Abstract
Secretion of proteins by rat parotid glands in response to parasympathetic nerve stimulation was studied in vivo during pentobarbitone anaesthesia. Parasympathetic stimulation (3-10 Hz) via the auriculotemporal nerve resulted in a copious flow of saliva low in protein. In contrast, sympathetic stimulation (5 Hz) via the cervical sympathetic trunk evoked saliva low in volume but high in protein. Nevertheless, the specific concentrations of amylase and peroxidase (mg/mg protein) and the ratio of amylase to peroxidase remained constant. Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis revealed a single, rapidly migrating protein band of unknown identity in proportionately greater amounts in parasympathetic saliva than in sympathetic saliva. Bilateral adrenalectomy led to reduced amylase and peroxidase secretion in response to parasympathetic stimulation both on a mg/ml and a mg/mg protein basis. SDS gel electrophoresis also demonstrated the decrease in specific amylase concentration following adrenalectomy. The ratio of amylase to peroxidase, however, was not significantly affected. Administration of 6-hydroxydopamine 17-72 h prior to adrenalectomy caused no further reduction in the secretion of amylase and peroxidase. Chronic sympathectomy of 2.5-4 months duration resulted in an increased protein secretion (mg/ml) by the parotid gland in response to parasympathetic stimulation. This increase was only slightly reduced by bilateral adrenalectomy. However, as observed in non-sympathectomized rats, adrenalectomy caused a significant reduction in the specific concentrations of both amylase and peroxidase, but did not affect the amylase to peroxidase ratios. We conclude that parasympathetic nerve stimulation of rat parotid glands after overnight starvation causes secretion of proteins in proportions similar to, but in significantly lower concentrations than those found in sympathetic saliva. Circulating catecholamines, however, influence the amount of amylase and peroxidase secreted by the rat parotid gland in response to parasympathetic nerve stimulation and account for most of the increased secretion of these enzymes following chronic sympathectomy.
Full text
PDF






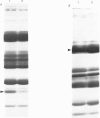
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Dawes C. The effects of electrical and pharmacological stimulation on the types of proteins secreted by rat parotid and submandibular glands. Arch Oral Biol. 1978;23(5):367–372. doi: 10.1016/0003-9969(78)90094-8. [DOI] [PubMed] [Google Scholar]
- Alm P., Ekström J. On the adrenergic innervation of the rat parotid gland. Experientia. 1977 Apr 15;33(4):523–524. doi: 10.1007/BF01922251. [DOI] [PubMed] [Google Scholar]
- Asking B., Delfs U., Emmelin N., Gjörstrup P. Amylase secretion from rat parotid glands as dependent on co-operation between sympathetic and parasympathetic nerves. Experientia. 1979 Oct 15;35(10):1336–1337. doi: 10.1007/BF01963993. [DOI] [PubMed] [Google Scholar]
- Asking B., Gjörstrup P. Amylase secretion in response to activation of different autonomic receptors in the rabbit parotid gland. Acta Physiol Scand. 1980 Aug;109(4):407–413. doi: 10.1111/j.1748-1716.1980.tb06613.x. [DOI] [PubMed] [Google Scholar]
- BURGEN A. S. TECHNIQUES FOR STIMULATING THE AURICULO-TEMPORAL NERVE AND RECORDING THE FLOW OF SALIVA. Int Ser Monogr Oral Biol. 1964;3:303–307. doi: 10.1016/b978-1-4832-2871-6.50026-6. [DOI] [PubMed] [Google Scholar]
- Brown C. L., Hanley M. R. The effects of substance P and related peptides on alpha-amylase release from rat parotid gland slices. Br J Pharmacol. 1981 Jun;73(2):517–523. doi: 10.1111/j.1476-5381.1981.tb10451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra T., Das R., Datta A. G. Role of thyroid gland on the peroxidase and iodinating enzymes of submaxillary gland. Eur J Biochem. 1977 Jan;72(2):259–263. doi: 10.1111/j.1432-1033.1977.tb11248.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Sensitization of the rat parotid gland to secretagogues following either parasympathetic denervation or sympathetic denervation or decentralization. Acta Physiol Scand. 1980 Mar;108(3):253–261. doi: 10.1111/j.1748-1716.1980.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Wahlestedt C. Supersensitivity to substance P and physalaemin in rat salivary glands after denervation or decentralization. Acta Physiol Scand. 1982 Aug;115(4):437–446. doi: 10.1111/j.1748-1716.1982.tb07102.x. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V. Substance P is a functional neurotransmitter in the rat parotid gland. J Physiol. 1983 Sep;342:483–498. doi: 10.1113/jphysiol.1983.sp014864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. R., Thulin A. Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res. 1975 Jun 9;159(2):179–193. doi: 10.1007/BF00219154. [DOI] [PubMed] [Google Scholar]
- Garrett J. R., Thulin A. Structural changes associated with parotid "degeneration secretion" after post-ganglionic sympathectomy in rats. Cell Tissue Res. 1975 Sep 16;162(1):1–12. doi: 10.1007/BF00223257. [DOI] [PubMed] [Google Scholar]
- Gjörstrup P. Amylase secretion in the rabbit parotid gland when stimulating the sympathetic nerves during parasympathetic activity. J Physiol. 1979 Nov;296:443–451. doi: 10.1113/jphysiol.1979.sp013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjörstrup P. Parotid secretion of fluid and amylase in rabbits during feeding. J Physiol. 1980 Dec;309:101–116. doi: 10.1113/jphysiol.1980.sp013497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop T. J., Garrett J. R. Effects of preganglionic sympathectomy on secretory changes in parotid acinar cells of rats in eating. Cell Tissue Res. 1974;154(2):135–150. doi: 10.1007/BF00223160. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Roberge M., Beaudoin A. R. Newly synthesized secretory proteins from pig pancreas are not released from a homogeneous granule compartment. Biochim Biophys Acta. 1982 Jun 16;716(3):331–336. doi: 10.1016/0304-4165(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Function of rat parotid gland after sympathectomy and total postganglionectomy. Am J Physiol. 1966 Oct;211(4):943–949. doi: 10.1152/ajplegacy.1966.211.4.943. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Sucanthapree C., Schneyer L. H., Jirakulsomchok D. Total salivary calcium and amylase output of rat parotid with electrical stimulation of autonomic innervation. Proc Soc Exp Biol Med. 1978 Dec;159(3):478–483. doi: 10.3181/00379727-159-40374. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Sucanthapree C., Schneyer L. H. Neural regulation of calcium and amylase of rat parotid saliva (39891). Proc Soc Exp Biol Med. 1977 Oct;156(1):132–135. doi: 10.3181/00379727-156-39891. [DOI] [PubMed] [Google Scholar]
- Speirs R. L., Herring J., Cooper W. D., Hardy C. C., Hind C. R. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch Oral Biol. 1974 Sep;19(9):747–752. doi: 10.1016/0003-9969(74)90161-7. [DOI] [PubMed] [Google Scholar]
- Sreebny L. M., Johnson D. A., Robinovitch M. R. Functional regulation of protein synthesis in the rat parotid gland. J Biol Chem. 1971 Jun 25;246(12):3879–3884. [PubMed] [Google Scholar]
- Thulin A., Garrett J. R. Secretory and structural effects of 6-hydroxy-dopamine on normal parotid glands of rats, and at different times after surgical sympathectomy. Q J Exp Physiol Cogn Med Sci. 1976 Jan;61(1):15–21. doi: 10.1113/expphysiol.1976.sp002330. [DOI] [PubMed] [Google Scholar]
- Torre J. C., Surgeon J. W. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method. Histochemistry. 1976 Oct 22;49(2):81–93. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia. 1968 Feb 15;24(2):155–156. doi: 10.1007/BF02146956. [DOI] [PubMed] [Google Scholar]