Abstract
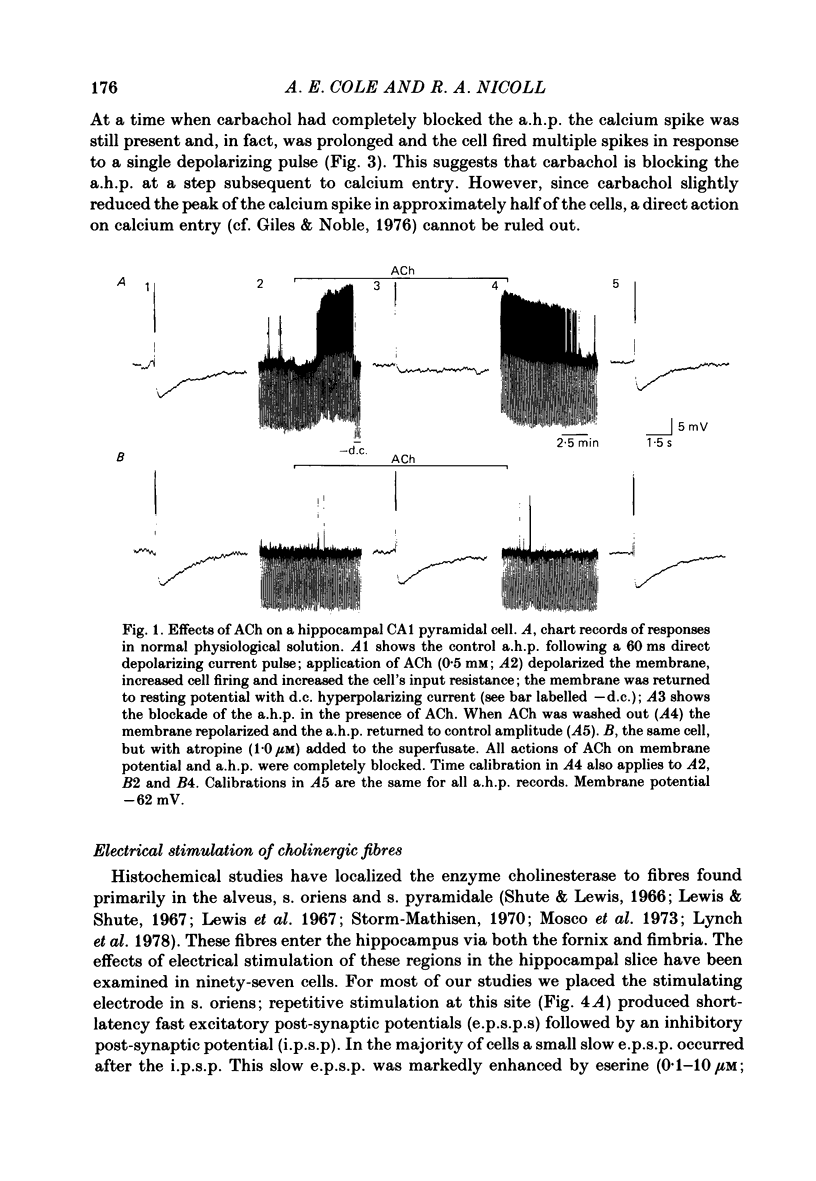
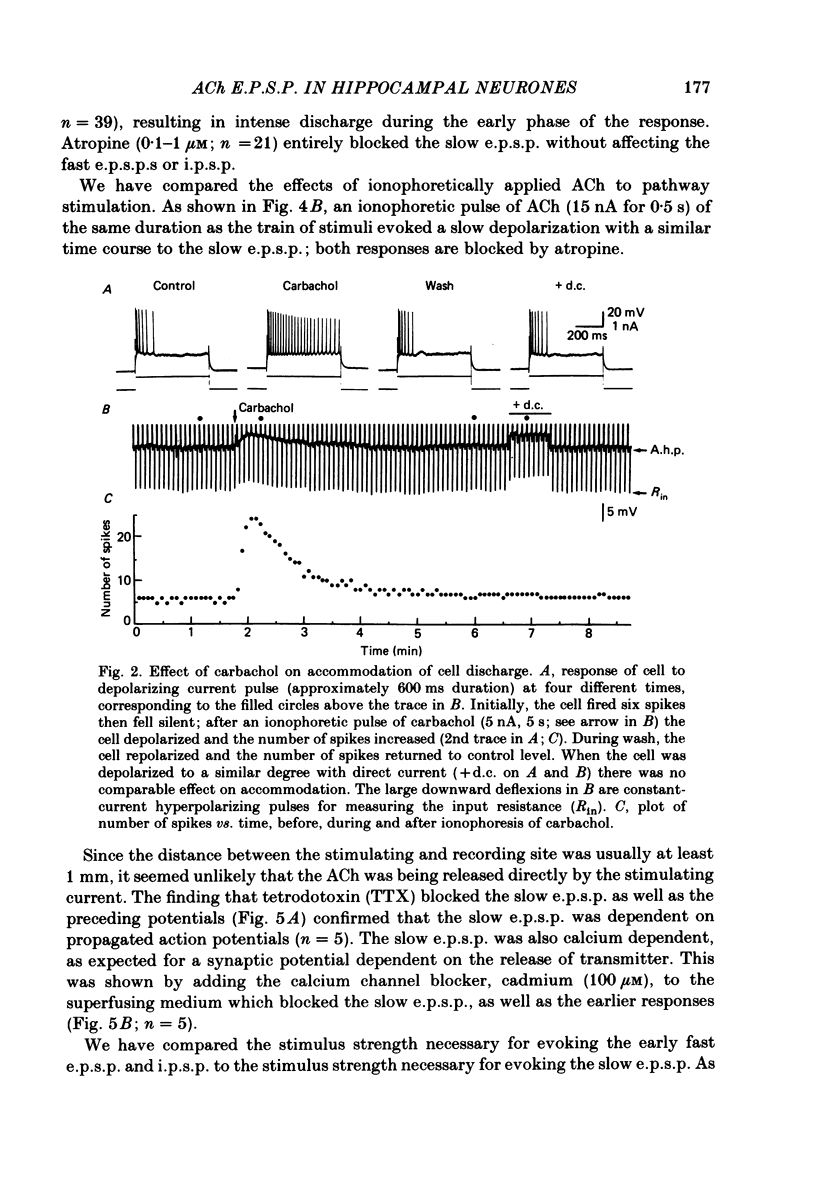
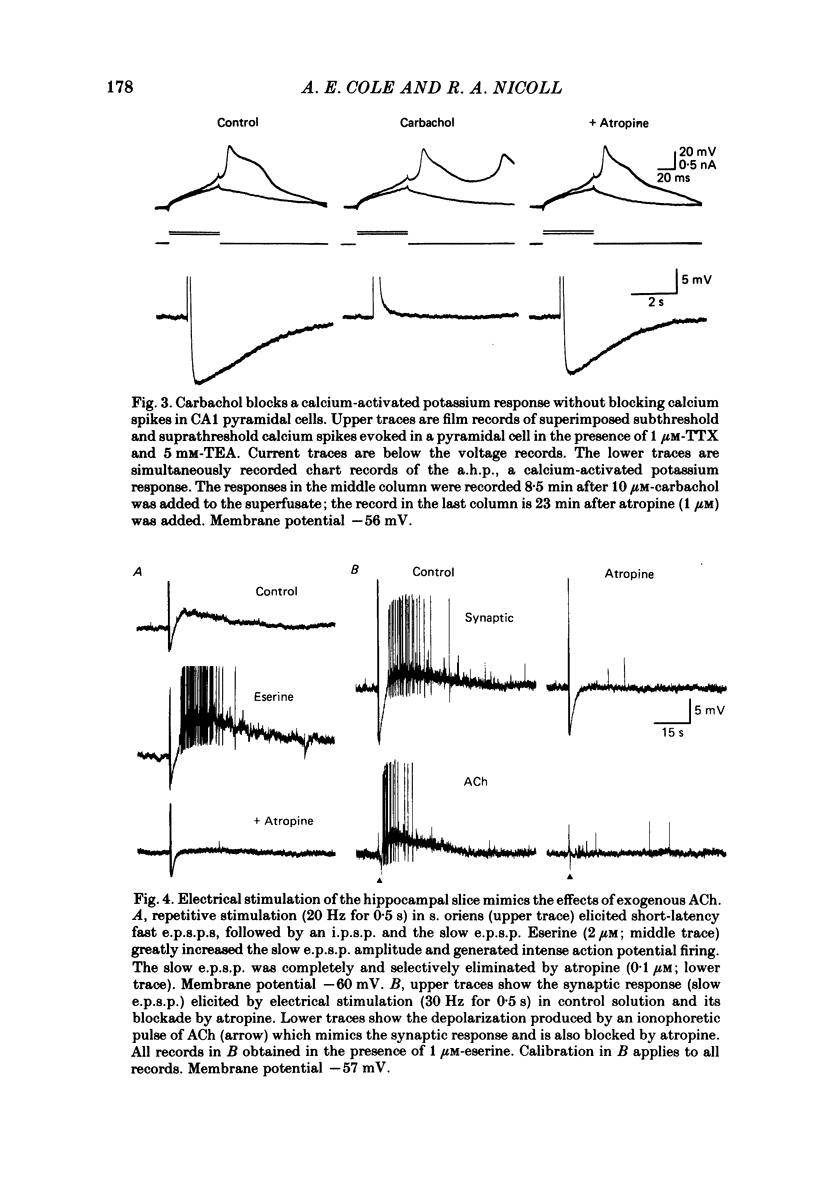
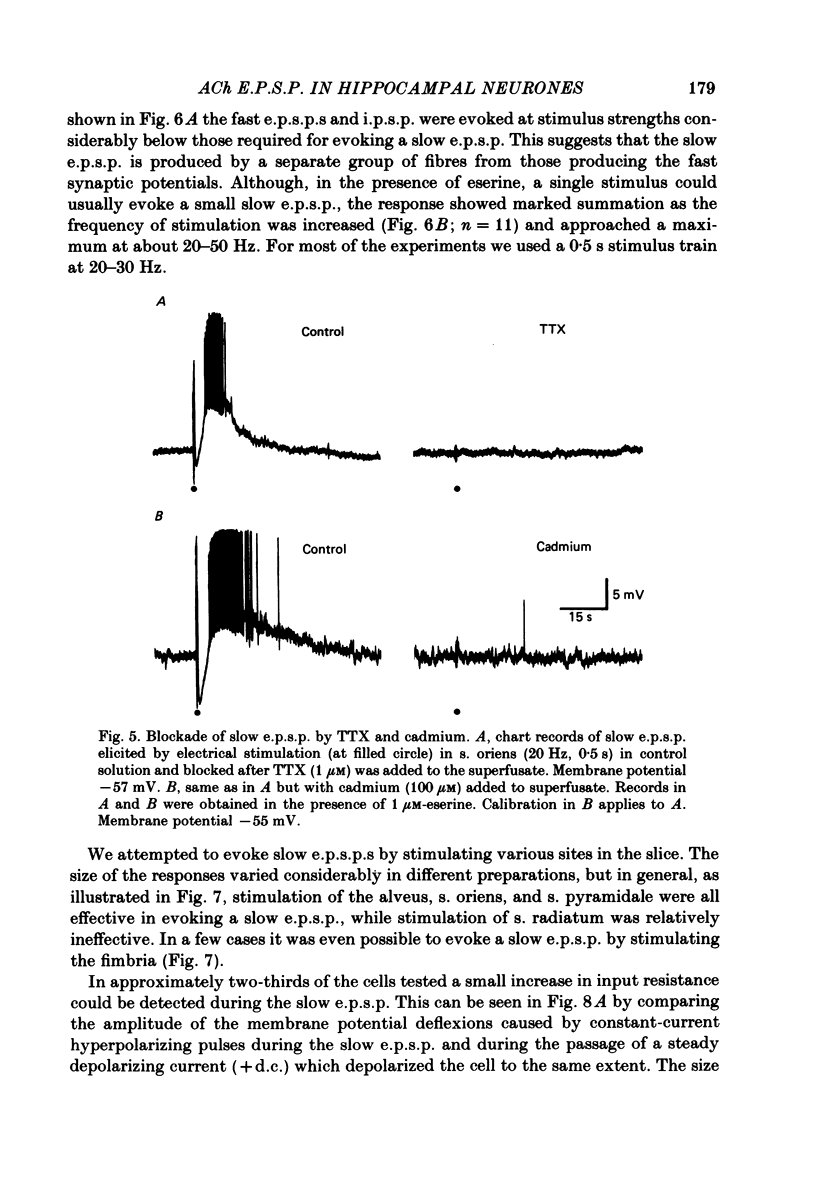
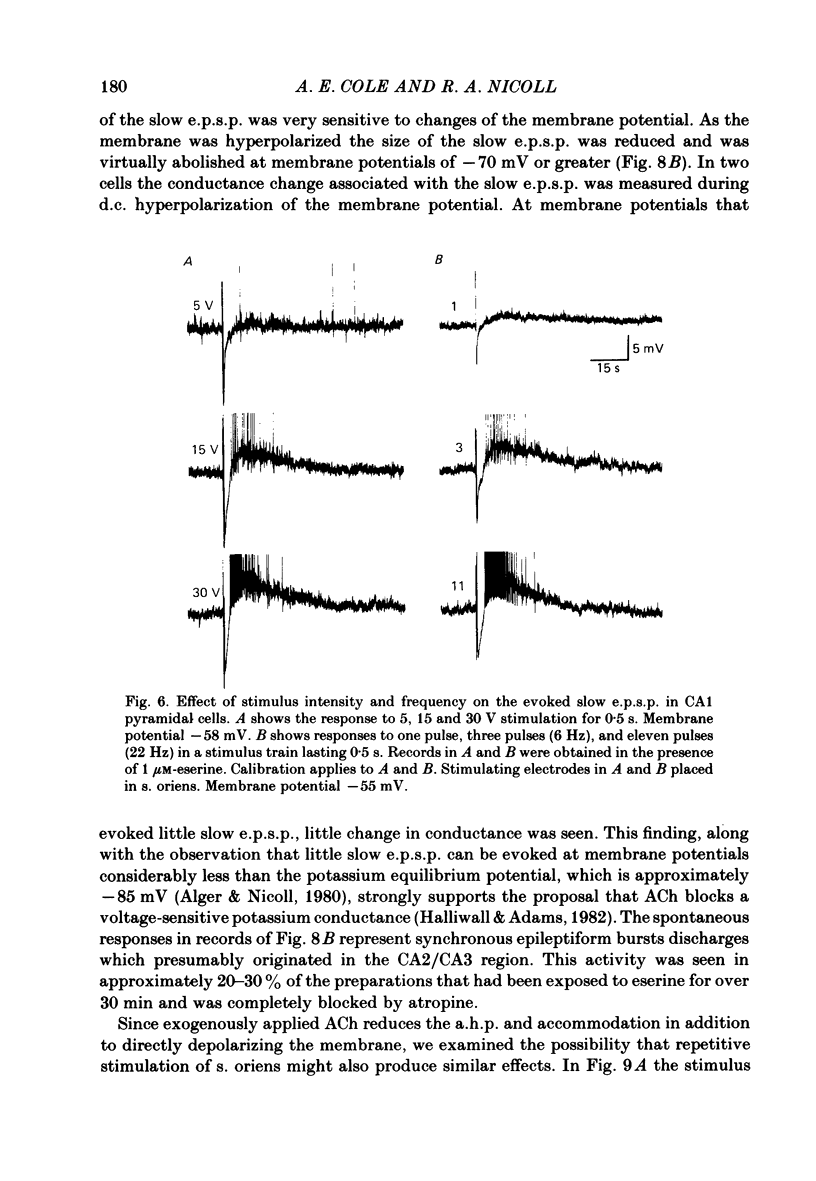
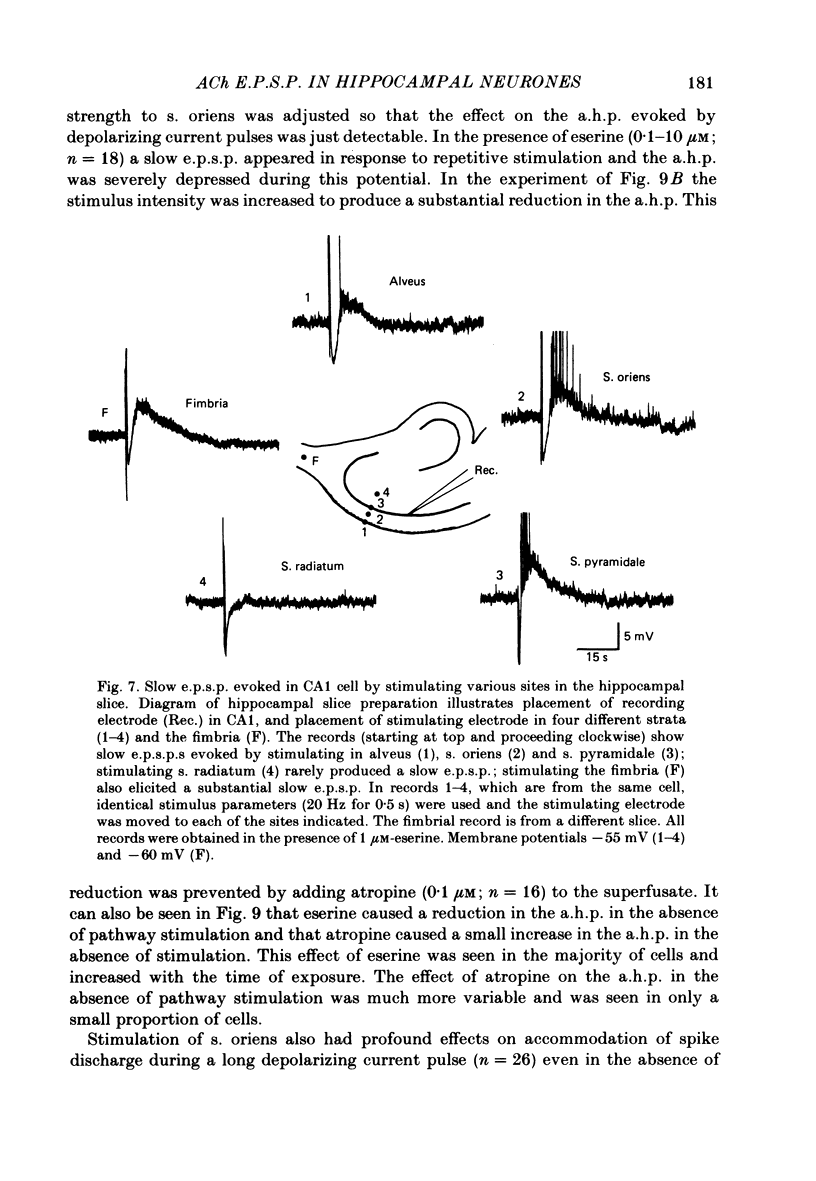
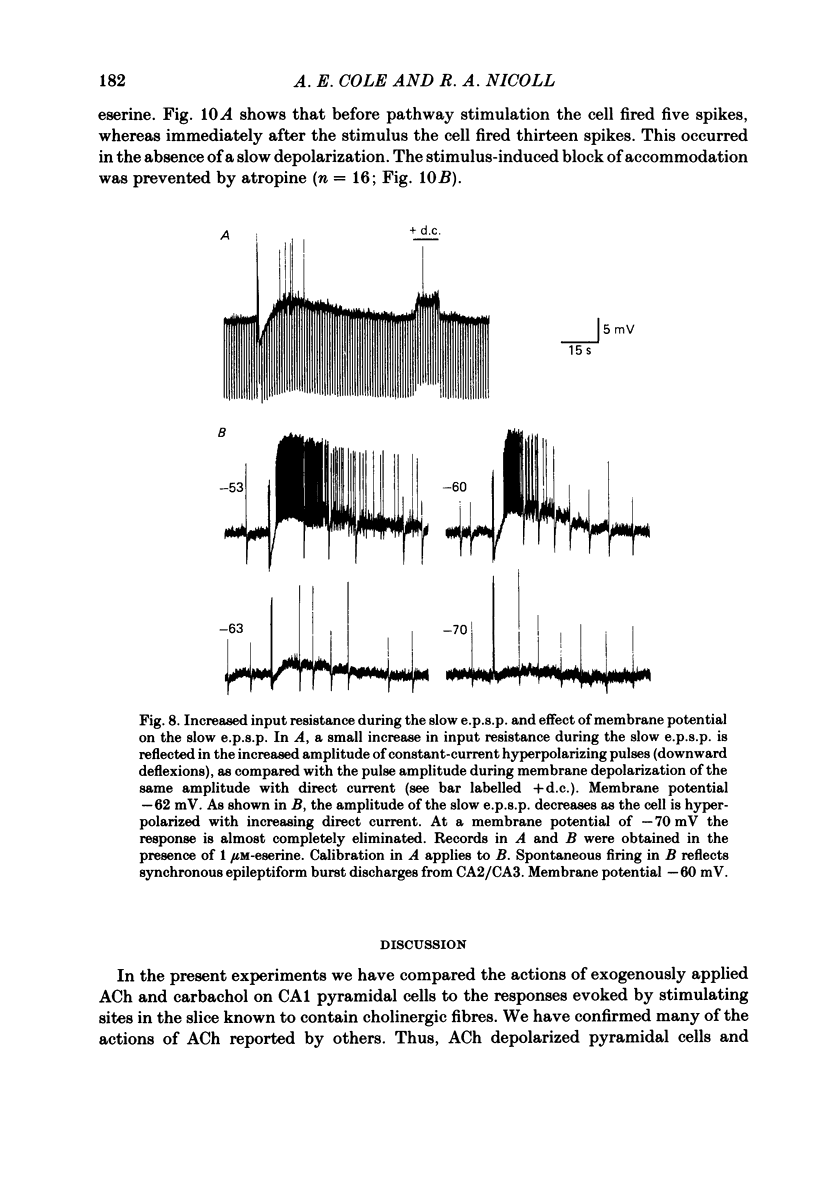
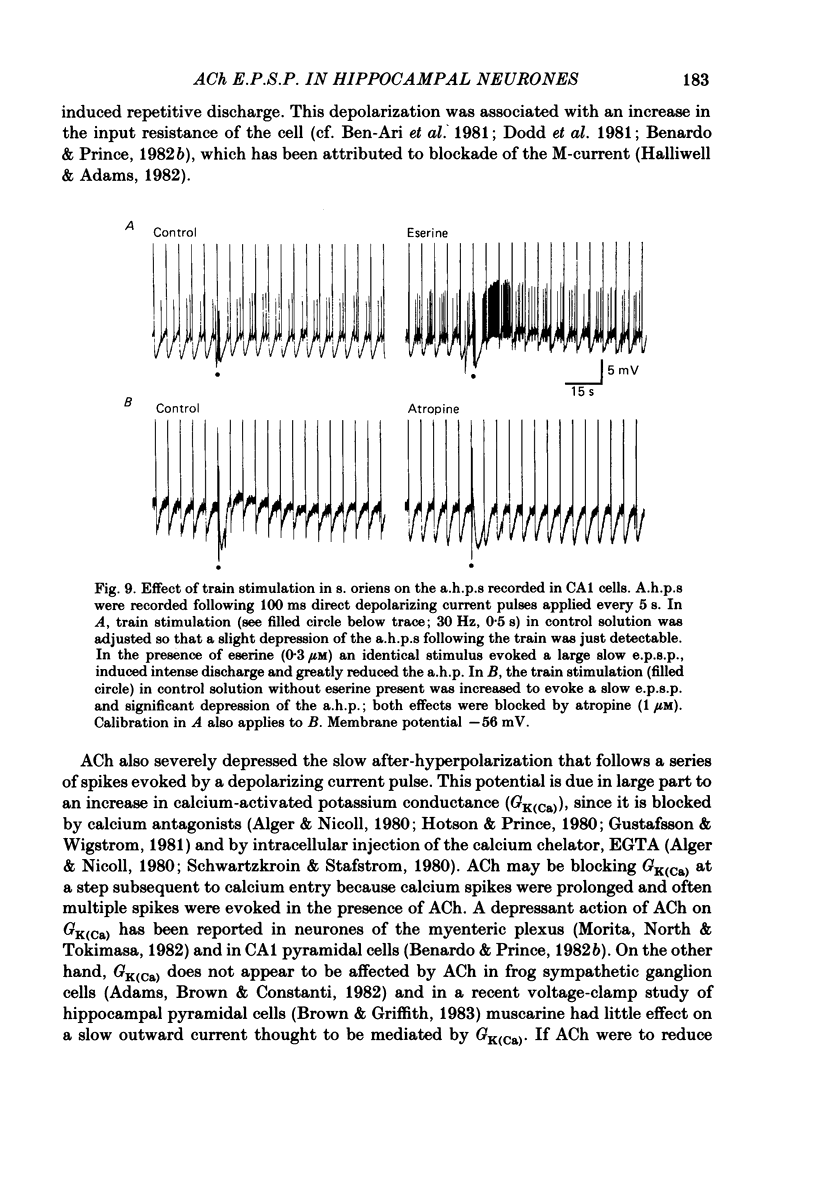
Intracellular recording from CA1 pyramidal cells in the hippocampal slice preparation was used to compare the action of exogenously applied acetylcholine (ACh) and cholinomimetics to the effect of electrically stimulating sites in the slice known to contain cholinergic fibres. ACh depolarized pyramidal cells with an associated increase in input resistance, blocked a calcium-activated potassium conductance (GK(Ca], and blocked accommodation of action potential discharge. All of these actions were blocked by the muscarinic antagonist, atropine. Repetitive electrical stimulation of stratum (s.) oriens evoked a series of fast excitatory post-synaptic potentials (e.p.s.p.s) followed by an inhibitory post-synaptic potential. These potentials were followed by a slow e.p.s.p. that lasted 20-30 s. The slow e.p.s.p. was selectively enhanced by eserine and blocked by atropine. Ionophoretic application of ACh closely mimicked the time course of the slow e.p.s.p. The slow e.p.s.p. was blocked by tetrodotoxin and cadmium, indicating that it was dependent on propagated action potentials and on calcium. Considerably higher stimulus strengths were needed to elicit a slow e.p.s.p. than to elicit the earlier synaptic potentials. The size of the slow e.p.s.p. was markedly increased by repetitive stimulation. Stimulation of the alveus, s. oriens, s. pyramidale and fimbria all evoked a slow e.p.s.p., while stimulation of s. radiatum was relatively ineffective. The input resistance of the cell increased during the slow e.p.s.p. Hyperpolarizing the cell decreased the size of the slow e.p.s.p. and at membrane potentials of -70 mV or greater, little response was recorded. Stimulation of s. oriens blocked GK(Ca) and accommodation of action potential discharge. These effects, which could be seen in the absence of any change in membrane potential, were enhanced by eserine and blocked by atropine. The present electrophysiological results establish that CA1 pyramidal cells receive a cholinergic input and demonstrate that this input can dramatically alter the firing properties of these neurones for tens of seconds in the absence of any marked effect on membrane potential. Such an action contrasts with previously characterized synaptic potentials in this region of the brain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol. 1982 Nov;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Krnjević K., Reiffenstein R. J., Reinhardt W. Inhibitory conductance changes and action of gamma-aminobutyrate in rat hippocampus. Neuroscience. 1981;6(12):2445–2463. doi: 10.1016/0306-4522(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic pharmacology of mammalian hippocampal pyramidal cells. Neuroscience. 1982 Jul;7(7):1703–1712. doi: 10.1016/0306-4522(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A. Physiological role of a slow, voltage-sensitive, synaptic response mediated by an identified serotonin-containing neurone. Q J Exp Physiol. 1982 Jan;67(1):179–183. doi: 10.1113/expphysiol.1982.sp002612. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Madison R., Davis J. N. A study of the rat septohippocampal pathway using anterograde transport of horseradish peroxidase. Neuroscience. 1981;6(10):1961–1973. doi: 10.1016/0306-4522(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Dodd J., Dingledine R., Kelly J. S. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981 Feb 23;207(1):109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Topographical and subcellular localization of choline acetyltransferase in rat hippocampal region. J Neurochem. 1970 Jul;17(7):1029–1037. doi: 10.1111/j.1471-4159.1970.tb02256.x. [DOI] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res. 1981 Feb 16;206(2):462–468. doi: 10.1016/0006-8993(81)90548-5. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Crawford G. D., Barber R. P., Salvaterra P. M., Vaughn J. E. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983 Apr 25;266(1):97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., Morita K., North R. A. Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. J Physiol. 1981 Nov;320:175–186. doi: 10.1113/jphysiol.1981.sp013942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., McGeer P. L., Peng J. H., McGeer E. G. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol. 1981 Aug 1;200(2):151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Ropert N. Electrophysiological and pharmacological characteristics of facilitation of hippocampal population spikes by stimulation of the medial septum. Neuroscience. 1982;7(9):2165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- MCLARDY T. Observations on the fornix of the monkey. I. Fiber studies. J Comp Neurol. 1955 Oct;103(2):327–343. doi: 10.1002/cne.901030207. [DOI] [PubMed] [Google Scholar]
- MORIN F. An experimental study of hypothalamic connections in the guinea pig. J Comp Neurol. 1950 Apr;92(2):193–213. doi: 10.1002/cne.900920206. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Meibach R. C., Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977 Jan 1;119(1):1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. Muscarinic agonists inactivate potassium conductance of guinea-pig myenteric neurones. J Physiol. 1982 Dec;333:125–139. doi: 10.1113/jphysiol.1982.sp014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosko S., Lynch G., Cotman C. W. The distribution of septal projections to the hippocampus of the rat. J Comp Neurol. 1973 Nov 15;152(2):163–174. doi: 10.1002/cne.901520204. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Muscarinic synaptic potentials in guinea-pig myenteric plexus neurones. J Physiol. 1982 Dec;333:151–156. doi: 10.1113/jphysiol.1982.sp014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain. 1966 Jun;89(2):317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- Rose A. M., Hattori T., Fibiger H. C. Analysis of the septo-hippocampal pathway by light and electron microscopic autoradiography. Brain Res. 1976 May 21;108(1):170–174. doi: 10.1016/0006-8993(76)90173-6. [DOI] [PubMed] [Google Scholar]
- Sastry B. R. Excitatory postsynaptic potentials in the mammalian central nervous system associated with an increase in the membrane resistance. Life Sci. 1980 Oct 13;27(15):1403–1407. doi: 10.1016/0024-3205(80)90404-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. Electron microscopy of cholinergic terminals and acetylcholinesterase-containing neurones in the hippocampal formation of the rat. Z Zellforsch Mikrosk Anat. 1966;69:334–343. doi: 10.1007/BF00406286. [DOI] [PubMed] [Google Scholar]
- Smith C. M. Acetylcholine release from the cholinergic septo-hippocampal pathway. Life Sci. 1974 Jun 1;14(11):2159–2166. doi: 10.1016/0024-3205(74)90098-8. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Quantitative histochemistry of acetylcholinesterase in rat hippocampal region correlated to histochemical staining. J Neurochem. 1970 Jun;17(6):739–750. doi: 10.1111/j.1471-4159.1970.tb03344.x. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Konishi S., Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7(9):2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Turner D. A., Schwartzkroin P. A. Steady-state electrotonic analysis of intracellularly stained hippocampal neurons. J Neurophysiol. 1980 Jul;44(1):184–199. doi: 10.1152/jn.1980.44.1.184. [DOI] [PubMed] [Google Scholar]
- VanderMaelen C. P., Aghajanian G. K. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980 Sep 25;287(5780):346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]




