Abstract
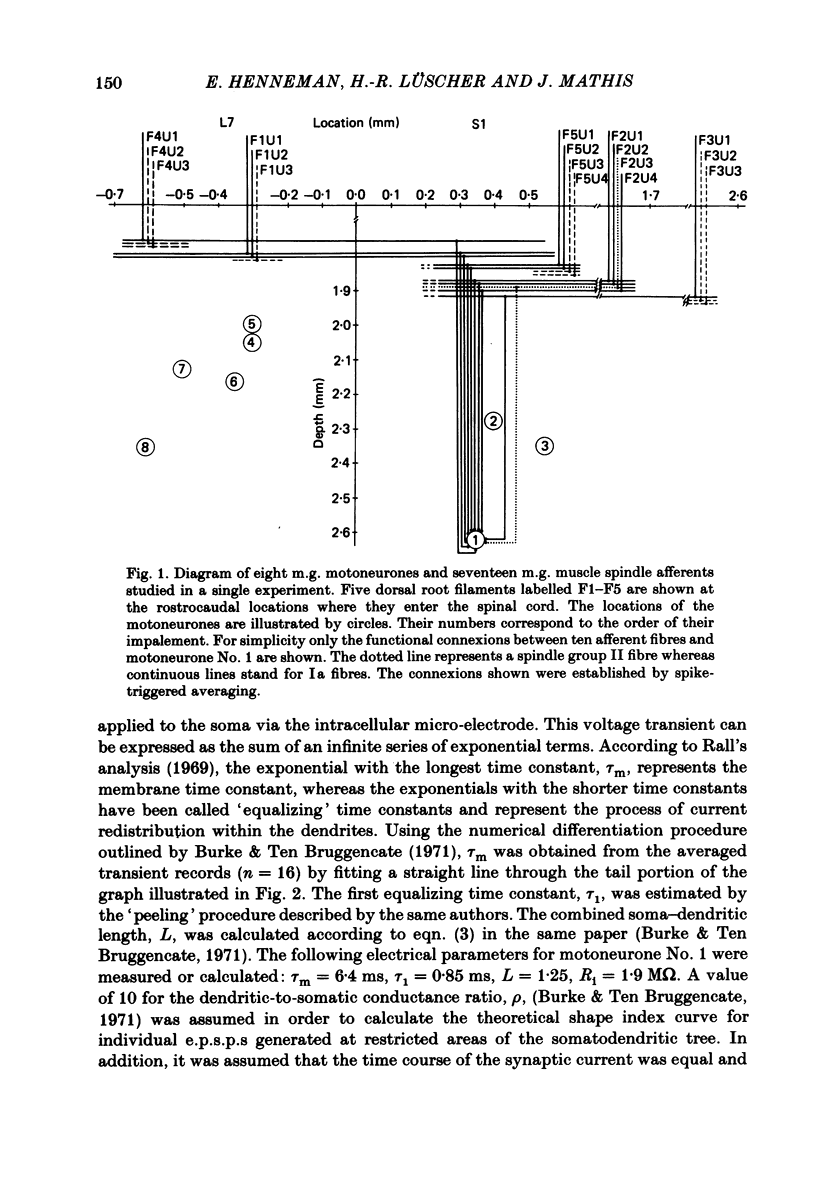
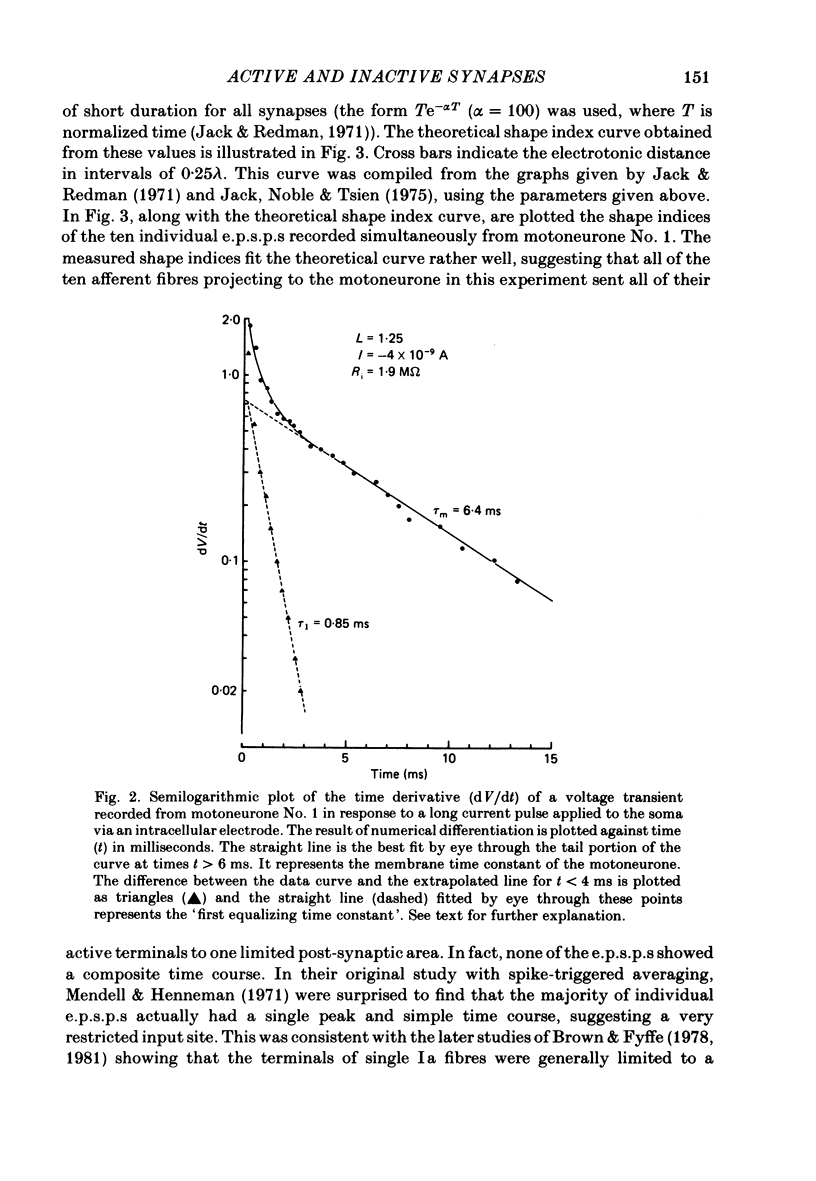
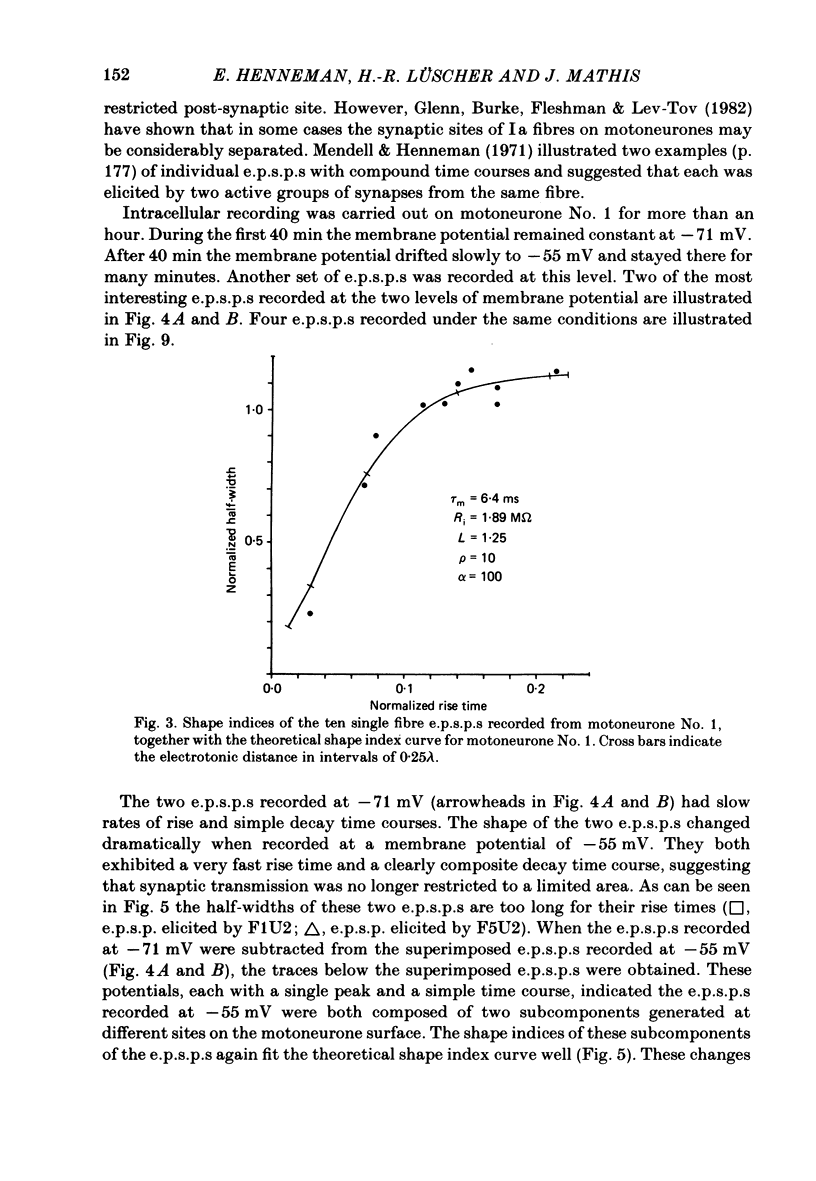
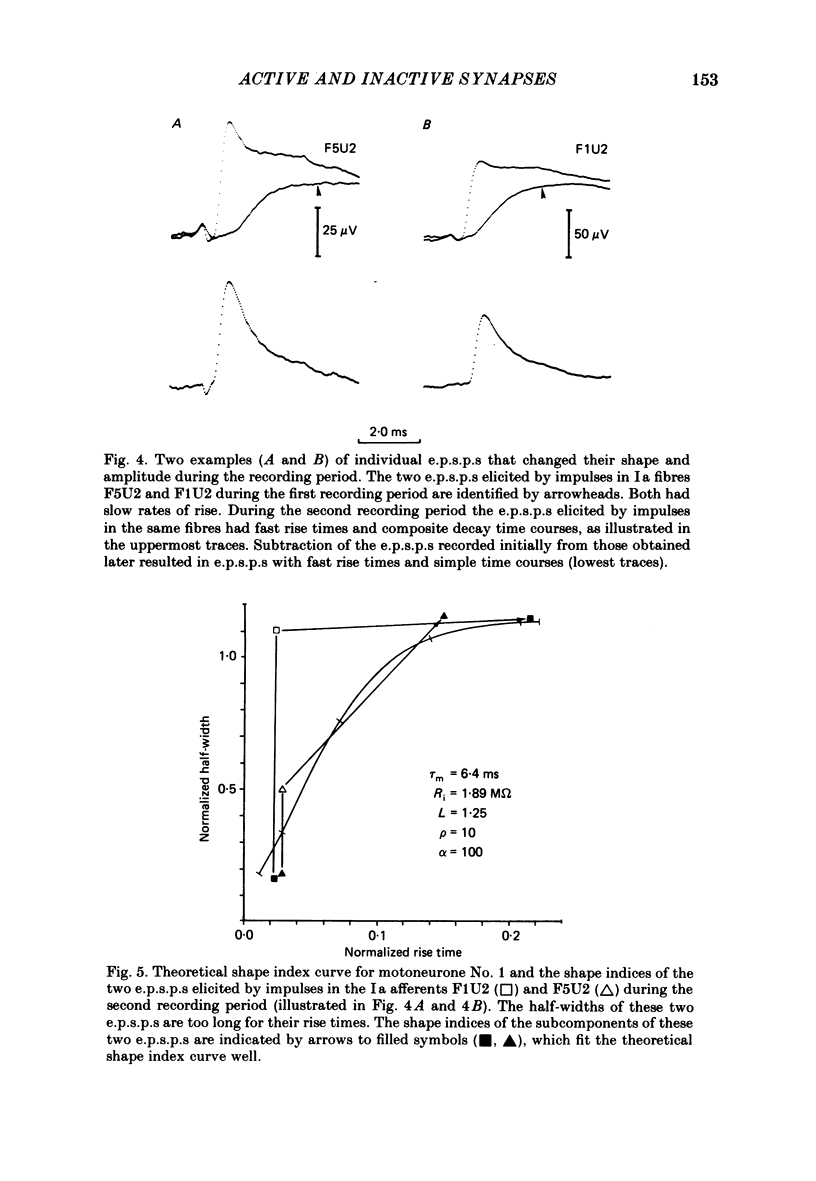
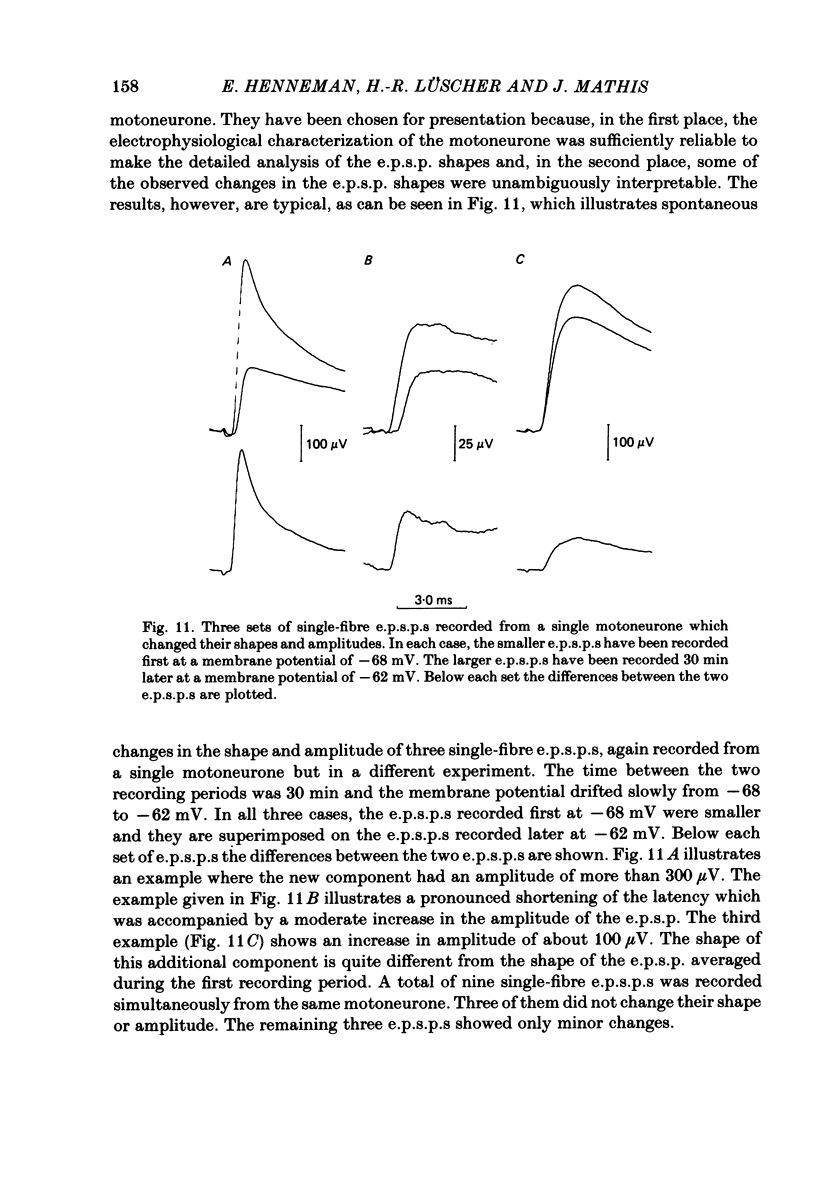
A technique is described for recording large numbers of individual or single-fibre excitatory post-synaptic potentials (e.p.s.p.s) from single motoneurones by means of spike-triggered averaging. The cable properties of the motoneurones were calculated from the decay time course of a voltage transient in the motoneurone following a current pulse applied to the soma. From this response a theoretical shape index curve was calculated. Most individual or single-fibre e.p.s.p.s elicited by impulses in different Ia fibres had simple decay time courses and shape indices that fitted the theoretical shape index curve of the motoneurone from which they were recorded very well. This suggested that the active terminals of these afferent fibres were located within limited post-synaptic areas. In a few cases the original amplitude, latency and shape of individual e.p.s.p.s changed dramatically when they were re-averaged 40 min later after the membrane potential had decreased, but was still at an acceptable level. E.p.s.p.s with simple decay time courses changed to e.p.s.p.s with composite decay time courses, presumably due to activation of previously silent synapses. The results suggest that impulses conducted in a single afferent fibre from a muscle spindle do not necessarily activate all of the synapses which the fibre forms on a motoneurone, but may repeatedly fail to activate some endings during prolonged periods of spike-triggered averaging, while consistently activating others. Evidence regarding the site of transmission failure and the possible mechanism of its relief is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Headley P. M. Electrical interaction between motoneurons and afferent terminals in cat spinal cord. J Neurophysiol. 1979 May;42(3):635–641. doi: 10.1152/jn.1979.42.3.635. [DOI] [PubMed] [Google Scholar]
- De Mey J. A critical review of light and electron microscopic immunocytochemical techniques used in neurobiology. J Neurosci Methods. 1983 Jan;7(1):1–18. doi: 10.1016/0165-0270(83)90014-6. [DOI] [PubMed] [Google Scholar]
- Decima E. E., Goldberg L. J. Antidromic electrical interaction between alpha motoneurons and presynamptic terminals. Brain Res. 1973 Jul 16;57(1):1–14. doi: 10.1016/0006-8993(73)90563-5. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo J., Rudomin P. The effects of gallamine on field and dorsal root potentials produced by antidromic stimulation of motor fibres in the frog spinal cord. Exp Brain Res. 1978 May 12;32(1):135–150. doi: 10.1007/BF00237397. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol. 1983 Jan;49(1):269–289. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Muheim M. H. Fabrication of well defined micropipette tips by hydrofluoric acid etching. Pflugers Arch. 1977 Nov 25;372(1):101–102. doi: 10.1007/BF00582213. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Collatos T. C., Niechaj A., Mendell L. M. Immediate increase in Ia-motoneuron synaptic transmission caudal to spinal cord transection. J Neurophysiol. 1979 May;42(3):655–664. doi: 10.1152/jn.1979.42.3.655. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Mechanisms of action potential propagation failure at sites of axon branching in the crayfish. J Physiol. 1980 Apr;301:243–259. doi: 10.1113/jphysiol.1980.sp013202. [DOI] [PMC free article] [PubMed] [Google Scholar]


