Abstract
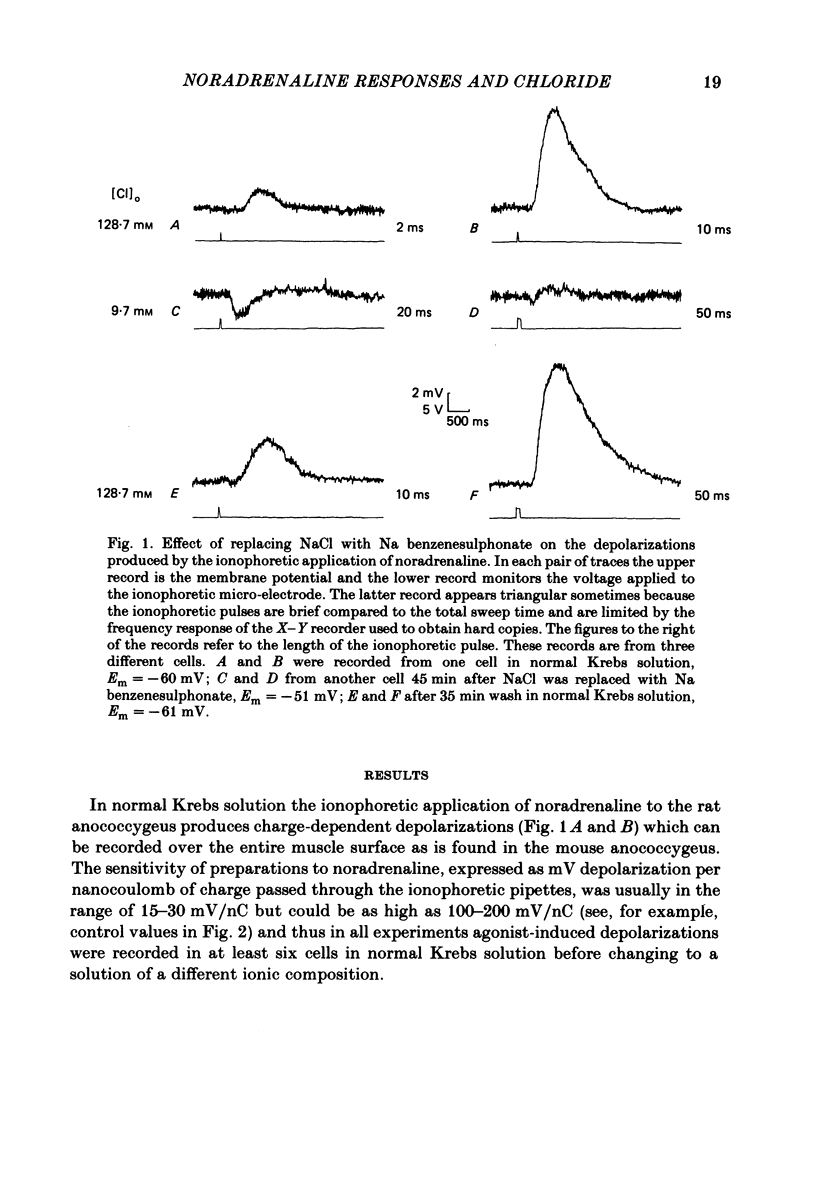
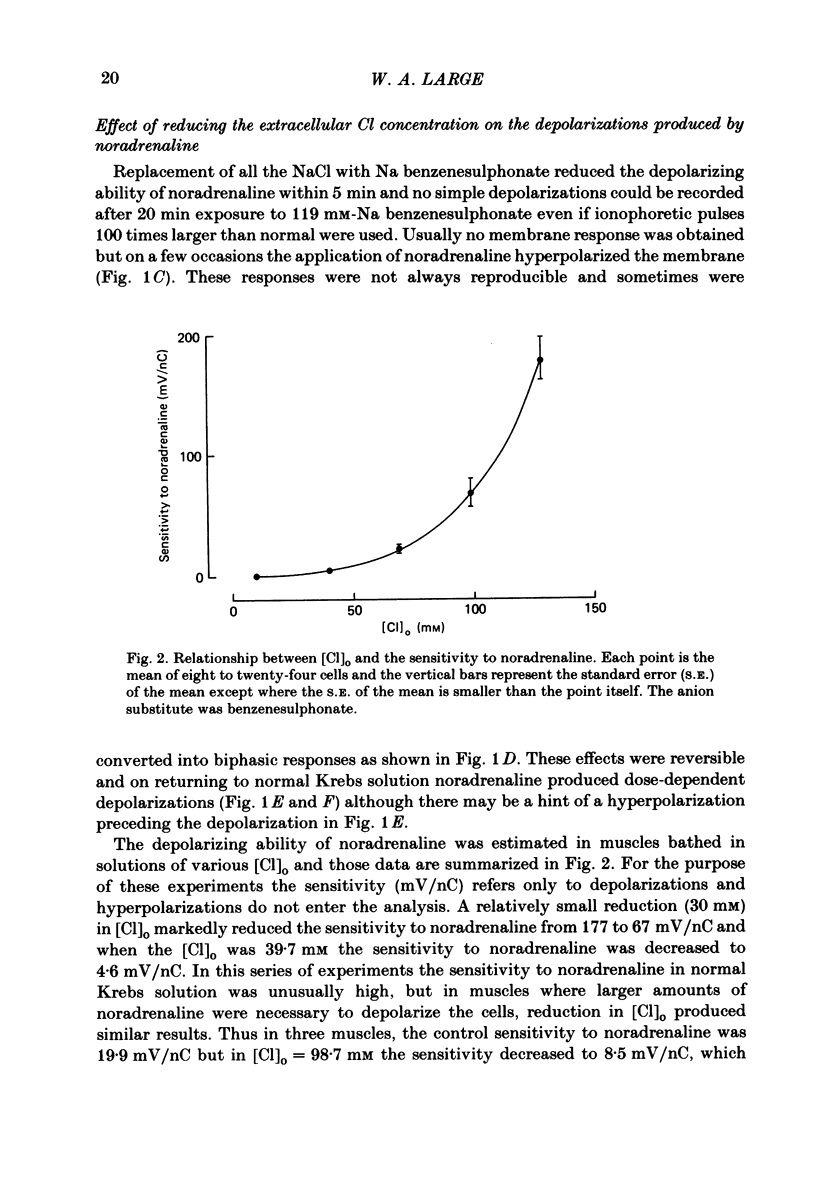
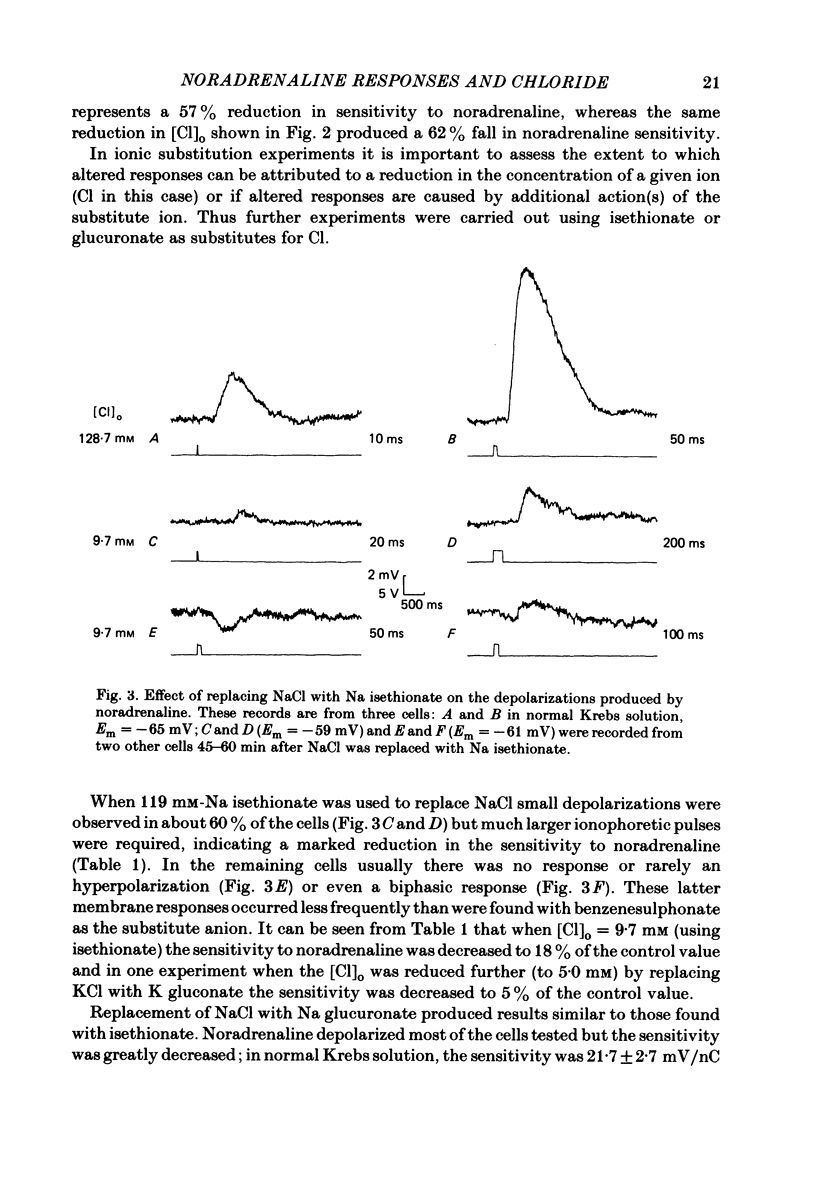
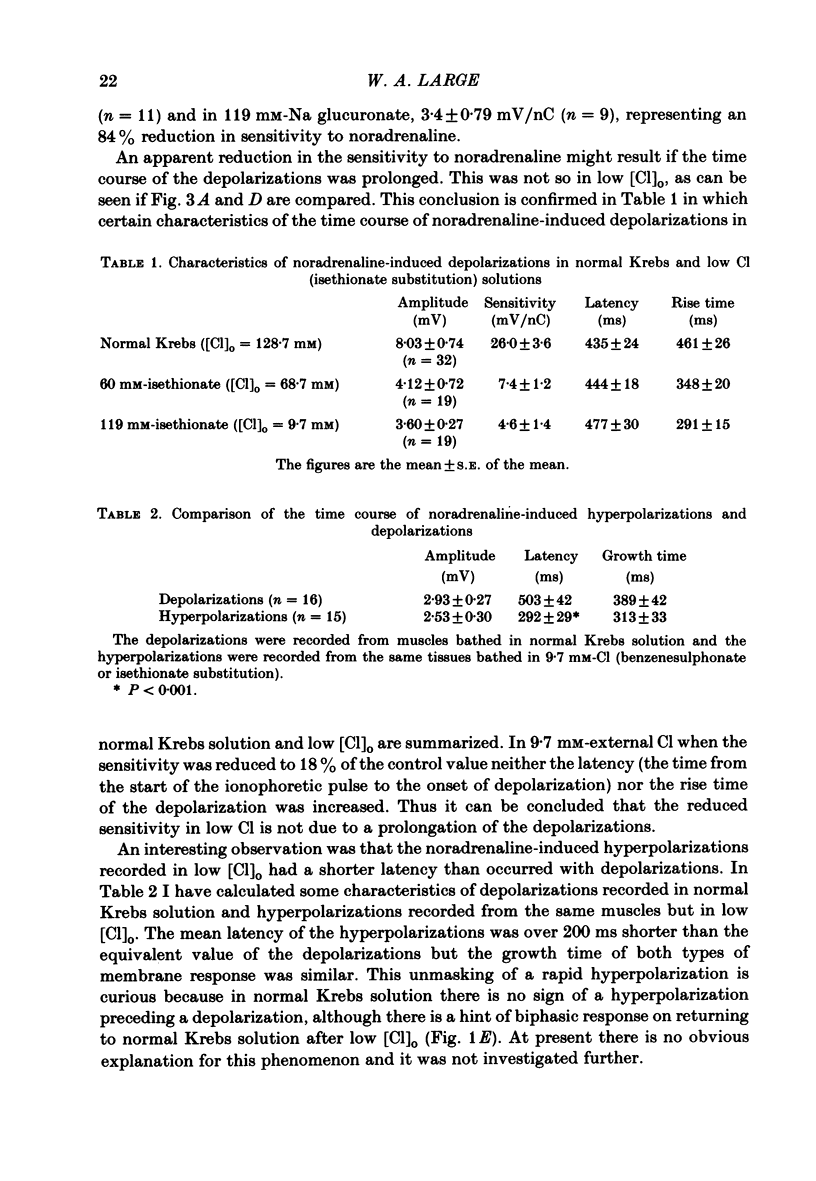
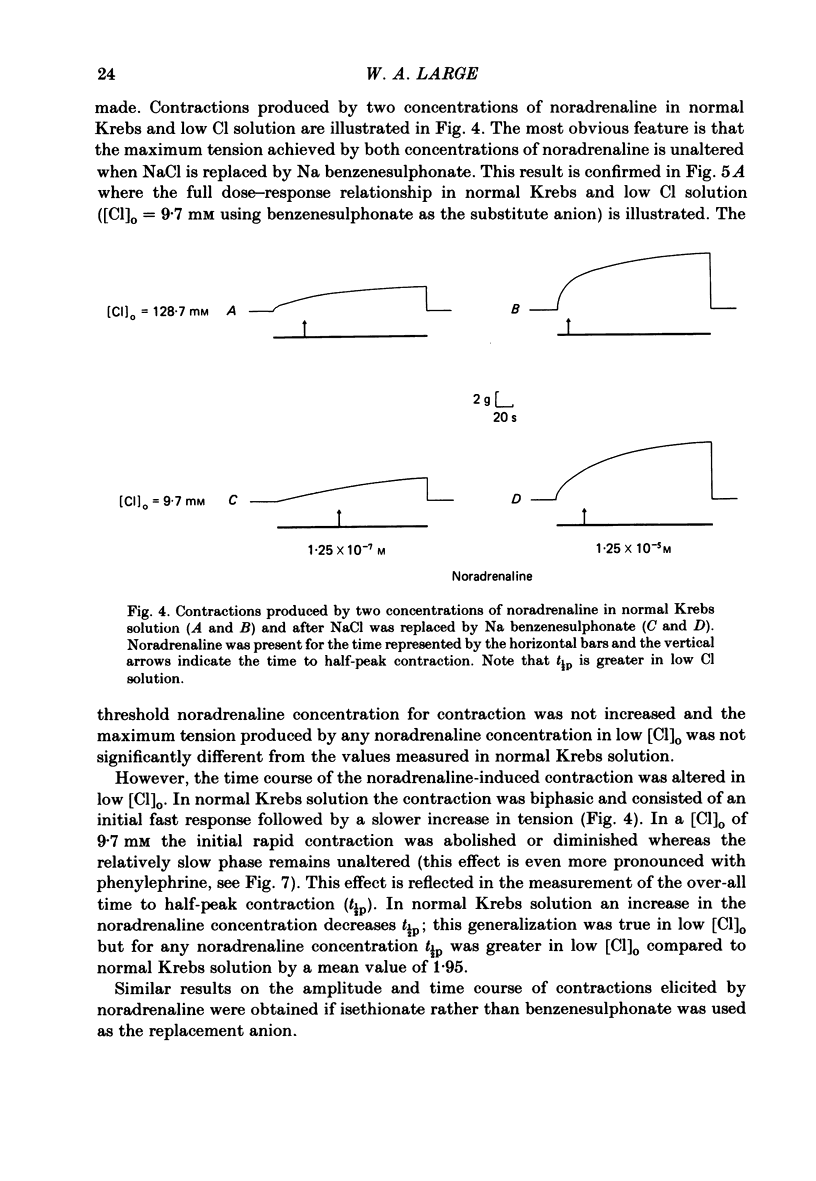
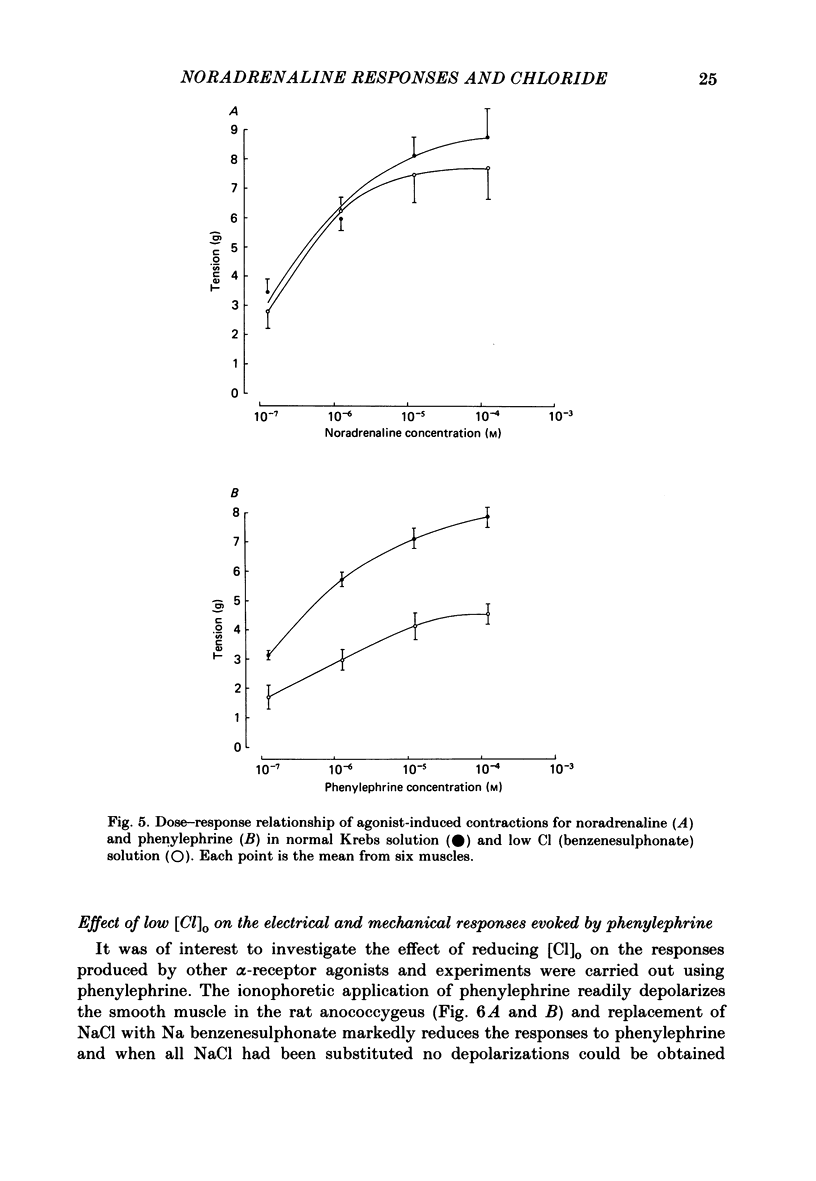
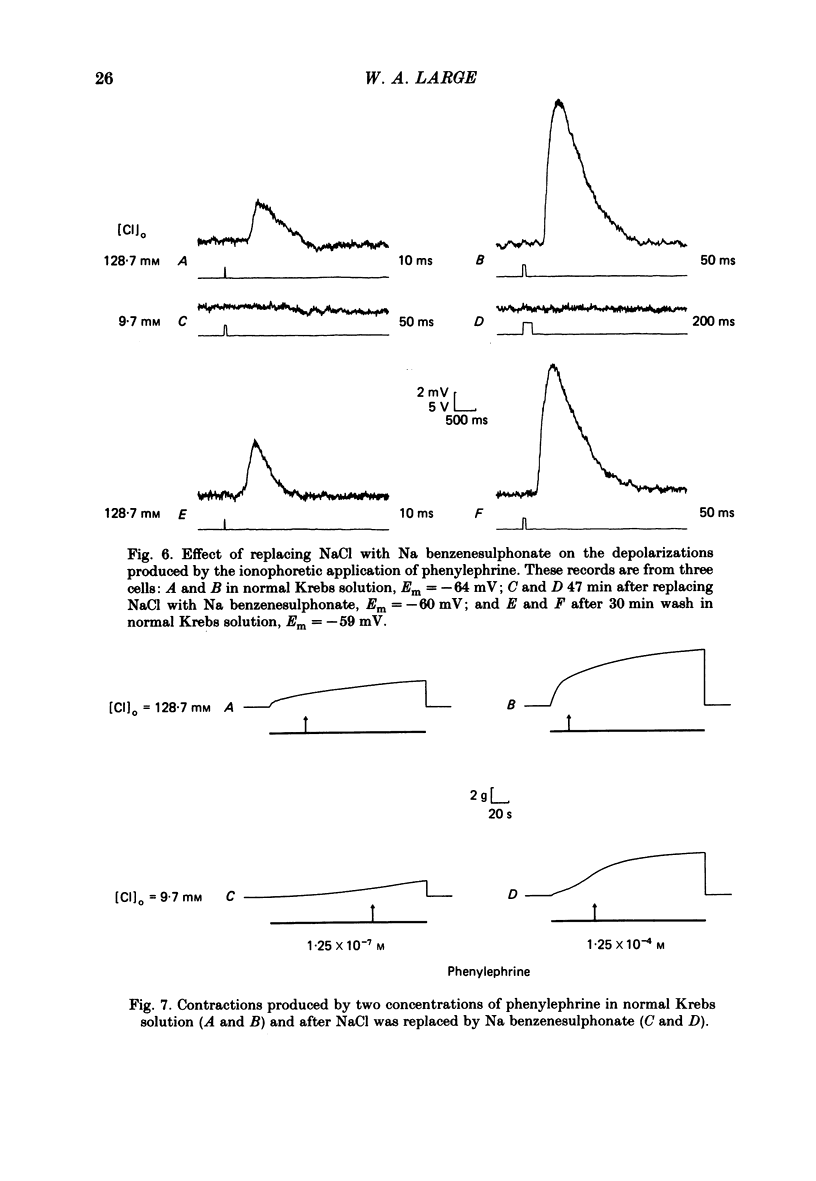
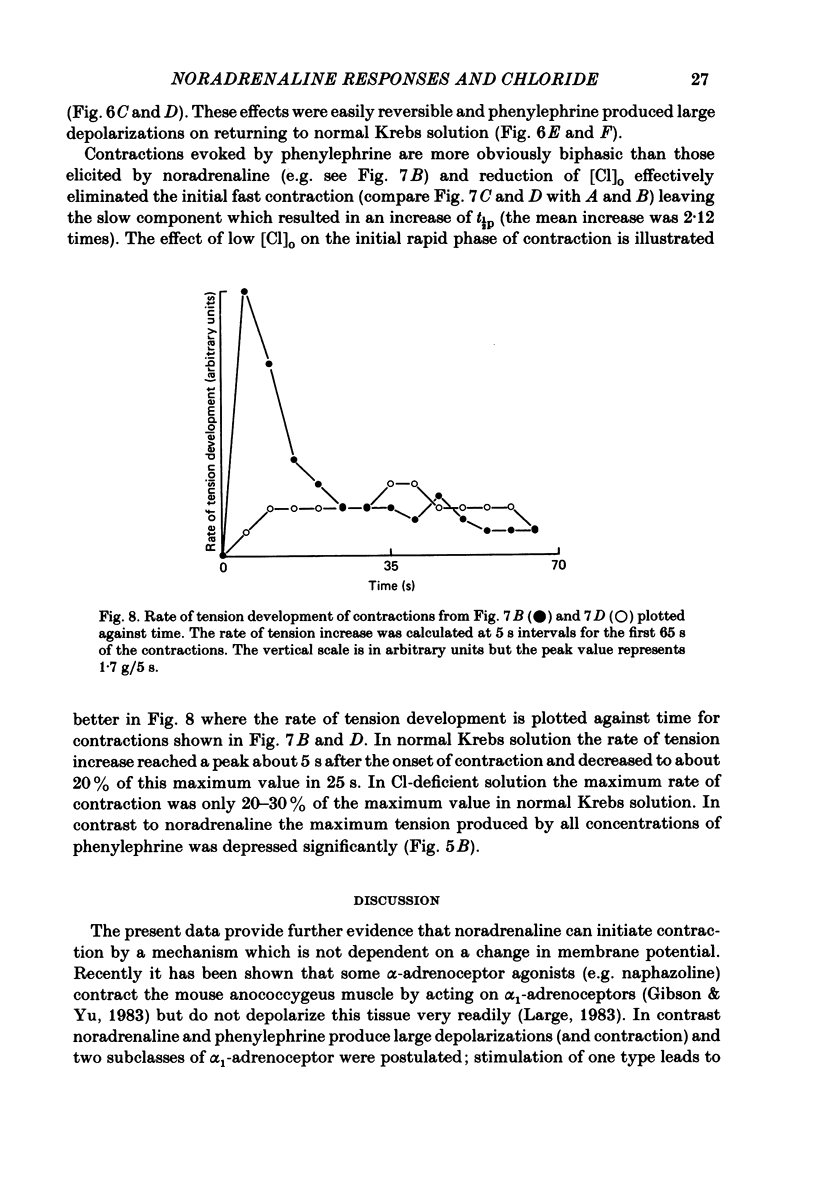
The effect of Cl removal on the depolarizations and contractions produced by activation of the alpha 1-adrenoceptor in the isolated rat anococcygeus was studied. In normal Krebs solution the ionophoretic application of noradrenaline and phenylephrine elicited large depolarizations; when NaCl was replaced with Na benzenesulphonate application of the agonists usually evoked no membrane response but sometimes a hyperpolarization or a biphasic membrane potential change was observed. Graded replacement of Cl by benzenesulphonate produced a progressive reduction in the depolarizing ability of noradrenaline and phenylephrine. When NaCl was replaced with Na isethionate or glucuronate, noradrenaline evoked small depolarizations in most of the cells but the sensitivity to noradrenaline (mV/nC) was reduced by over 80%. In other cells either no potential change or hyperpolarization was observed. In solutions containing low Cl the resting membrane potential was slightly depolarized by a mean value of 5-10 mV (depending on the anion substitute) and the membrane resistance was unaltered. In normal Krebs solution noradrenaline and phenylephrine evoked biphasic contractile responses but when NaCl was replaced by Na benzenesulphonate the initial rapid contraction was diminished greatly or abolished. However, the maximum tension produced by any concentration of noradrenaline was unaltered in low Cl solution although the contractions produced by phenylephrine were depressed in low Cl media. These data are further evidence that stimulation of the alpha 1-adrenoceptor in smooth muscle can produce contraction which is not mediated by a change in membrane potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983 Mar;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Szurszewski J. H. The stimulant action of noradrenaline (alpha-action) on guinea-pig myometrium compared with that of acetylcholine. Proc R Soc Lond B Biol Sci. 1974 Jan 29;185(1079):225–262. doi: 10.1098/rspb.1974.0018. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle cells of the rat anococcygeus muscle. J Physiol. 1975 Feb;245(1):49–62. doi: 10.1113/jphysiol.1975.sp010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. R., Starke K. Postsynaptic alpha-adrenoceptor subtypes in rabbit blood vessels and rat anococcygeus muscle studied in vitro. J Cardiovasc Pharmacol. 1981 Jul-Aug;3(4):854–866. doi: 10.1097/00005344-198107000-00019. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Smith C. F., Walker J. M. Selectivity of blocking agents for pre-and postsynaptic alpha-adrenoceptors. Br J Pharmacol. 1977 May;60(1):91–96. doi: 10.1111/j.1476-5381.1977.tb16752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Yu O. Pharmacology of postsynaptic alpha-adrenoreceptors in the mouse anococcygeus muscle. J Auton Pharmacol. 1983 Mar;3(1):1–6. doi: 10.1111/j.1474-8673.1983.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A. Membrane potential responses of the mouse anococcygeus muscle to ionophoretically applied noradrenaline. J Physiol. 1982 May;326:385–400. doi: 10.1113/jphysiol.1982.sp014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A. Membrane potential responses to ionophoretically applied alpha-adrenoceptor agonists in the mouse anococcygeus muscle. Br J Pharmacol. 1983 May;79(1):233–243. doi: 10.1111/j.1476-5381.1983.tb10517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaribuchi T., Ito Y., Kuriyama H. Effects of catecholamines on the guinea-pig vas deferens in various ionic environments. Jpn J Physiol. 1971 Dec;21(6):691–708. doi: 10.2170/jjphysiol.21.691. [DOI] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlström B. A. A study on the action of noradrenaline on ionic content and sodium, potassium and chloride effluxes in the rat portal vein. Acta Physiol Scand. 1973 Dec;89(4):522–530. doi: 10.1111/j.1748-1716.1973.tb05545.x. [DOI] [PubMed] [Google Scholar]


