Abstract
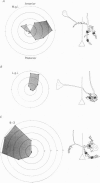
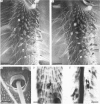
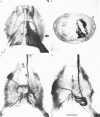
The structural relationship between the afferent projection and the dendrites of the interneurones was examined in the cercal-to-giant interneurone system of the cricket using intracellular recording and dye injection techniques. The physiology of the sensory neurones beneath the cercal filiform hairs was investigated by placing a recording pipette over the end of a cut hair and using movements of the pipette to characterize the directionality of the receptor. Most of the filiform receptors could be classified as belonging to one of four major types. Each type is sensitive to a different wind direction and is confined to particular regions of the cercus. The location of the terminal arborizations of each type of sensory cell was revealed by staining with cobalt chloride. Single cells were stained reliably by placing a dye-filled pipette over a cut hair. Each physiological receptor type arborizes in a different region of the central nervous system. Therefore the neuropile is functionally divided according to wind direction. The dendrites of three identified interneurones were examined in the context of this afferent projection. It was found that each of these neurones has dendrites in regions of neuropile corresponding to different wind directions. By searching for unitary synaptic potentials in identified interneurones, it was possible to show a strong correlation between anatomical overlap of primary afferent and interneurone and the existence of a synaptic connexion. Further, when there was no overlap, no synaptic potentials were seen. Therefore the over-all excitatory receptive field of an interneurone could be predicted by examining its dendritic structure. Each of the three identified interneurones examined in this study was found to have a directional response that matched the response predicted on the basis of its anatomy.
Full text
PDF

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. P., Altman J. S. A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res. 1977 Dec 16;138(2):359–363. doi: 10.1016/0006-8993(77)90753-3. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. Dendritic trees and cutaneous receptive fields of adjacent spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:429–440. doi: 10.1113/jphysiol.1980.sp013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLONNIER M. THE TANGENTIAL ORGANIZATION OF THE VISUAL CORTEX. J Anat. 1964 Jul;98:327–344. [PMC free article] [PubMed] [Google Scholar]
- Callec J. J., Guillet J. C., Pichon Y., Boistel J. Further studies on synaptic transmission in insects. II. Relations between sensory information and its synaptic integration at the level of a single giant axon in the cockroach. J Exp Biol. 1971 Aug;55(1):123–149. doi: 10.1242/jeb.55.1.123. [DOI] [PubMed] [Google Scholar]
- Daley D. L. Neural basis of wind-receptive fields of cockroach giant interneurons. Brain Res. 1982 Apr 22;238(1):211–216. doi: 10.1016/0006-8993(82)90785-5. [DOI] [PubMed] [Google Scholar]
- Edwards J. S., Palka J. The cerci and abdominal giant fibres of the house cricket, Acheta domesticus. I. Anatomy and physiology of normal adults. Proc R Soc Lond B Biol Sci. 1974 Jan 22;185(1078):83–103. doi: 10.1098/rspb.1974.0007. [DOI] [PubMed] [Google Scholar]
- Gnatzy W., Schmidt K. Die Feinstruktur der Sinneshaare auf den Cerci von Gryllus bimaculatus Deg. (Saltatoria, Gryllidae). I. Faden- und Keulenhaare. Z Zellforsch Mikrosk Anat. 1971;122(2):190–209. [PubMed] [Google Scholar]
- Gnatzy W., Tautz J. Ultrastructure and mechanical properties of an insect mechanoreceptor: stimulus-transmitting structures and sensory apparatus of the cercal filiform hairs of Gryllus. Cell Tissue Res. 1980;213(3):441–463. doi: 10.1007/BF00237890. [DOI] [PubMed] [Google Scholar]
- Kirk M. D., Waldrop B., Glantz R. M. A quantitative correlation of contour sensitivity with dendritic density in an identified visual neuron. Brain Res. 1983 Sep 12;274(2):231–237. doi: 10.1016/0006-8993(83)90700-x. [DOI] [PubMed] [Google Scholar]
- Levine R. B., Truman J. W. Metamorphosis of the insect nervous system: changes in morphology and synaptic interactions of identified neurones. Nature. 1982 Sep 16;299(5880):250–252. doi: 10.1038/299250a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto S. G., Murphey R. K. Sensory deprivation during development decreases the responsiveness of cricket giant interneurones. J Physiol. 1977 Jun;268(2):533–548. doi: 10.1113/jphysiol.1977.sp011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall B., Murphey R. K. The morphology of cricket giant interneurons. J Neurobiol. 1974;5(6):565–580. doi: 10.1002/neu.480050607. [DOI] [PubMed] [Google Scholar]
- Murphey R. K., Bacon J. P., Sakaguchi D. S., Johnson S. E. Transplantation of cricket sensory neurons to ectopic locations: arborizations and synaptic connections. J Neurosci. 1983 Apr;3(4):659–672. doi: 10.1523/JNEUROSCI.03-04-00659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey R. K., Jacklet A., Schuster L. A topographic map of sensory cell terminal arborizations in the cricket CNS; correlation with birthday and position in a sensory array. J Comp Neurol. 1980 May 1;191(1):53–64. doi: 10.1002/cne.901910103. [DOI] [PubMed] [Google Scholar]
- Murphey R. K., Levine R. B. Mechanisms responsible for changes observed in response properties of partially deafferented insect interneurons. J Neurophysiol. 1980 Feb;43(2):367–382. doi: 10.1152/jn.1980.43.2.367. [DOI] [PubMed] [Google Scholar]
- Murphey R. K. The structure and development of a somatotopic map in crickets: the cercal afferent projection. Dev Biol. 1981 Dec;88(2):236–246. doi: 10.1016/0012-1606(81)90167-6. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Stein R. B., Malhotra S. K. Properties of action potentials from insect motor nerve fibres. J Exp Biol. 1970 Oct;53(2):299–316. doi: 10.1242/jeb.53.2.299. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Shankland M. Development of a sensory afferent projection in the grasshopper embryo. II. Growth and branching of peripheral sensory axons within the central nervous system. J Embryol Exp Morphol. 1981 Aug;64:187–209. [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Tieman S. B., Hirsch H. V. Exposure to lines of only one orientation modifies dendritic morphology of cells in the visual cortex of the cat. J Comp Neurol. 1982 Nov 10;211(4):353–362. doi: 10.1002/cne.902110403. [DOI] [PubMed] [Google Scholar]
- YOUNG J. Z. Regularities in the retina and optic lobes of octopus in relation to form discrimination. Nature. 1960 Jun 11;186:836–839. doi: 10.1038/186836a0. [DOI] [PubMed] [Google Scholar]