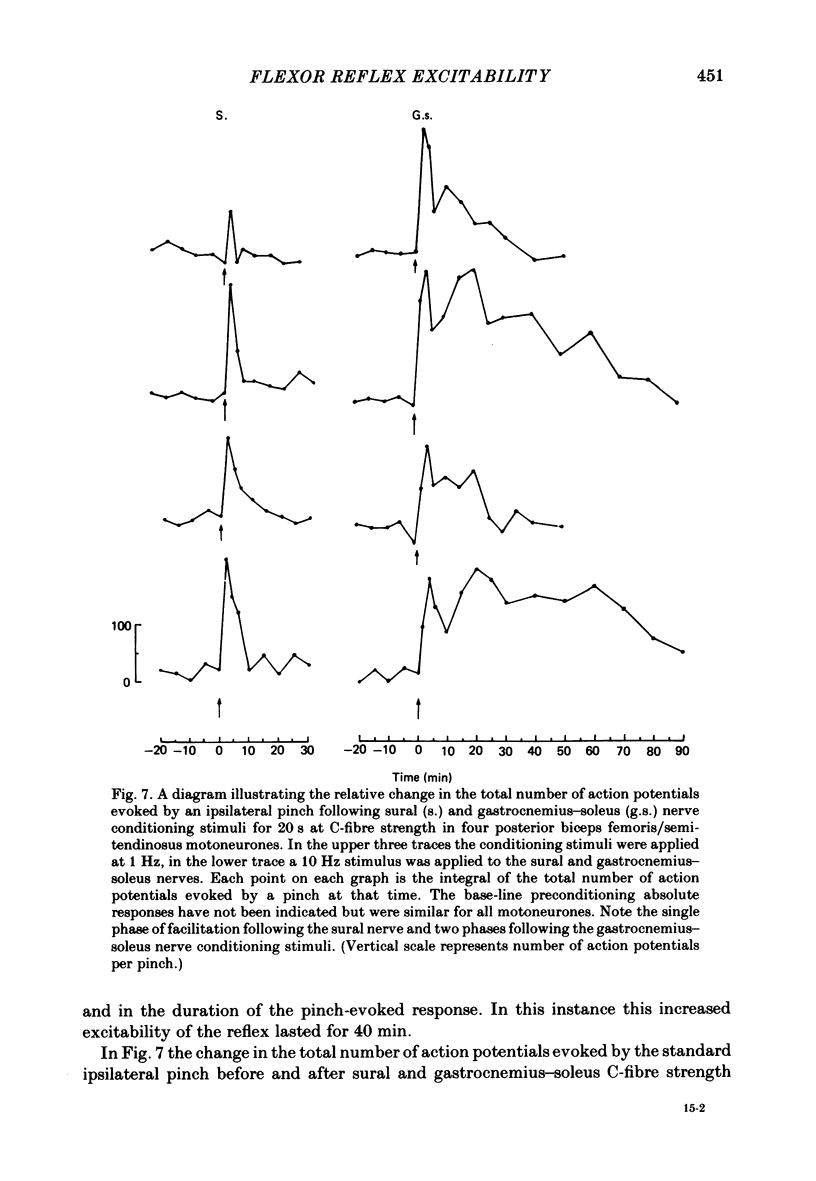
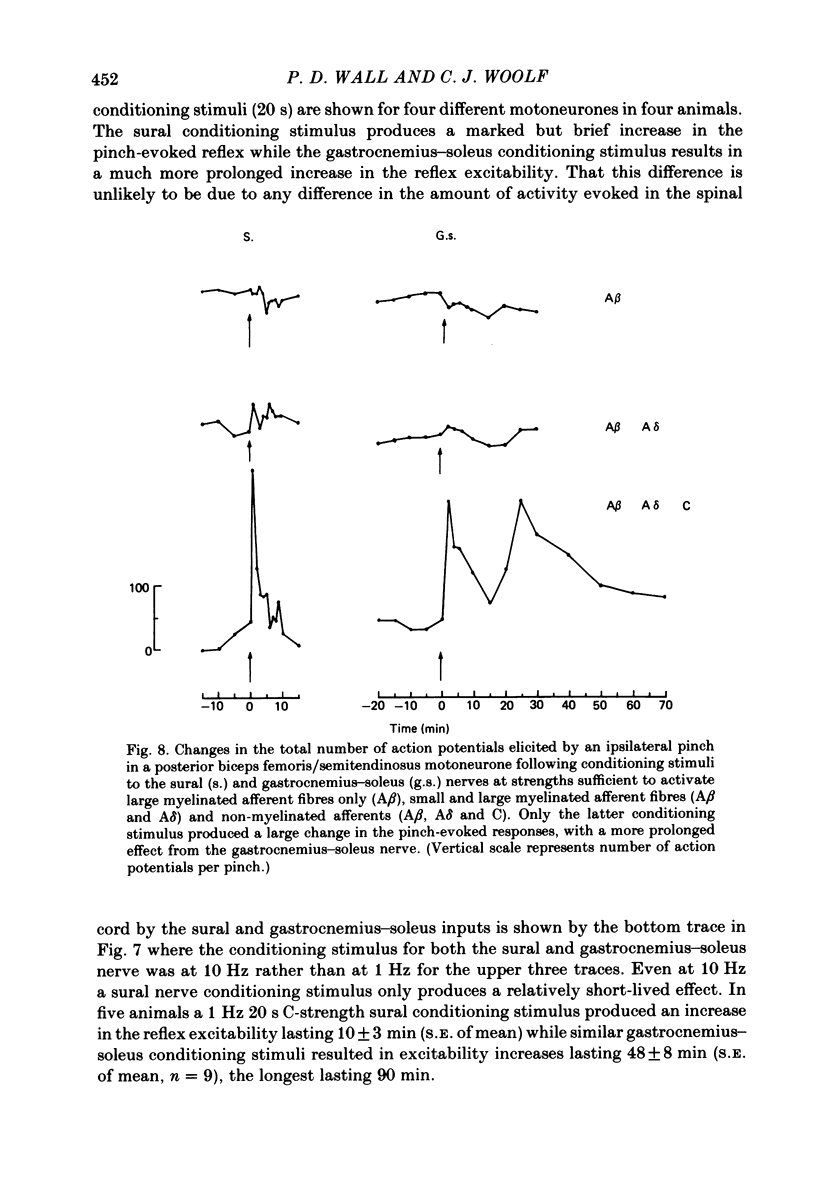
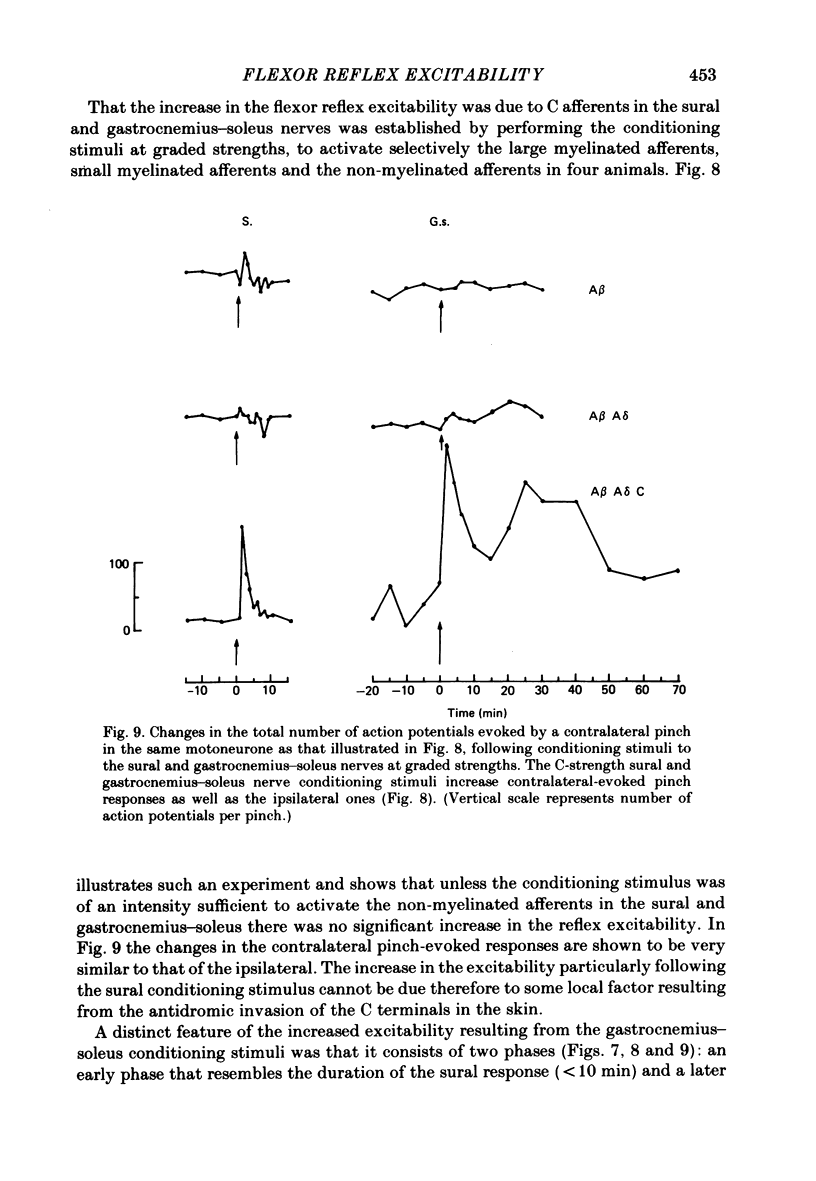
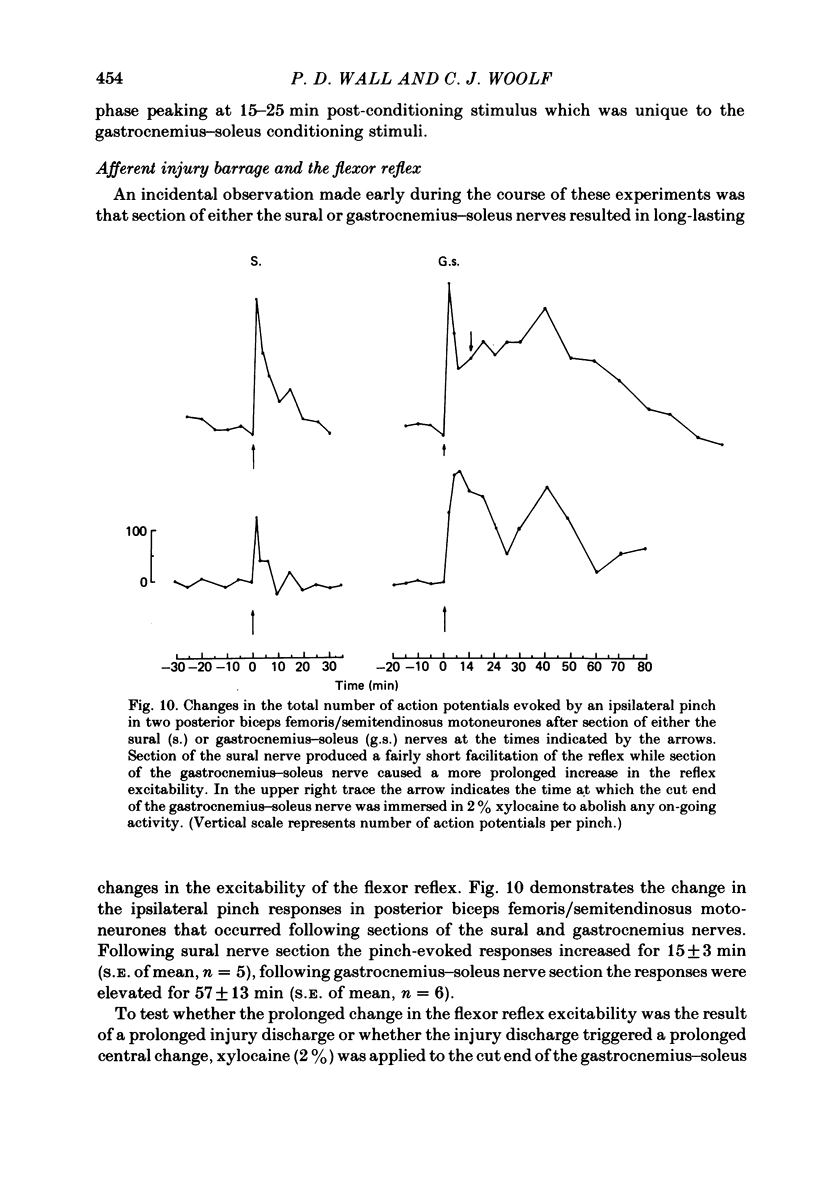
Stimulation of cutaneous afferent fibres in the sural nerve and muscle afferent fibres in the gastrocnemius-soleus nerve at a strength that excites C axons produces a delayed and long-lasting burst of activity in posterior biceps femoris/semitendinosus flexor motoneurones. Following a 20 s stimulation at 1 Hz to the sural nerve the flexor motoneurones continue to fire for 20 s while a similar stimulus to gastrocnemius-soleus nerve results in an after-discharge lasting three times longer. Using stimuli to the sural and gastrocnemius-soleus nerves as conditioning stimuli (20 s, 1 Hz) changes in the excitability of the flexor reflex were measured by recording the discharge evoked by a test sural nerve stimulus or by a standard pinch to the ipsilateral and contralateral toes. Prior to any conditioning stimulus the flexor reflex remained stable for prolonged periods. Conditioning stimuli at strengths that activated large myelinated afferent fibres only, or large and small myelinated afferent fibres, failed to produce more than a very transient alteration in the reflex excitability. Conditioning stimuli at C-fibre strength to the sural nerve produced a marked increase in the excitability of the reflex for 10 min. C-fibre strength gastrocnemius-soleus nerve conditioning stimuli resulted in a similar increase in excitability followed by a second phase of facilitation peaking at 20-30 min and lasting for up to 90 min. The afferent barrage initiated by cutting the sural and gastrocnemius-soleus nerves resulted in similar patterns of reflex excitability increases with the muscle nerve resulting in a more prolonged effect than the cutaneous nerve. The results show that a brief C-afferent fibre input into the spinal cord can produce a prolonged increase in the excitability of the flexion reflex and that muscle C-afferent fibres evoke longer-lasting changes than cutaneous C fibres. The differences in the time course of the post-conditioning effects may be related to the well-described differences in the sensory consequences of injury to skin versus deep tissue.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brushart T. M., Mesulam M. M. Transganglionic demonstration of central sensory projections from skin and muscle with HRP-lectin conjugates. Neurosci Lett. 1980 Apr;17(1-2):1–6. doi: 10.1016/0304-3940(80)90051-8. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett. 1983 Nov 11;41(3):233–238. doi: 10.1016/0304-3940(83)90456-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The spread of sensitization of polymodal nociceptors in the rabbit from nearby injury and by antidromic nerve stimulation. J Physiol. 1979 Dec;297(0):207–216. doi: 10.1113/jphysiol.1979.sp013035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Wall P. D. The laminar organization of dorsal horn cells responding to peripheral C fibre stimulation. Exp Brain Res. 1980;41(1):36–44. doi: 10.1007/BF00236677. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Schmidt R. F., Willis W. D. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979 Jan;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G., Arvidsson J., Robertson B., Ygge J. Transganglionic transport of horseradish peroxidase in primary sensory neurons. Neurosci Lett. 1979 Apr;12(1):23–28. doi: 10.1016/0304-3940(79)91474-5. [DOI] [PubMed] [Google Scholar]
- Kniffki K. D., Schomburg E. D., Steffens H. Effects from fine muscle and cutaneous afferents on spinal locomotion in cats. J Physiol. 1981;319:543–554. doi: 10.1113/jphysiol.1981.sp013925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Jänig W. The cell bodies of origin of sympathetic and sensory axons in some skin and muscle nerves of the cat hindlimb. J Comp Neurol. 1983 Feb 20;214(2):115–130. doi: 10.1002/cne.902140202. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Waxman S., Basbaum A. I. Ongoing activity in peripheral nerve: injury discharge. Exp Neurol. 1974 Dec;45(3):576–589. doi: 10.1016/0014-4886(74)90163-0. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. C-primary afferent fibre mediated inhibitions in the dorsal horn of the decerebrate-spinal rat. Exp Brain Res. 1983;51(2):283–290. doi: 10.1007/BF00237204. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 Dec 15;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Swett J. E. The cutaneous contribution to the hamstring flexor reflex in the rat: an electrophysiological and anatomical study. Brain Res. 1984 Jun 15;303(2):299–312. doi: 10.1016/0006-8993(84)91216-2. [DOI] [PubMed] [Google Scholar]


