Abstract
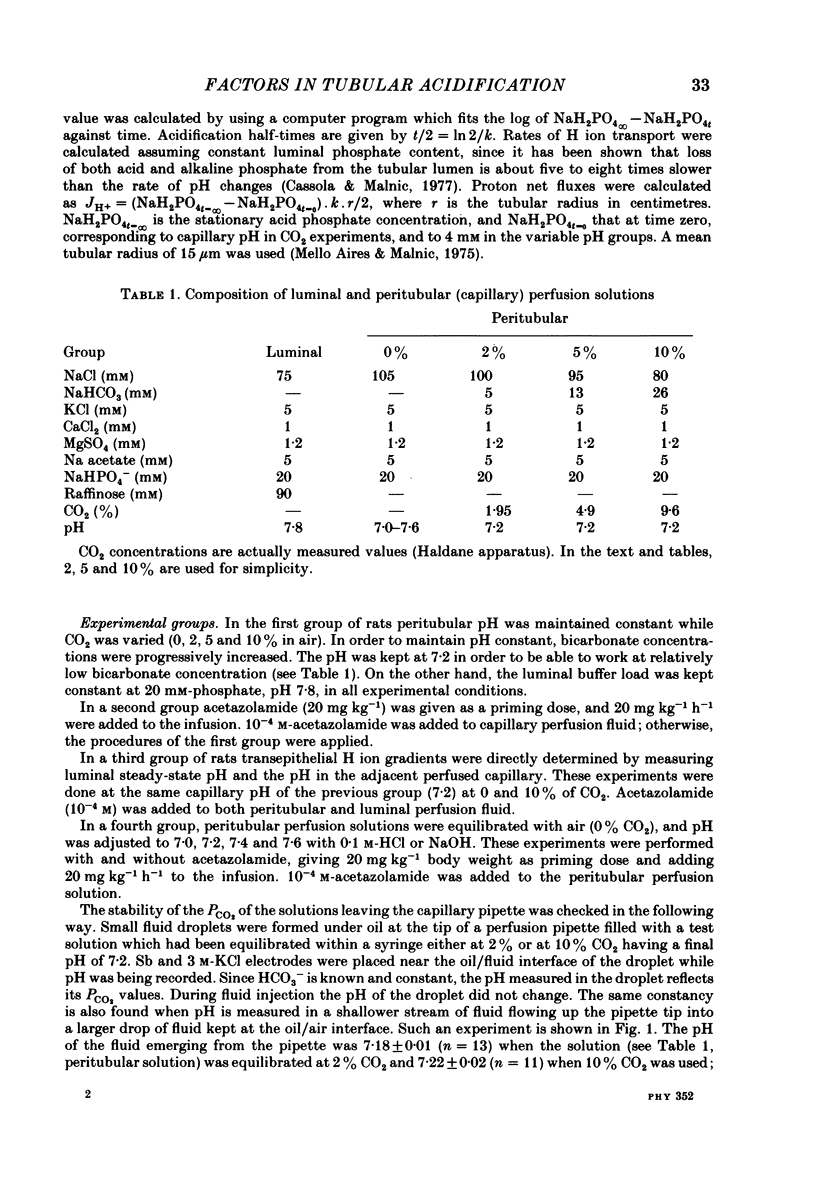
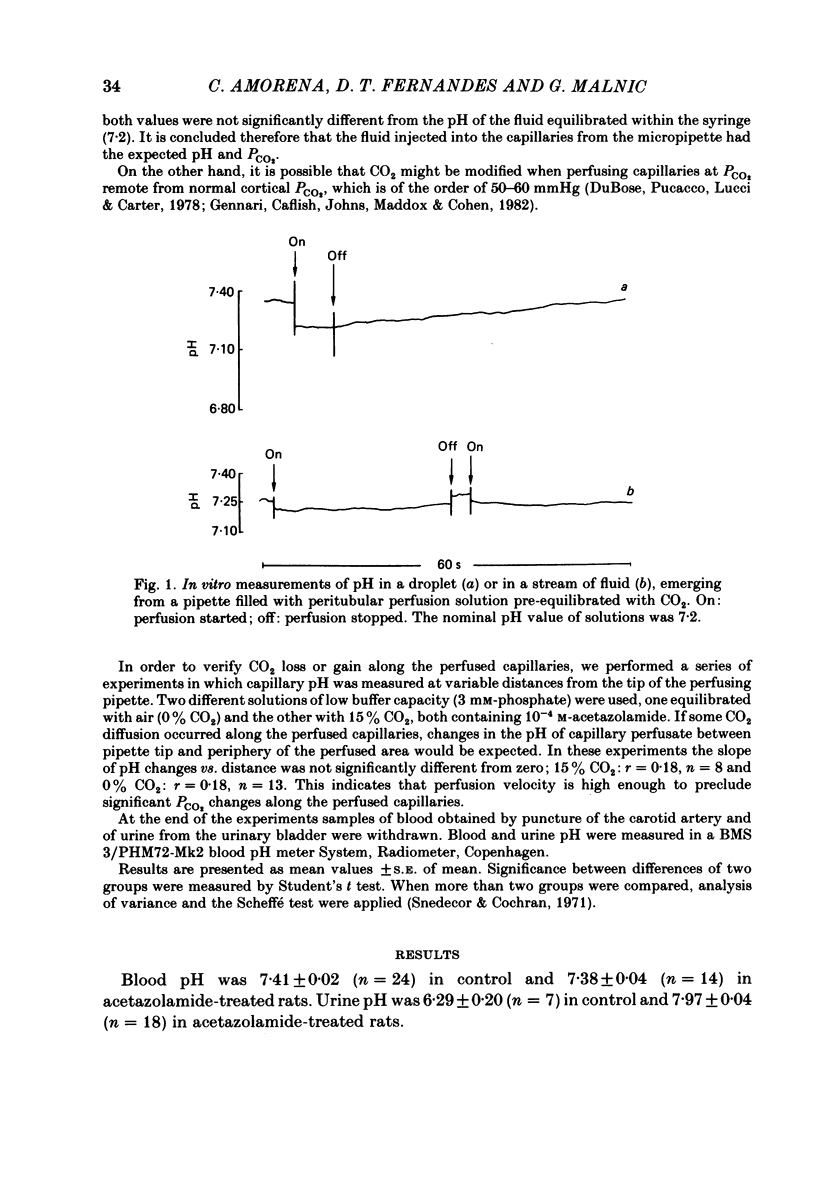
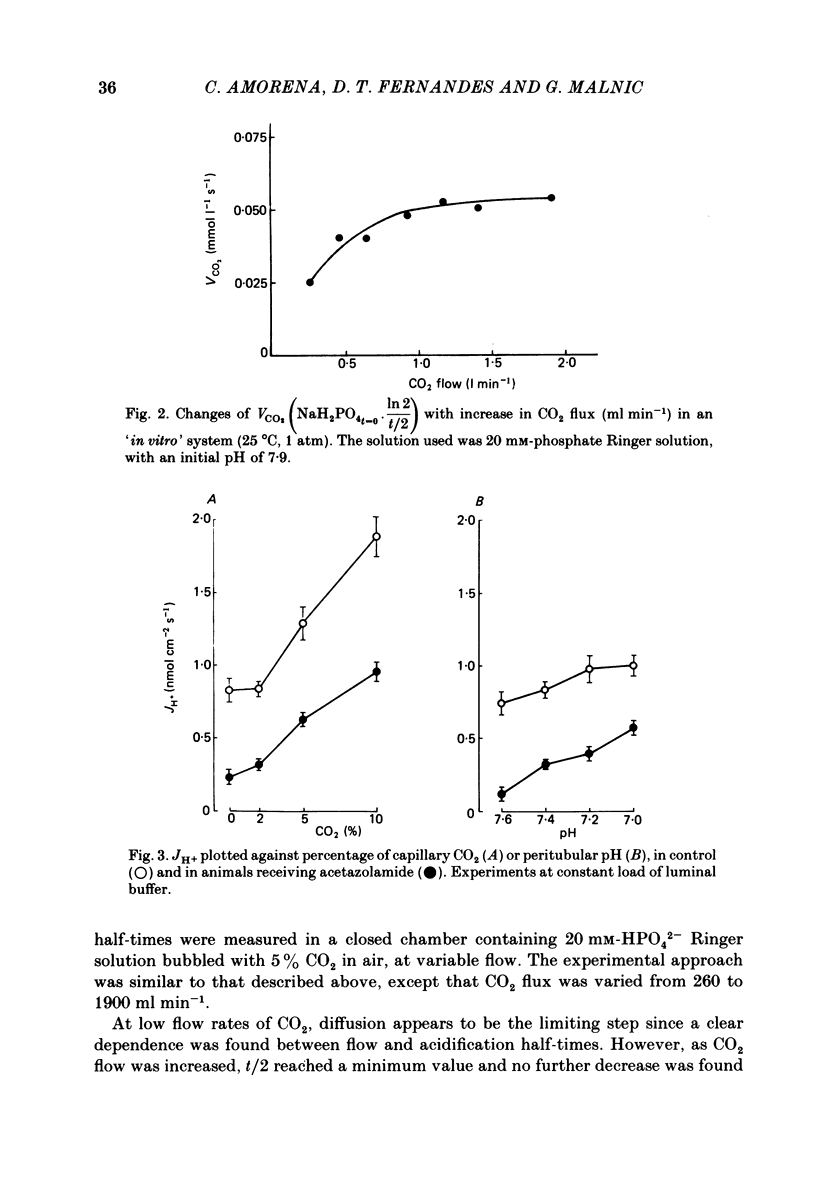
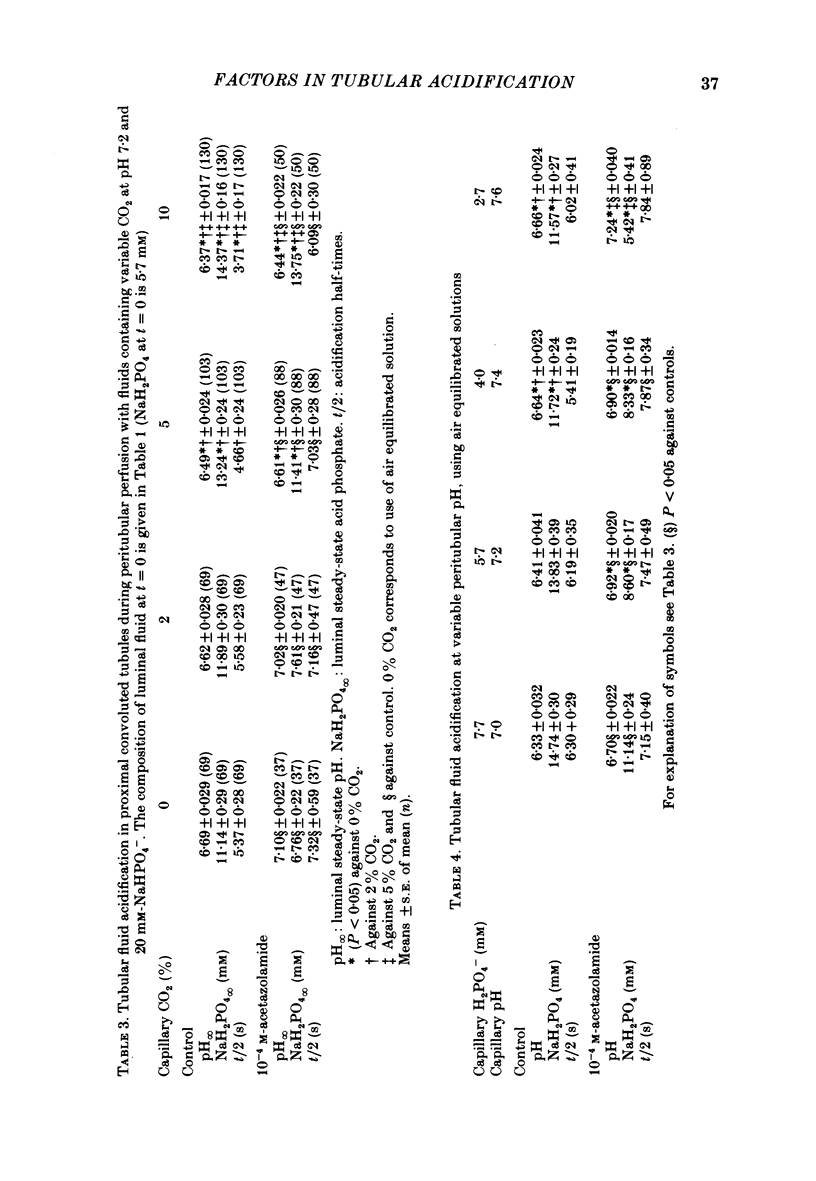
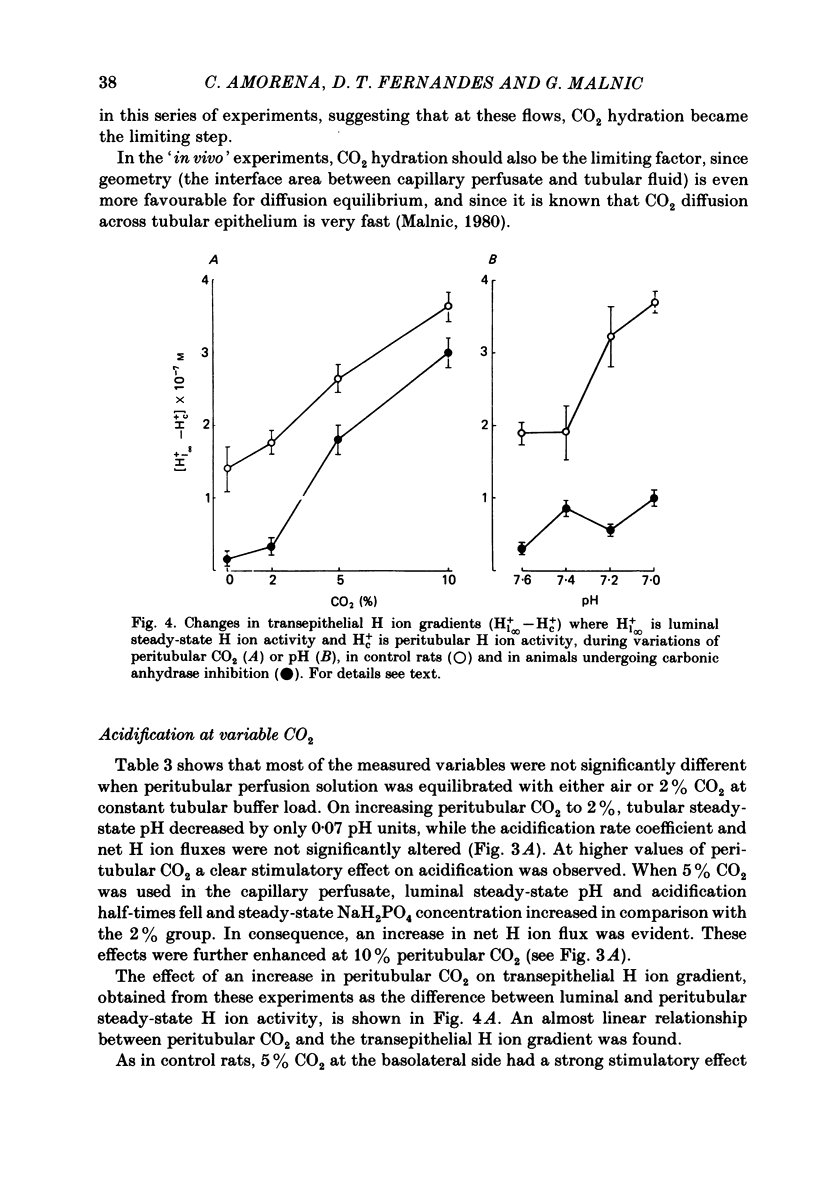
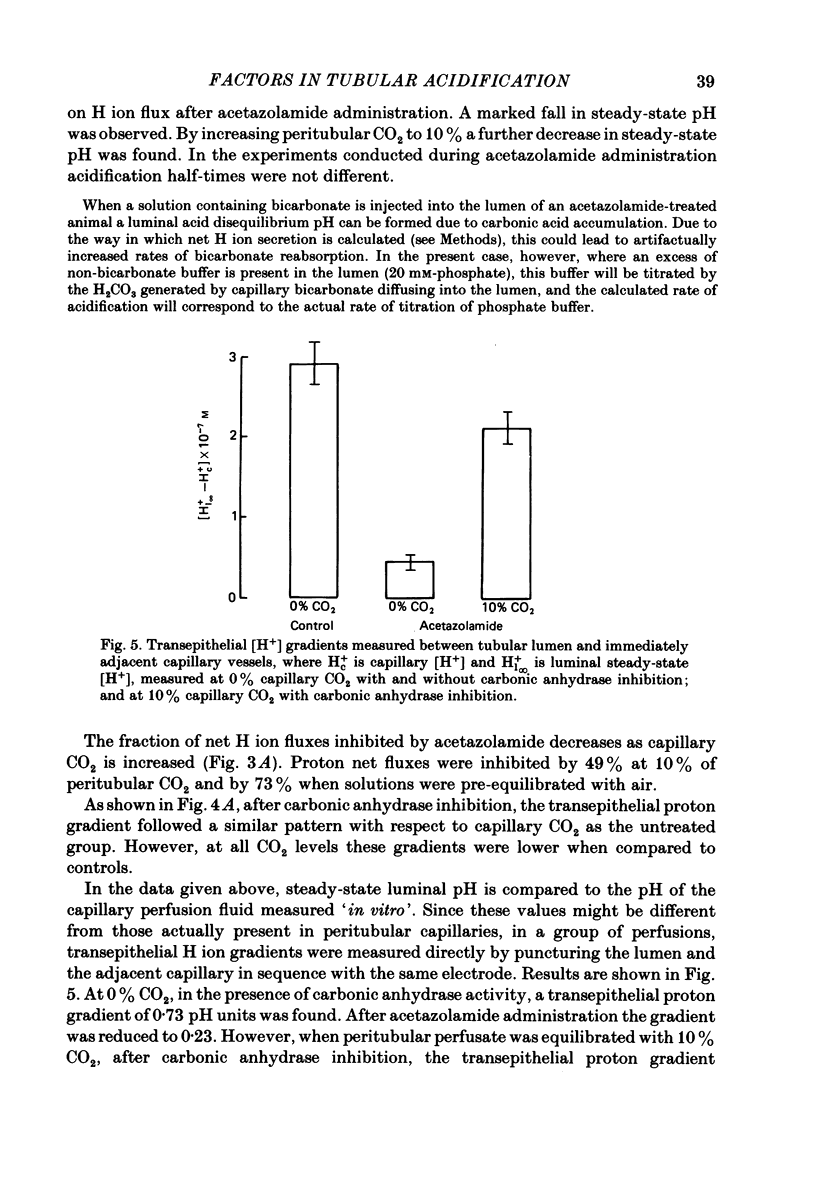
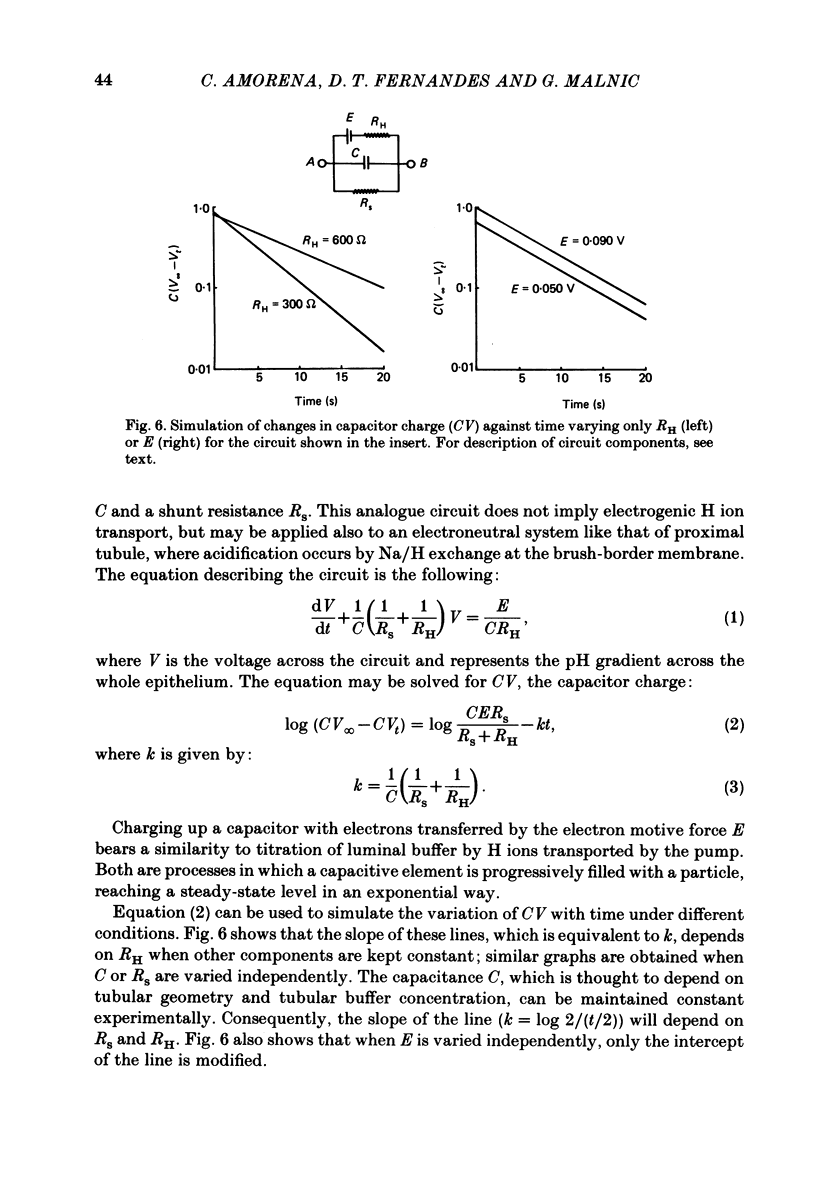
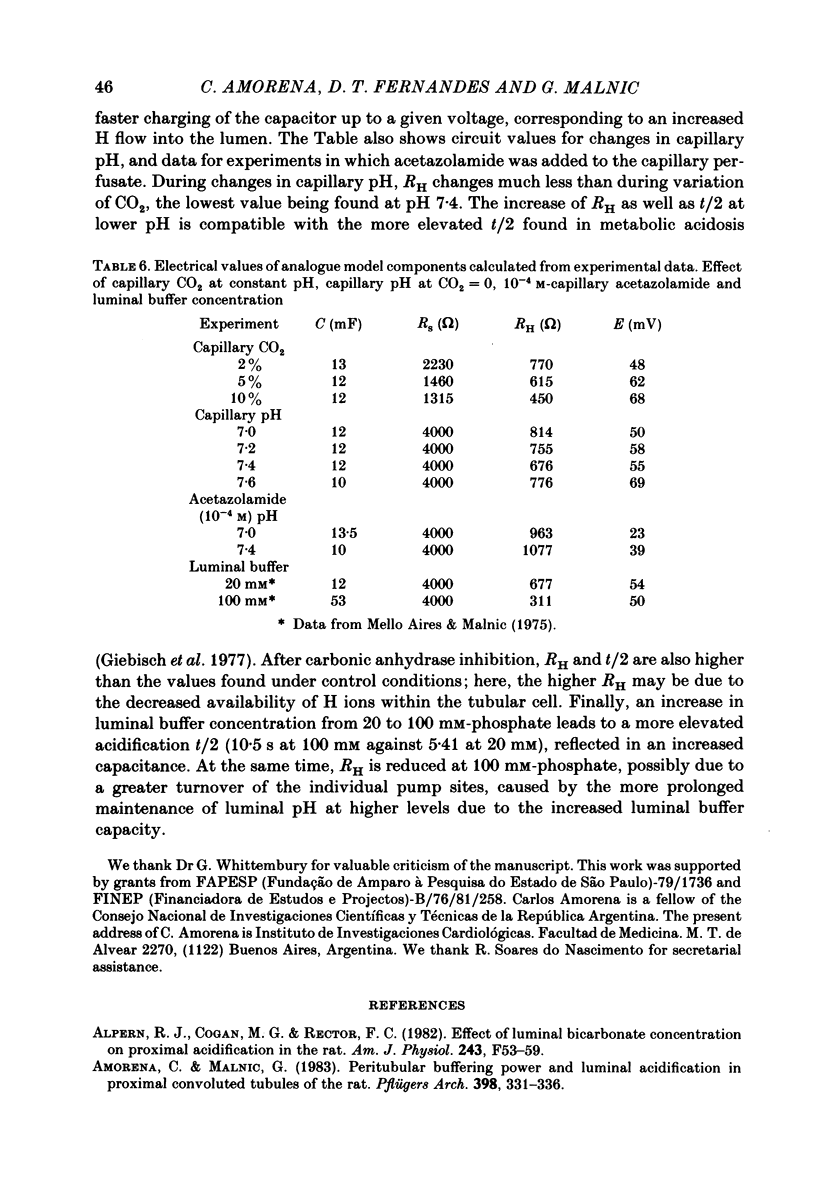
The effect of peritubular PCO2 and pH changes within the physiological range on proximal tubular acidification of non-bicarbonate (phosphate) buffer was evaluated with and without carbonic anhydrase inhibition by stopped-flow microperfusion and Sb micro-electrode techniques. Luminal steady-state pH was reduced from 6.69 to 6.37 and H ion fluxes (JH+) increased from 0.63 to 1.57 nmol cm-2 s-1 by increasing capillary CO2 from 0 to 9.6% at pH 7.2. After acetazolamide a marked, although attenuated, effect of CO2 on acidification was still observed; JH+ increased from 0.088 nmol cm-2 s-1 at 0% CO2 to 0.78 at 9.6% CO2. Most of this effect can be explained by titration of luminal buffer by CO2, uncatalysed CO2 hydration and H2CO3 recirculation. An increase in capillary CO2 reduced acidification half-times (t/2), which, according to an analogue circuit model, may be due to increased H ion access to the pump. Peritubular pH changes at 0% CO2 also modified tubular acidification, increasing JH+ from 0.73 nmol cm-2 s-1 at pH 7.6 to 0.99 at pH 7.0. After acetazolamide, JH+ still increased from 0.11 nmol cm-2 s-1 at pH 7.6 to 0.57 at pH 7.0. In conclusion, both peritubular CO2 changes at constant pH and pH changes at 0% CO2 were effective to modify JH+, in the presence and absence of carbonic anhydrase activity. In the studied range, capillary CO2 induced larger changes in JH+ than pH. The data show substrate (H ion) is a limiting factor for tubular H ion secretion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Cogan M. G., Rector F. C., Jr Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am J Physiol. 1982 Jul;243(1):F53–F59. doi: 10.1152/ajprenal.1982.243.1.F53. [DOI] [PubMed] [Google Scholar]
- Amorena C., Malnic G. Peritubular buffering power and luminal acidification in proximal convoluted tubules of the rat. Pflugers Arch. 1983 Sep;398(4):331–336. doi: 10.1007/BF00657243. [DOI] [PubMed] [Google Scholar]
- Cassola A. C., Giebisch G., Malnic G. Mechansims and components of renal tubular acidification. J Physiol. 1977 Jun;267(3):601–624. doi: 10.1113/jphysiol.1977.sp011828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola A. C., Malnic G. Phosphate transfer and tubular pH during renal stopped flow microperfusion experiments in the rat. Pflugers Arch. 1977 Jan 17;367(3):249–255. doi: 10.1007/BF00581362. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Biagi B., Giebisch G. Control mechanisms of bicarbonate transport across the rat proximal convoluted tubule. Am J Physiol. 1982 May;242(5):F532–F543. doi: 10.1152/ajprenal.1982.242.5.F532. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Malnic G., Giebisch G. Passive driving forces of proximal tubular fluid and bicarbonate transport: gradient dependence of H+ secretion. Am J Physiol. 1983 Nov;245(5 Pt 1):F622–F633. doi: 10.1152/ajprenal.1983.245.5.F622. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Warnock D. G., Lin E. T., Rector F. C., Jr Effect of acetazolamide on bicarbonate reabsorption in the proximal tubule of the rat. Am J Physiol. 1979 Dec;237(6):F447–F454. doi: 10.1152/ajprenal.1979.237.6.F447. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Steinmetz P. R. Control of active proton transport in turtle urinary bladder by cell pH. J Gen Physiol. 1980 Sep;76(3):381–393. doi: 10.1085/jgp.76.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho E. M., Malnic G. H in cortical peritubular capillaries of rat kidney. Pflugers Arch. 1976 Jun 22;363(3):211–217. doi: 10.1007/BF00594603. [DOI] [PubMed] [Google Scholar]
- Gennari F. J., Caflisch C. R., Johns C., Maddox D. A., Cohen J. J. PCO2 measurements in surface proximal tubules and peritubular capillaries of the rat kidney. Am J Physiol. 1982 Jan;242(1):F78–F85. doi: 10.1152/ajprenal.1982.242.1.F78. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., De Mello G. B., De Mello Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol. 1977 Jun;267(3):571–599. doi: 10.1113/jphysiol.1977.sp011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F., Quehenberger P., Greger R., Silbernagl S., Stockinger P. Evidence for a bicarbonate leak in the proximal tubule of the rat kidney. Pflugers Arch. 1980 Aug;386(3):239–244. doi: 10.1007/BF00587474. [DOI] [PubMed] [Google Scholar]
- Malnic G. CO2 equilibria in renal tissue. Am J Physiol. 1980 Oct;239(4):F307–F318. doi: 10.1152/ajprenal.1980.239.4.F307. [DOI] [PubMed] [Google Scholar]
- Malnic G., Vieira F. L. The antimony microelectrode in kidney micropuncture. Yale J Biol Med. 1972 Jun-Aug;45(3-4):356–367. [PMC free article] [PubMed] [Google Scholar]
- Malnic G., de Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol. 1971 Jun;220(6):1759–1767. doi: 10.1152/ajplegacy.1971.220.6.1759. [DOI] [PubMed] [Google Scholar]
- Mello Aires M., Malnic G. Peritubular pH and PCO'2 in renal tubular acidification. Am J Physiol. 1975 Jun;228(6):1766–1774. doi: 10.1152/ajplegacy.1975.228.6.1766. [DOI] [PubMed] [Google Scholar]
- PITTS R. F. Mechanisms for stabilizing the alkaline reserves of the body. Harvey Lect. 1952;48:172–209. [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., ROBERTS A. D., Jr, SMITH J. S. The role of plasma CO2 tension and carbonic anhydrase activity in the renal reabsorption of bicarbonate. J Clin Invest. 1960 Nov;39:1706–1721. doi: 10.1172/JCI104193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C. R., de Mello G. B., Mangili O. C., Malnic G. H+ ion secretion in proximal tubule of low-Co2/HCO-3 perfused isolated rat kidney. Pflugers Arch. 1982 Mar;393(1):63–70. doi: 10.1007/BF00582393. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Berry C. A., Rector F. C., Jr Effect of luminal and peritubular HCO3(-) concentrations and PCO2 on HCO3(-) reabsorption in rabbit proximal convoluted tubules perfused in vitro. J Clin Invest. 1982 Sep;70(3):639–649. doi: 10.1172/JCI110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J. Na+-dependent H+ efflux from proximal tubule: evidence for reversible Na+-H+ exchange. Am J Physiol. 1981 Oct;241(4):F380–F385. doi: 10.1152/ajprenal.1981.241.4.F380. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H. H+ current response to CO2 and carbonic anhydrase inhibition in turtle bladder. Am J Physiol. 1976 Aug;231(2):565–572. doi: 10.1152/ajplegacy.1976.231.2.565. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. CO2 requirements for H+ secretion by the isolated turtle bladder. Am J Physiol. 1971 Jun;220(6):2051–2057. doi: 10.1152/ajplegacy.1971.220.6.2051. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]



