Abstract
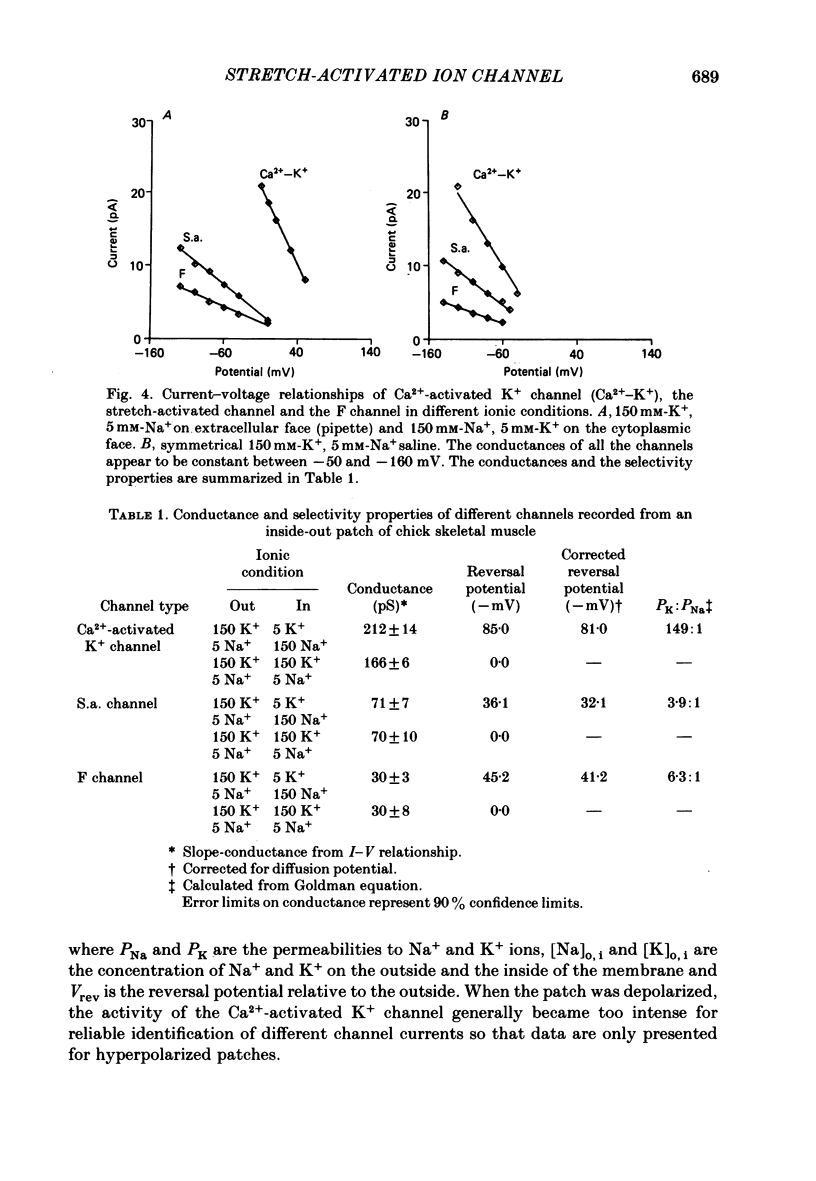
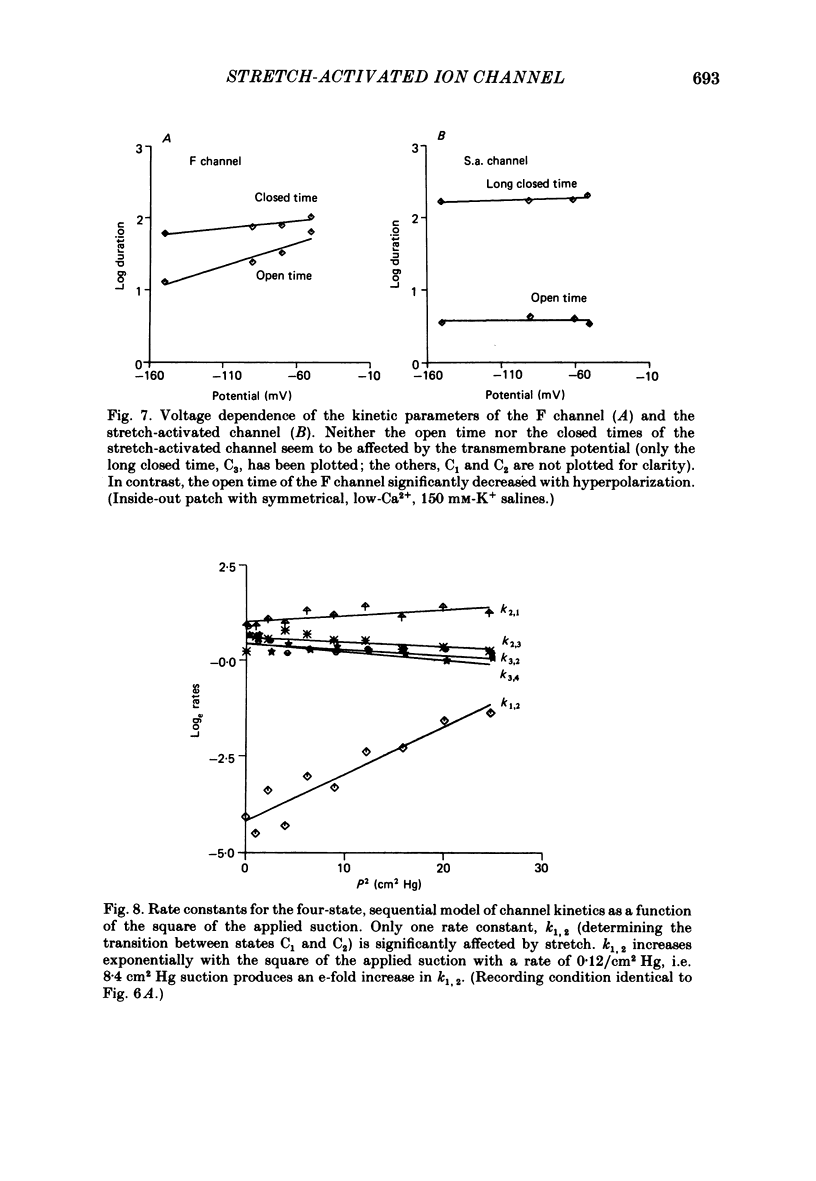
The membrane of tissue-cultured chick pectoral muscle contains an ionic channel which is activated by membrane stretch. Nicotinic channels and Ca2+-activated K+ channels are not affected by stretch. In 150 mM-external K+ and 150 mM-internal Na+ the channel has a conductance of 70 pS, linear current-voltage relationship between -50 and -140 mV and a reversal potential of +30 mV. Kinetic analysis of single-channel records indicates that there are one open (O) and three closed (C) states. The data can be fitted by the reaction scheme: C1-C2-C3-O. Only the rate constant that governs the C1-C2 transition (k1,2) is stretch-sensitive. None of the rates are voltage-sensitive. The rate constant k1,2 varies with the square of the tension as k1, 2 = k0 X e alpha T2, where alpha is a constant describing the sensitivity to stretch and T is the tension. A typical value of alpha is 0.08 (dyn cm-1)-2. Following exposure to cytochalasin B the channel becomes more sensitive to stretch. The stretch-sensitivity constant, alpha, increases from 0.08 to 2.4 (dyn cm-1)-2. The probability of the channel being open is strongly dependent upon the extracellular K+ concentration. With a suction of 2 cmHg the probability increases from 0.004 in normal saline (5 mM-K+) to 0.26 in 150 mM-K+. The channel appears to gather force from a large area of membrane (greater than 3 X 10(5) A2), probably by a cytochalasin-resistant cytoskeletal network.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Sachs F. Patch clamp studies of single ionic channels. Annu Rev Biophys Bioeng. 1984;13:269–302. doi: 10.1146/annurev.bb.13.060184.001413. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D., Rydqvist B. Crayfish stretch receptor: an investigation with voltage-clamp and ion-sensitive electrodes. J Physiol. 1978 Nov;284:155–179. doi: 10.1113/jphysiol.1978.sp012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Ottoson D., Rydqvist B., Swerup C. The permeability of the transducer membrane of the crayfish stretch receptor to calcium and other divalent cations. Neuroscience. 1981;6(7):1455–1460. doi: 10.1016/0306-4522(81)90200-1. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot G., Wong B. S., Binstock L., Ehrenstein G. Reversal potentials corresponding to mechanical stimulation and leakage current in Myxicola giant axons. Biochim Biophys Acta. 1981 Dec 7;649(2):487–491. doi: 10.1016/0005-2736(81)90440-5. [DOI] [PubMed] [Google Scholar]
- HUBBARD S. J. A study of rapid mechanical events in a mechanoreceptor. J Physiol. 1958 Apr 30;141(2):198–218. doi: 10.1113/jphysiol.1958.sp005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Ottoson D. Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. J Physiol. 1975 Oct;252(1):259–281. doi: 10.1113/jphysiol.1975.sp011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., GOLDMAN D. E. The effects of mechanical stimulation on some electrical properties of axons. J Gen Physiol. 1962 Nov;46:297–313. doi: 10.1085/jgp.46.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian P. L., Schacht J. Sound stimulates labeling of polyphosphoinositides in the auditory organ of the noctuid moth. J Neurochem. 1980 Mar;34(3):709–712. doi: 10.1111/j.1471-4159.1980.tb11201.x. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klie J. W., Wellhöner H. H. Voltage clamp studies on the stretch response in the neuron of the slowly adapting crayfish stretch receptor. Pflugers Arch. 1973 Aug 17;342(2):93–104. doi: 10.1007/BF00587840. [DOI] [PubMed] [Google Scholar]
- Korn H., Triller A., Mallet A., Faber D. S. Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science. 1981 Aug 21;213(4510):898–901. doi: 10.1126/science.6266015. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J., Minna J. An active electrical response in fibroblasts. J Gen Physiol. 1972 Jul;60(1):58–71. doi: 10.1085/jgp.60.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4702–4706. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S. Effects of some organic cations on generator potential of crayfish stretch receptor. J Gen Physiol. 1968 Aug;52(2):363–386. doi: 10.1085/jgp.52.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., McConnaughey W. B., Elson E. L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Bush B. M. Coxal muscle receptors in the crab: the receptor current and some properties of the receptor nerve fibres. J Exp Biol. 1971 Apr;54(2):515–524. doi: 10.1242/jeb.54.2.515. [DOI] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Saum W. R., Ayachi S., Brown A. M. Actions of sodium and potassium ions on baroreceptors of normotensive and spontaneously hypertensive rats. Circ Res. 1977 Dec;41(6):768–774. doi: 10.1161/01.res.41.6.768. [DOI] [PubMed] [Google Scholar]
- TERZUOLO C. A., WASHIZU Y. Relation between stimulus strength, generator potential and impulse frequency in stretch receptor of Crustacea. J Neurophysiol. 1962 Jan;25:56–66. doi: 10.1152/jn.1962.25.1.56. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Steponkus P. L. The stress-strain relation of the plasma membrane of isolated plant protoplasts. Biochim Biophys Acta. 1981 May 20;643(3):663–668. doi: 10.1016/0005-2736(81)90363-1. [DOI] [PubMed] [Google Scholar]



