Abstract
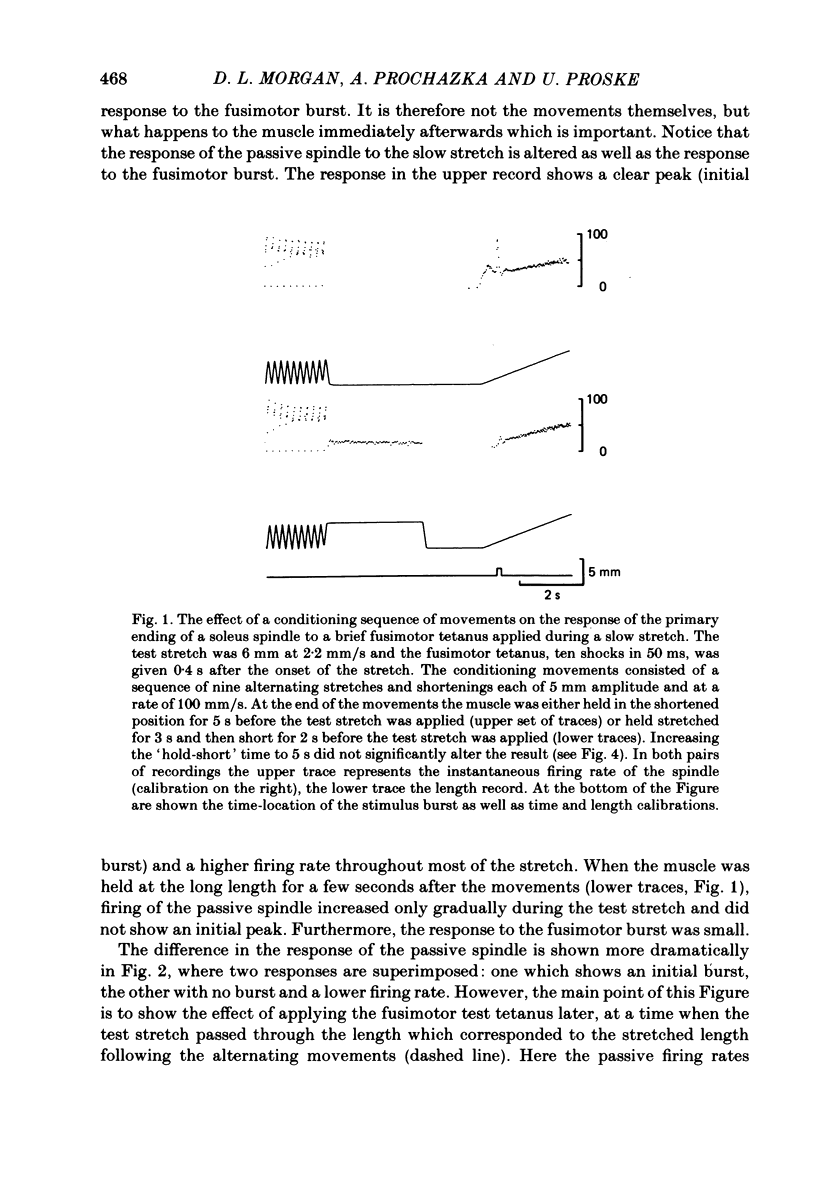
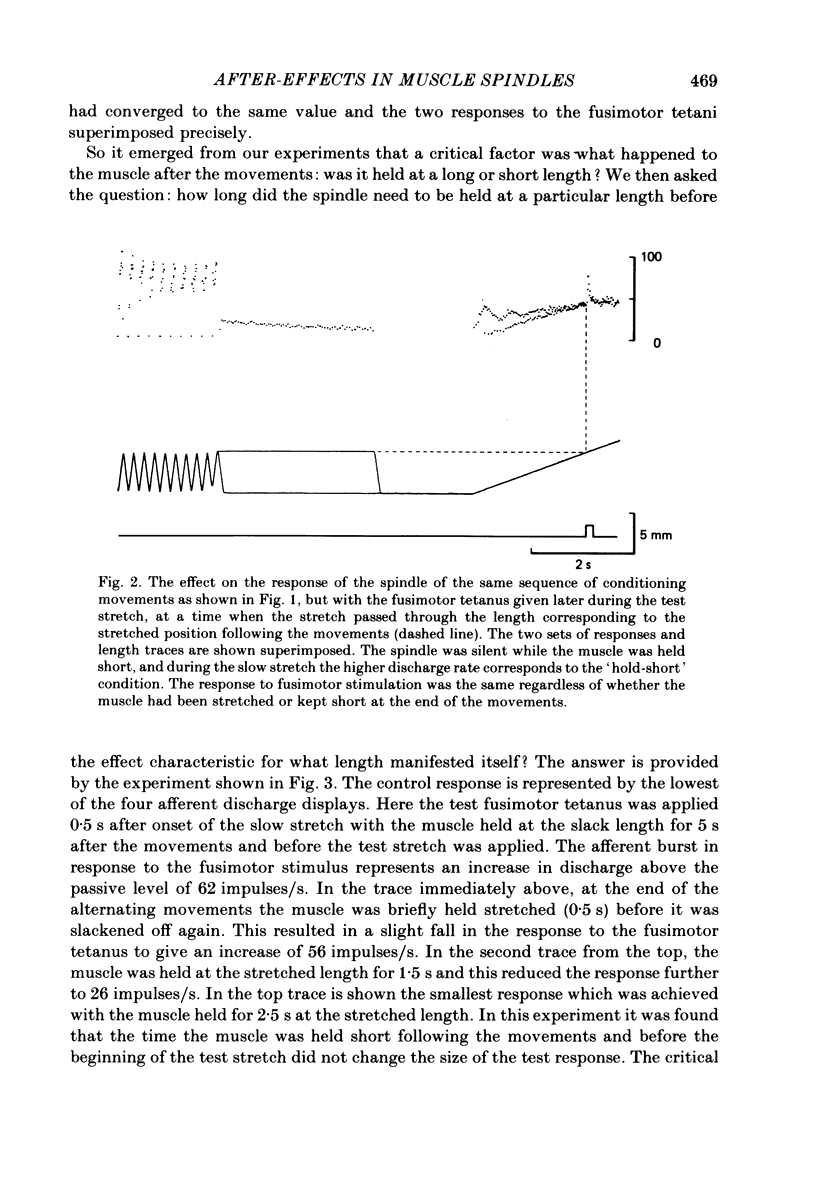
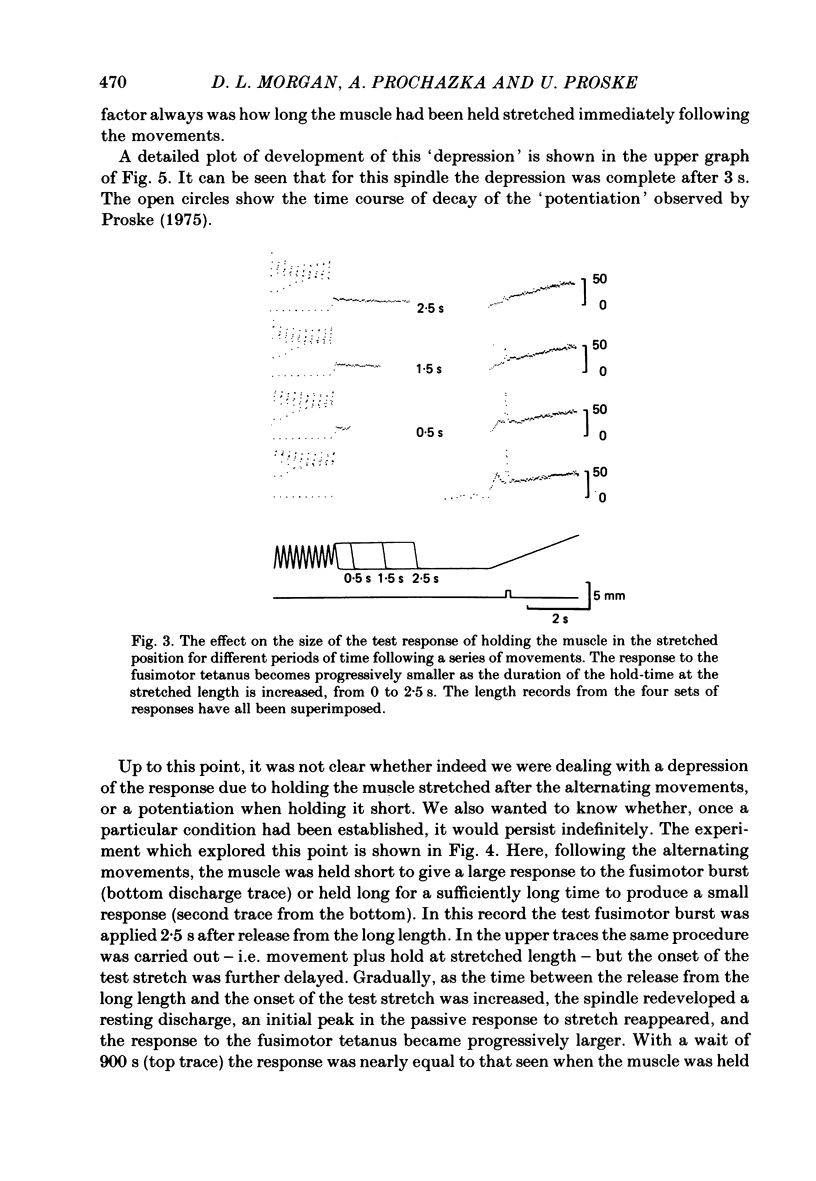
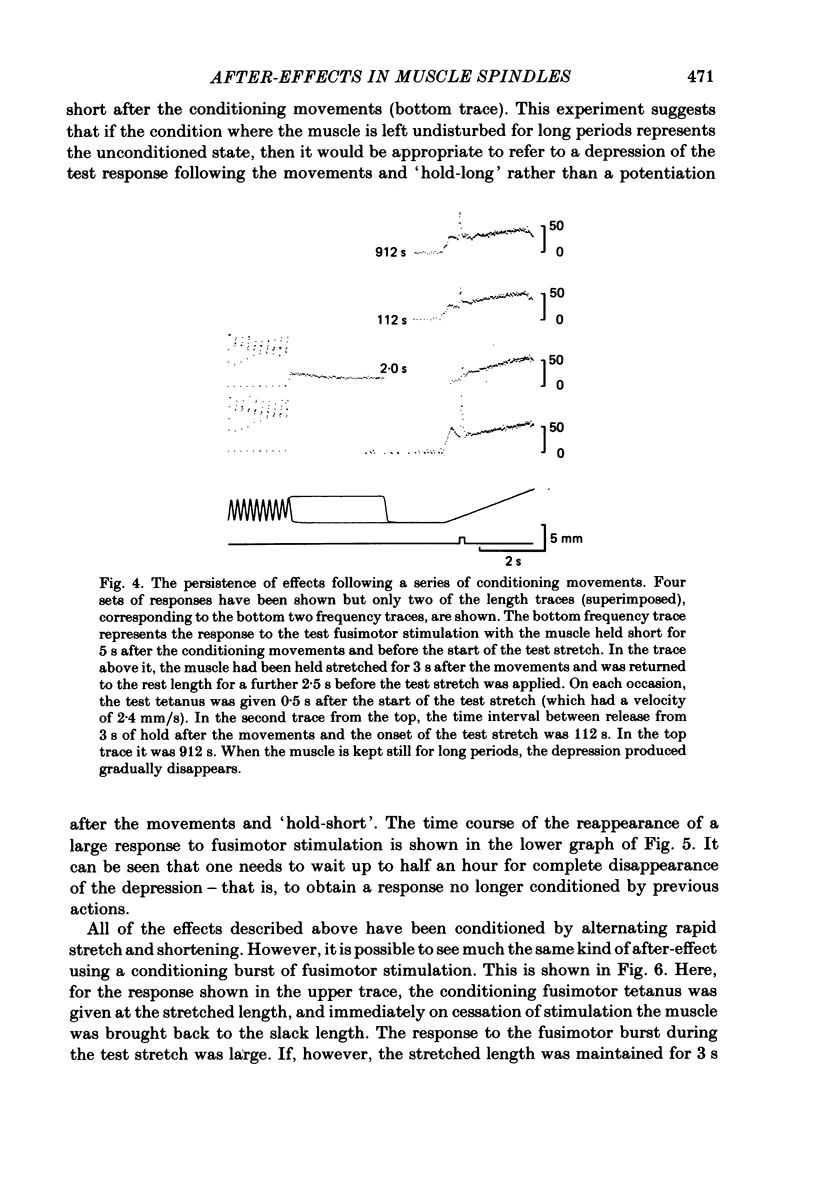
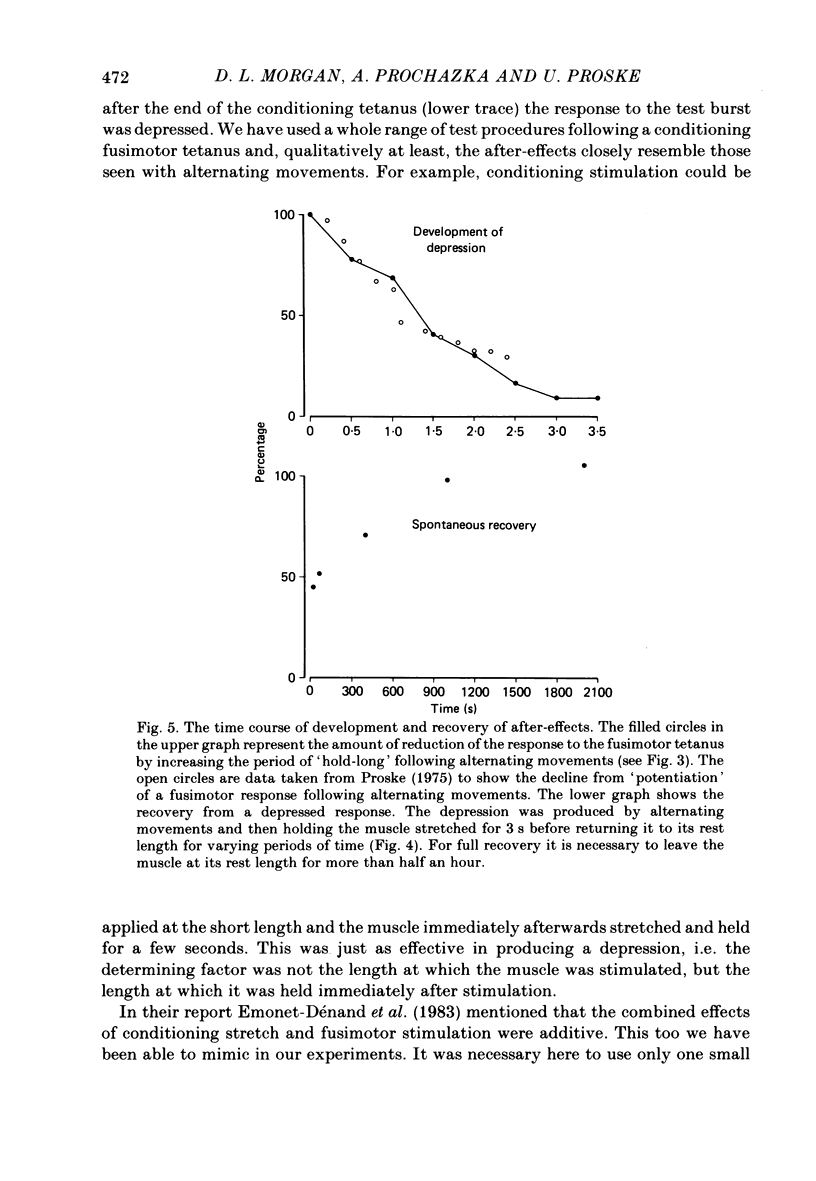
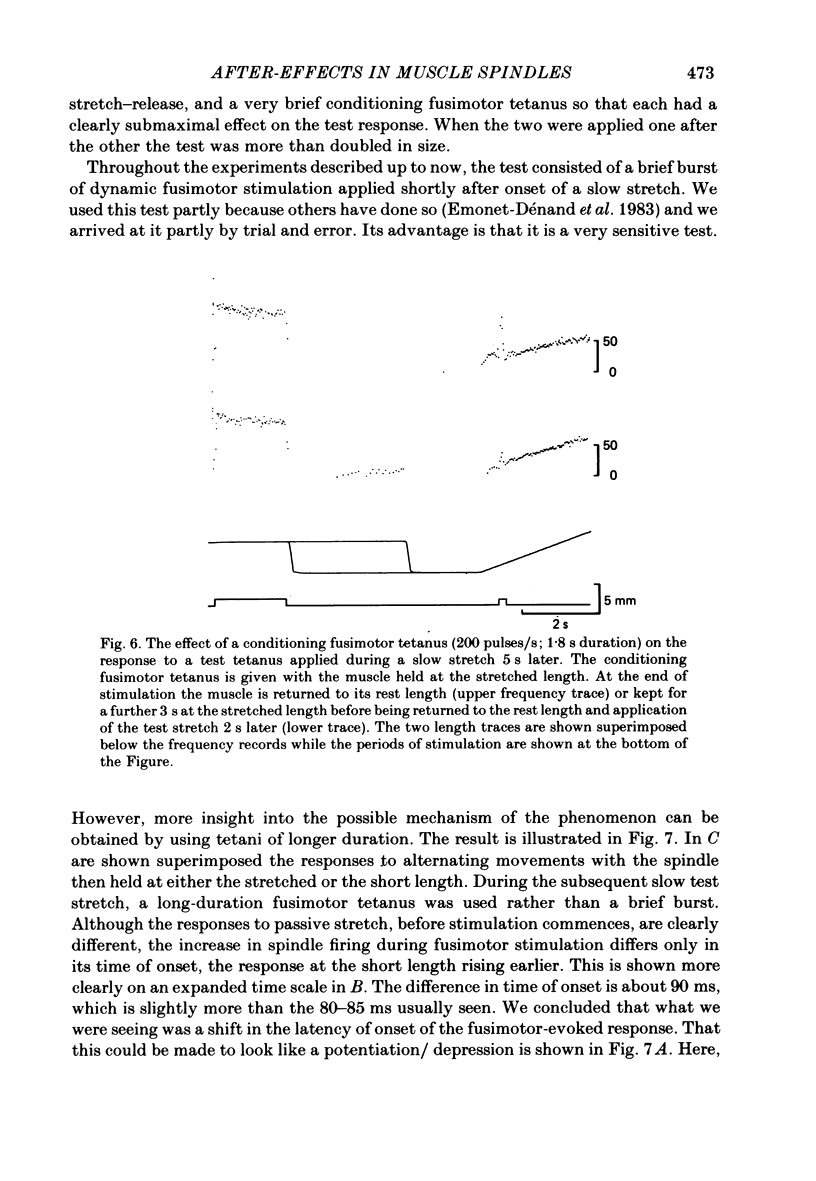
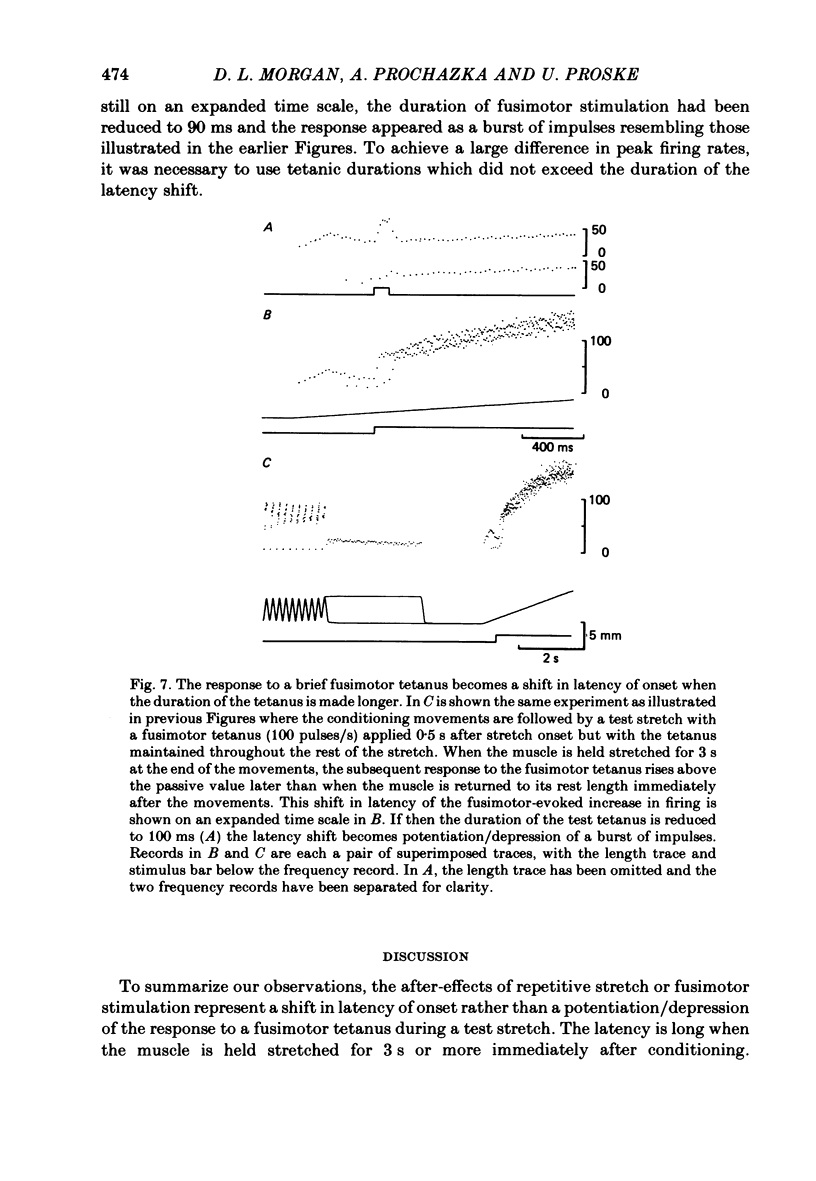
These experiments explore the after-effects of repetitive movements and dynamic fusimotor stimulation on the responses of primary endings of soleus muscle spindles in the anaesthetized cat. If immediately following a series of conditioning stretch and shortening movements, the muscle was held at the stretched length for 3 s before being returned to its rest length, the subsequent response to a brief dynamic fusimotor tetanus given during a slow test stretch produced only a small increase in spindle firing. If, on the other hand, the muscle was returned to its rest length immediately after the movements, the fusimotor tetanus evoked a much larger afferent burst. This difference in the size of the burst could only be observed if the fusimotor tetanus was given soon after onset of the test stretch. If it was delayed and given at a time when the test stretch passed through the length at which the muscle had been held stretched after the movements, there was no difference in the size of the afferent burst. If following the movements the muscle was held stretched for less than 3 s, the response to the subsequent tetanus was not fully depressed. Once the depressed condition had been achieved, the muscle had to be left undisturbed for up to half an hour before the response had recovered its original fully undepressed size. Conditioning repetitive stimulation of the fusimotor fibre was just as effective as using alternating movements in producing the effects. If the test tetanus, which was normally ten shocks in 50 ms, was made longer, the change in size of the impulse burst became a change in latency of onset of the response to the tetanus. Holding the muscle stretched at the end of the conditioning movements/tetanus produced a delay in onset of the response to the test tetanus without significantly altering its size. These observations have been interpreted as arising from development of stable cross-bridges between actin and myosin filaments in the intrafusal fibres. During repetitive movements or fusimotor stimulation, stable bridges become detached and during the subsequent 3 s they re-attach, at the length at which the muscle is being held after conditioning.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuthnott E. R., Ballard K. J., Boyd I. A., Gladden M. H., Sutherland F. I. The ultrastructure of cat fusimotor endings and their relationship to foci of sarcomere convergence in intrafusal fibres. J Physiol. 1982 Oct;331:285–309. doi: 10.1113/jphysiol.1982.sp014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A. The muscle spindle controversy. Sci Prog. 1981 Summer;67(266):205–221. [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol. 1951 Apr;113(2-3):283–297. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Ottoson D. Initial burst of primary endings of isolated mammalian muscle spindles. J Neurophysiol. 1976 Mar;39(2):324–330. doi: 10.1152/jn.1976.39.2.324. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Sollins M. R., Julian F. J. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature. 1976 Apr 15;260(5552):619–621. doi: 10.1038/260619a0. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Quick D. C. Stretch-induced contraction of intrafusal muscle in cat muscle spindle. J Neurosci. 1981 Oct;1(10):1069–1074. doi: 10.1523/JNEUROSCI.01-10-01069.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Gregory J. E. The time-course of recovery of the initial burst of primary endings of muscle spindles. Brain Res. 1977 Feb;121(2):358–361. doi: 10.1016/0006-8993(77)90159-7. [DOI] [PubMed] [Google Scholar]
- Proske U. Stretch-evoked potentiation of responses of muscle spindles in the cat. Brain Res. 1975 May 2;88(2):378–383. doi: 10.1016/0006-8993(75)90403-5. [DOI] [PubMed] [Google Scholar]
- Siegman M. J., Butler T. M., Mooers S. U., Davies R. E. Crossbridge attachment, resistance to stretch, and viscoelasticity in resting mammalian smooth muscle. Science. 1976 Jan 30;191(4225):383–385. doi: 10.1126/science.128819. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Proske U. Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. J Neurophysiol. 1981 Aug;46(2):250–259. doi: 10.1152/jn.1981.46.2.250. [DOI] [PubMed] [Google Scholar]


