Abstract
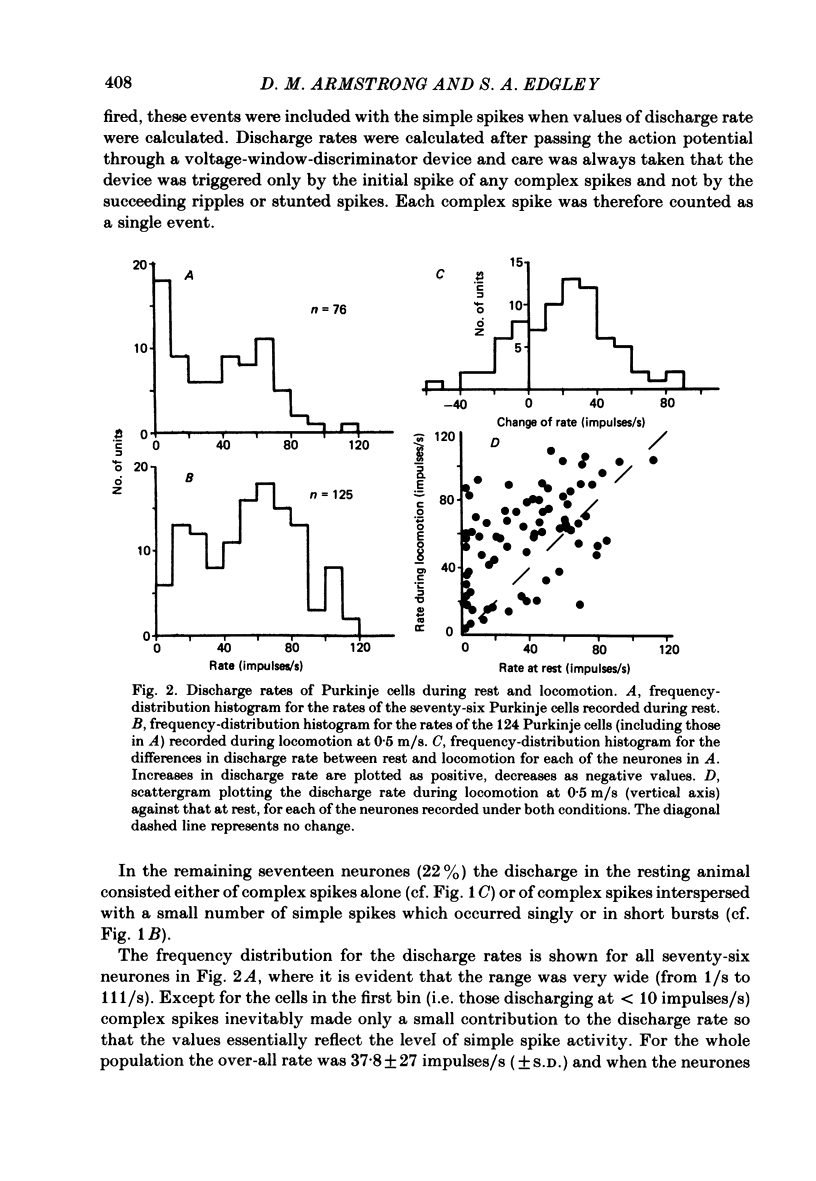
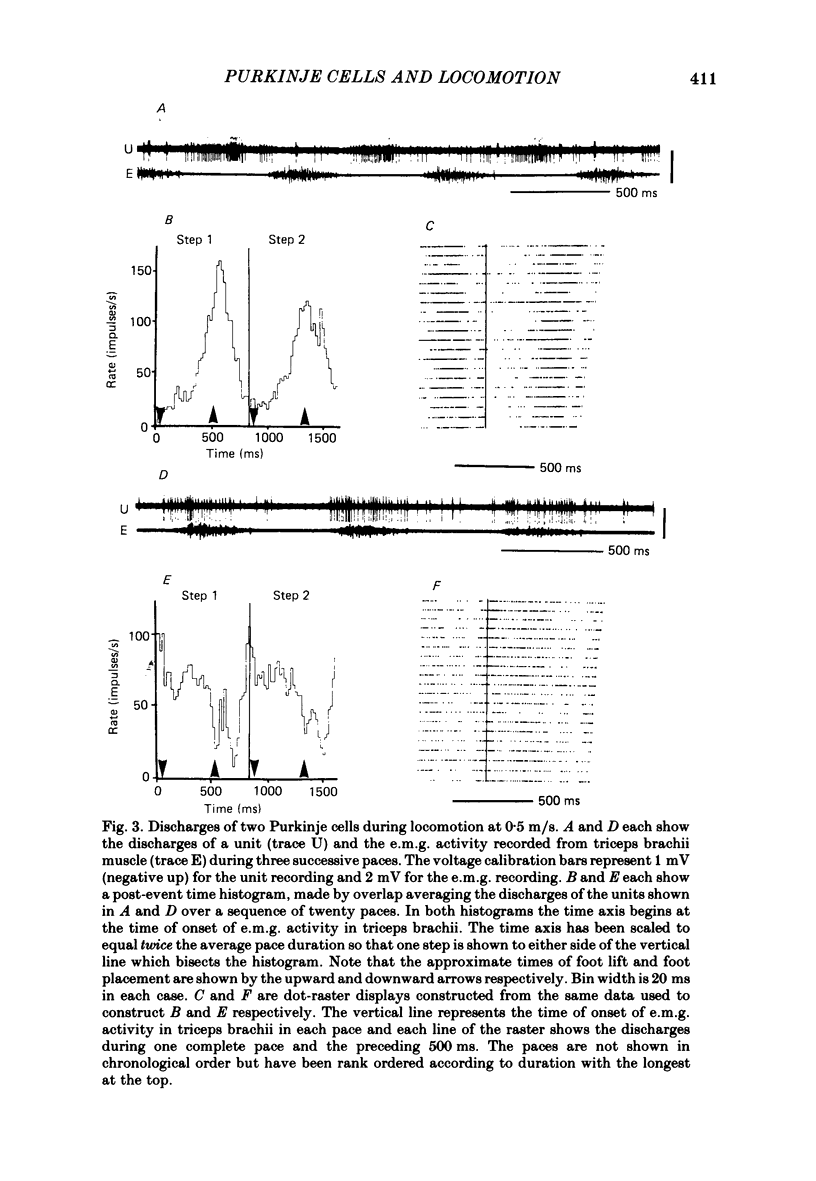
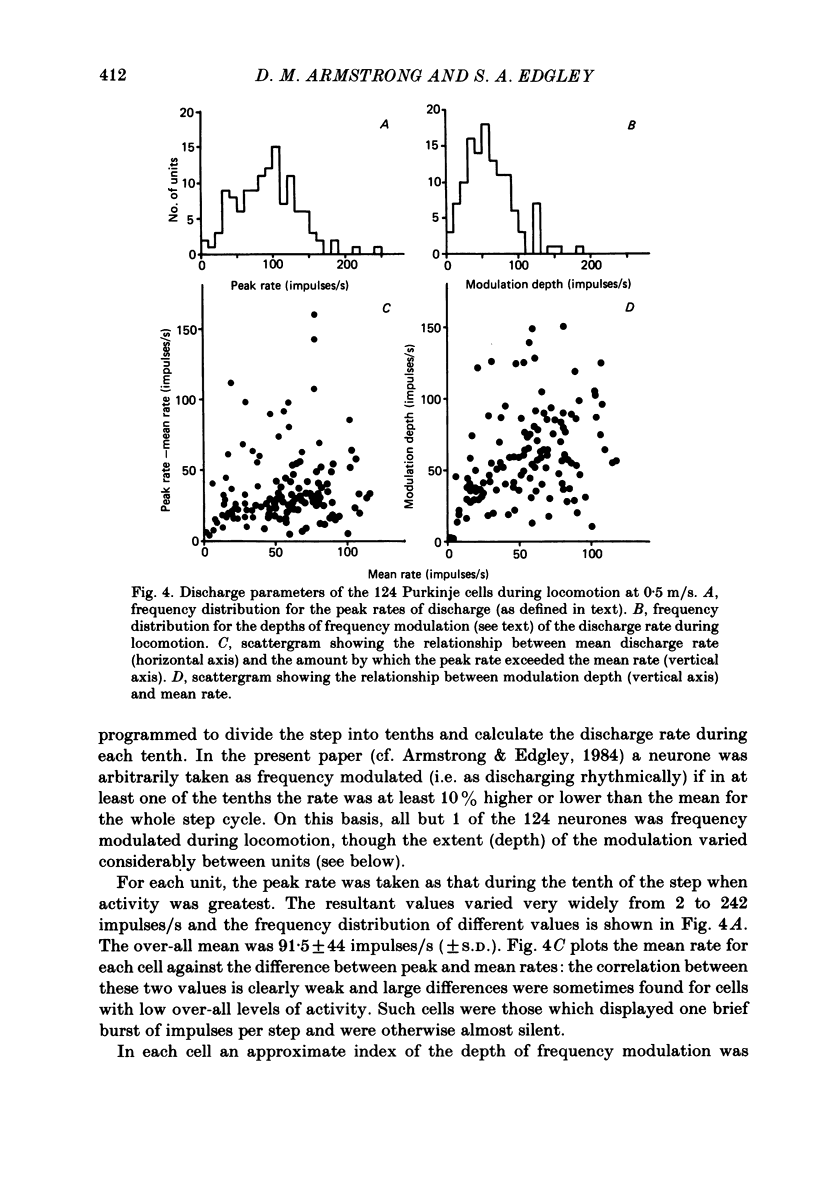
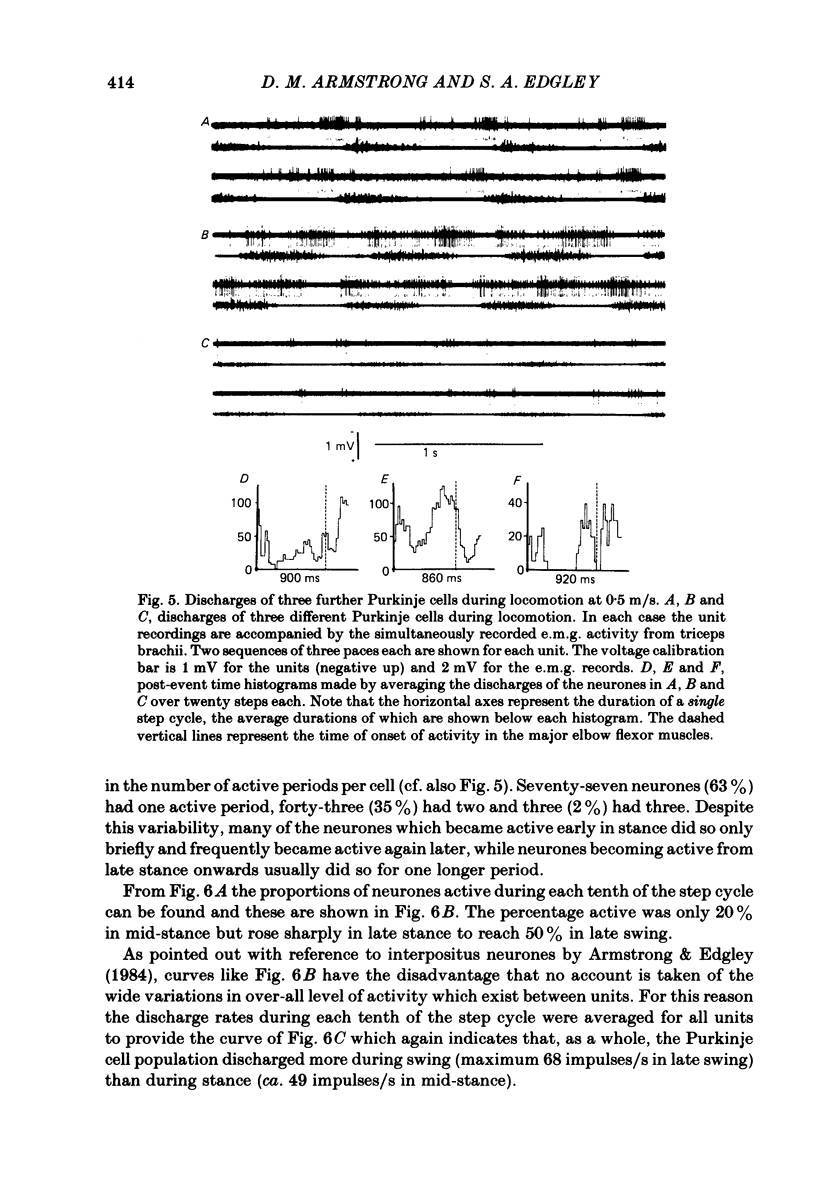
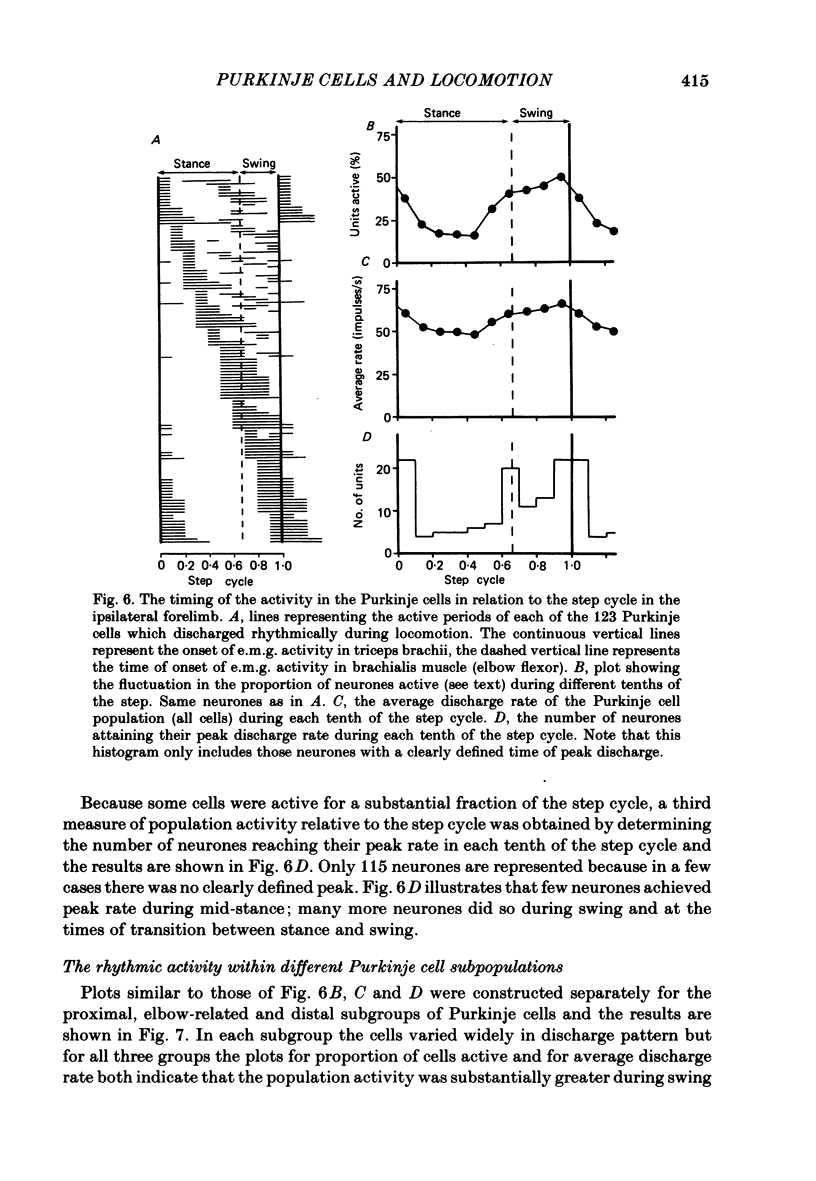
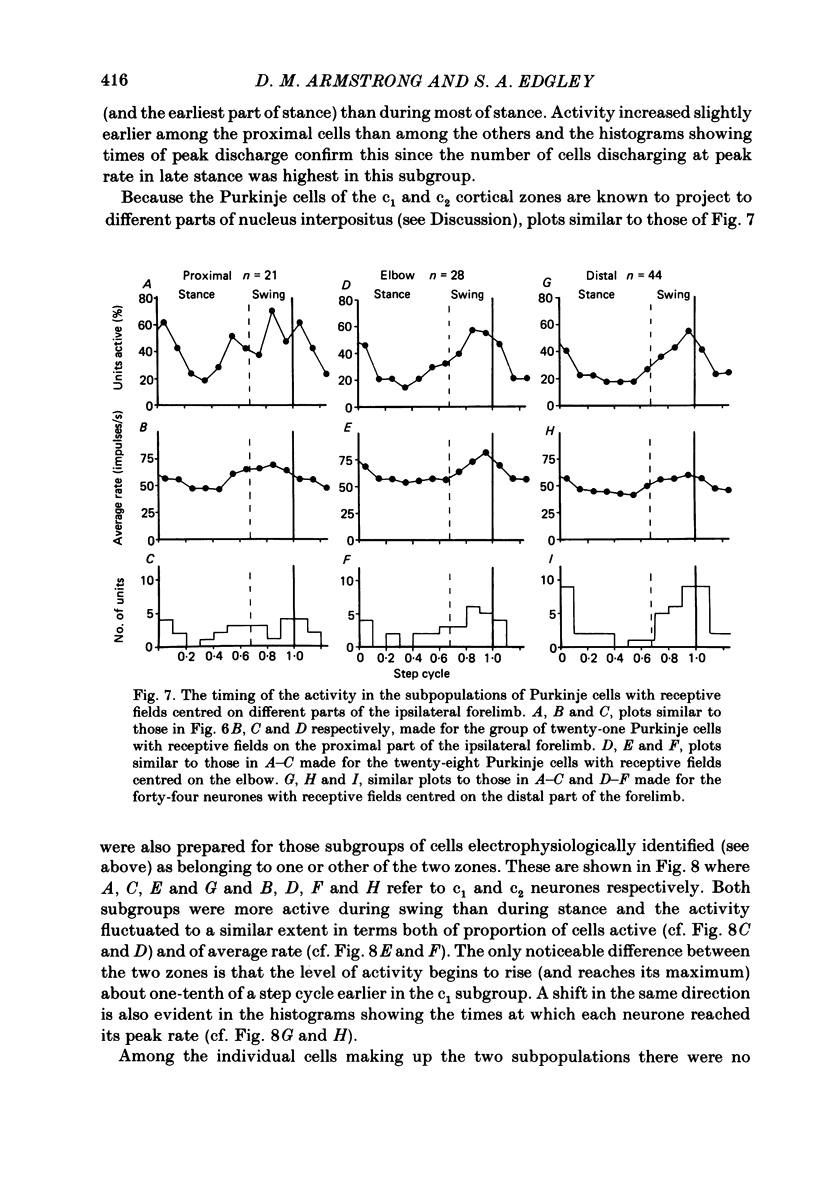
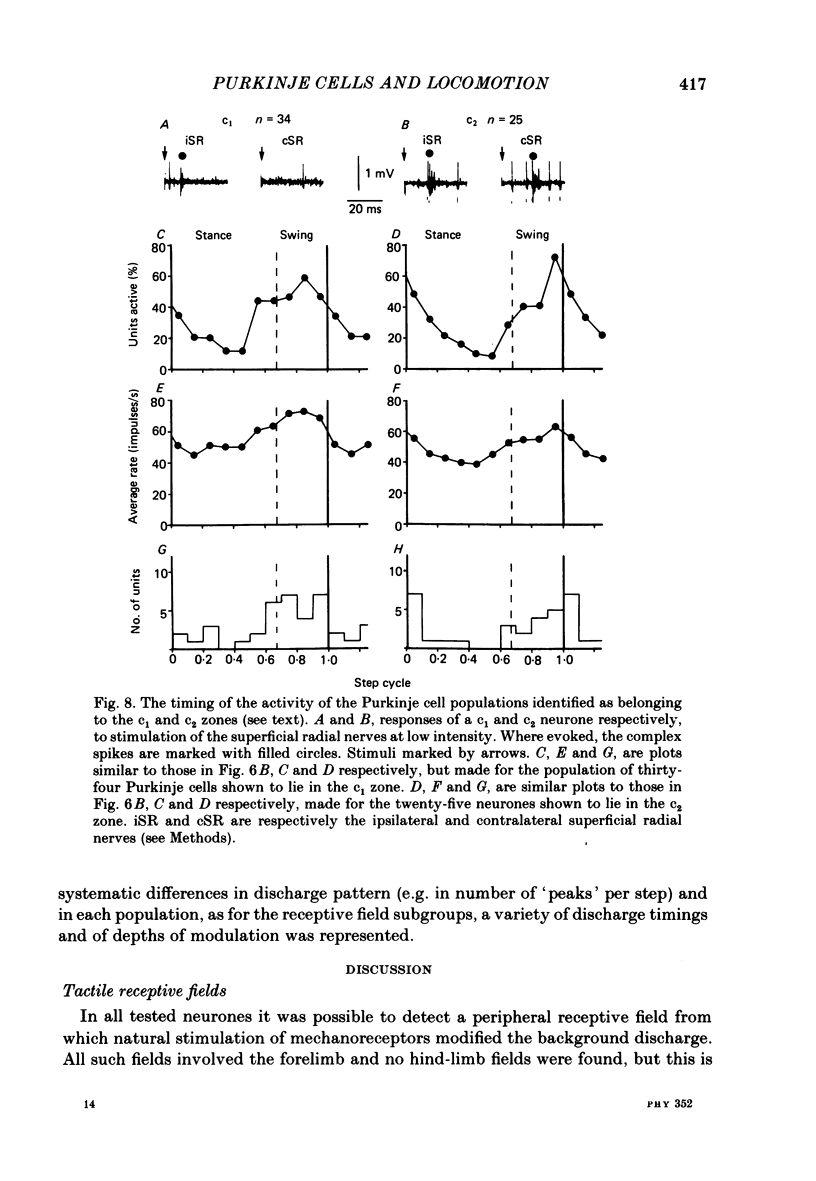
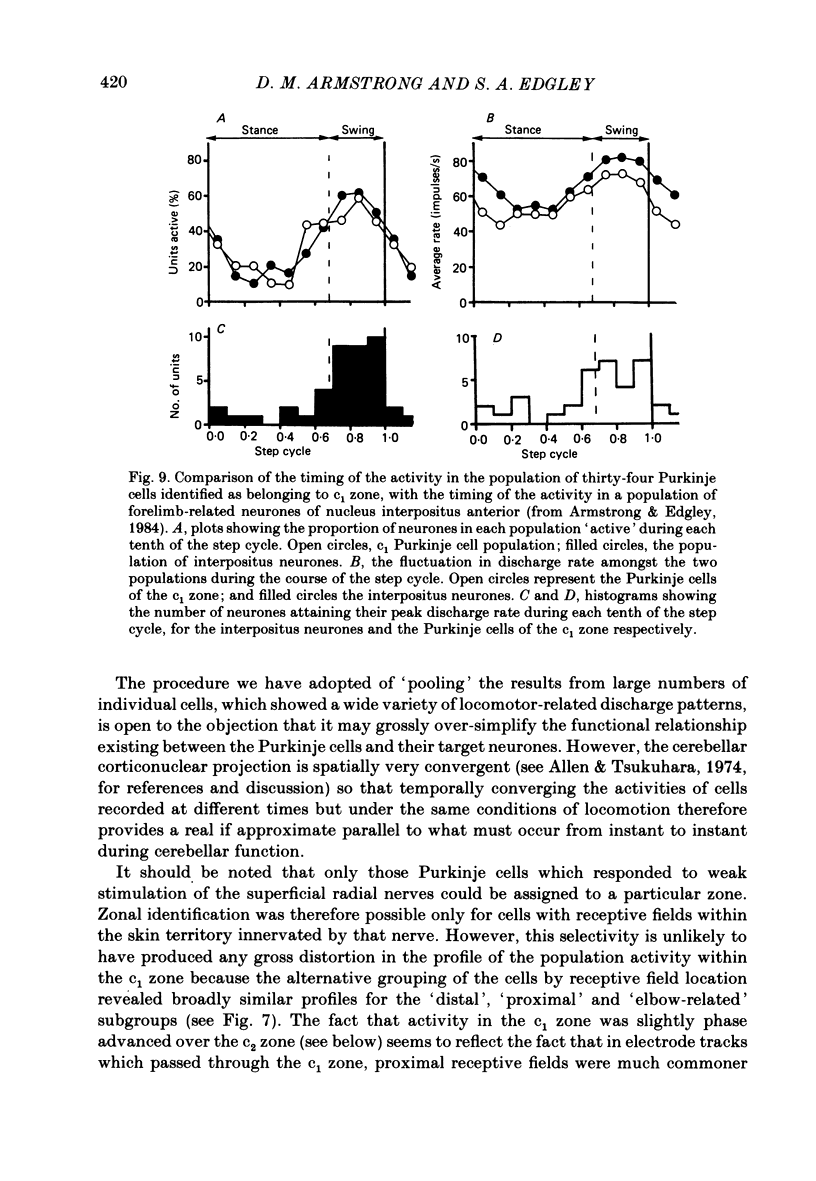
Extracellular recordings were made from 124 Purkinje cells in the paravermal part of lobule V of the cerebellum in cats walking steadily at a speed of 0.5 m/s on a moving belt. All cells tested had a tactile receptive field from which simple spikes could be evoked and 96% of these were on the ipsilateral forelimb. Seventy-six of the cells were also studied whilst the animals sat or lay quietly without movement. Complex spikes were discharged at 1-2/s and these were accompanied by simple spikes in fifty-nine cells (78%); in the remaining cells there were no or few simple spikes. The over-all mean discharge rate (including both types of spike) was 37.8 +/- 27 impulses/s (+/- S.D.). During locomotion all cells discharged both types of spike and the over-all mean rate was 57.6 +/- 29 impulses/s (+/- S.D.). In all cells but one, the frequency of the simple spikes was modulated rhythmically in time with the stepping movements but the phasing relative to the step cycle varied widely between cells. Peak rates also varied widely, the average being 91.5 +/- 44 impulses/s (+/- S.D.). Most cells (63%) generated one period of accelerated discharge per step but others generated two (35%) or three (2%) such periods. Despite the individual variations in discharge timing the population as a whole was considerably more active during the swing than the stance phase of the step cycle in the ipsilateral forelimb (68 impulses/s as compared with 49 impulses/s on average). Thirty-four cells were electrophysiologically identified as lying in the c1 zone of the cortex and twenty-five as being in the c2 zone (nomenclature of Oscarsson, 1980). During locomotion, the population activity in the two zones differed slightly: activity in the c1 population was phase advanced by approximately one-tenth of the step cycle. The results are discussed, with particular emphasis on the finding that population activity in the Purkinje cells of the c1 zone fluctuated during the step cycle in parallel with that in the part of nucleus interpositus to which they project.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984 Jun;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Dietrichs E. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of horseradish peroxidase. III. The anterior lobe. Anat Embryol (Berl) 1981;162(2):223–247. doi: 10.1007/BF00306494. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. The cerebellum as a computer: patterns in space and time. J Physiol. 1973 Feb;229(1):1–32. doi: 10.1113/jphysiol.1973.sp010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Haines D. E., Rubertone J. A. Cerebellar corticonuclear fibers of the dorsal culminate lobule (anterior lobe--lobule V) in a prosimian primate, Galago senegalensis. J Comp Neurol. 1979 Aug 1;186(3):321–341. doi: 10.1002/cne.901860303. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J. A., McCarley R. W. Spontaneous discharge rates of cat cerebellar Purkinje cells in sleep and waking. Electroencephalogr Clin Neurophysiol. 1972 Nov;33(5):457–469. doi: 10.1016/0013-4694(72)90210-6. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. J Physiol. 1969 Aug;203(3):641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano N. Changes of simple and complex spike activity of cerebellar purkinje cells with sleep and waking. Science. 1970 Dec 18;170(3964):1325–1327. doi: 10.1126/science.170.3964.1325. [DOI] [PubMed] [Google Scholar]
- Mortimer J. A. Temporal sequence of cerebellar Purkinje and nuclear activity in relation to the acoustic startle response. Brain Res. 1973 Feb 28;50(2):457–462. doi: 10.1016/0006-8993(73)90751-8. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Udo M., Kamei H., Matsukawa K., Tanaka K. Interlimb coordination in cat locomotion investigated with perturbation. II. Correlates in neuronal activity of Deiter's cells of decerebrate walking cats. Exp Brain Res. 1982;46(3):438–447. doi: 10.1007/BF00238638. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Minoda K., Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res. 1981;41(3-4):292–300. doi: 10.1007/BF00238886. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980 Jul;44(1):119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]
- Udo M., Oda Y., Tanaka K., Horikawa J. Cerebellar control of locomotion investigated in cats: discharges from Deiters' neurones, EMG and limb movements during local cooling of the cerebellar cortex. Prog Brain Res. 1976;44:445–459. doi: 10.1016/S0079-6123(08)60751-7. [DOI] [PubMed] [Google Scholar]


