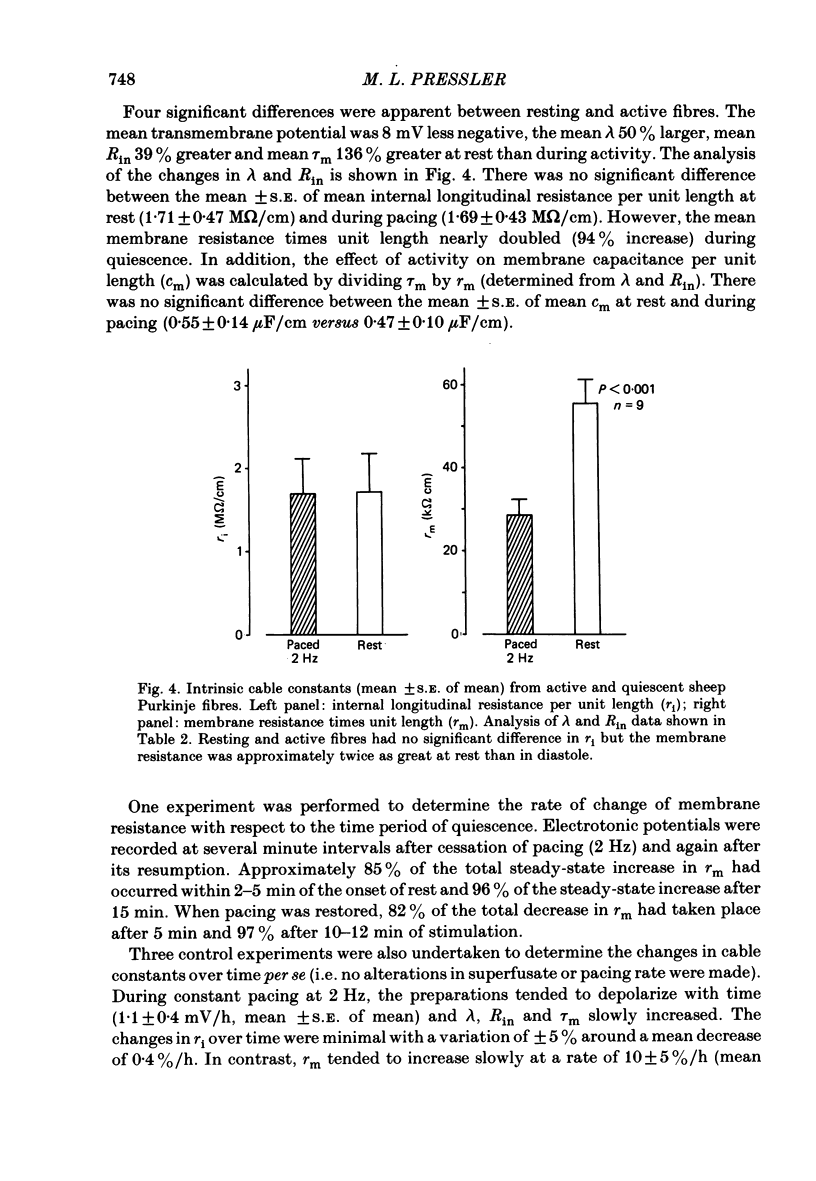
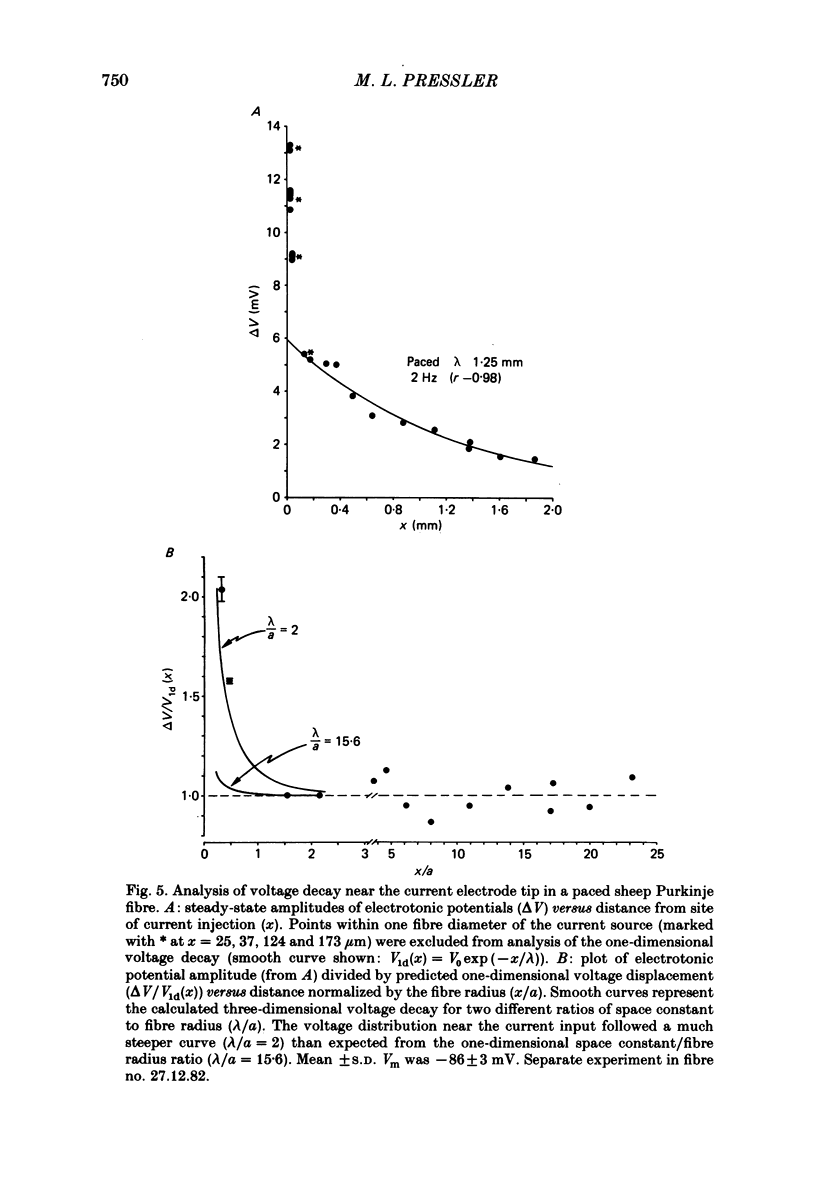
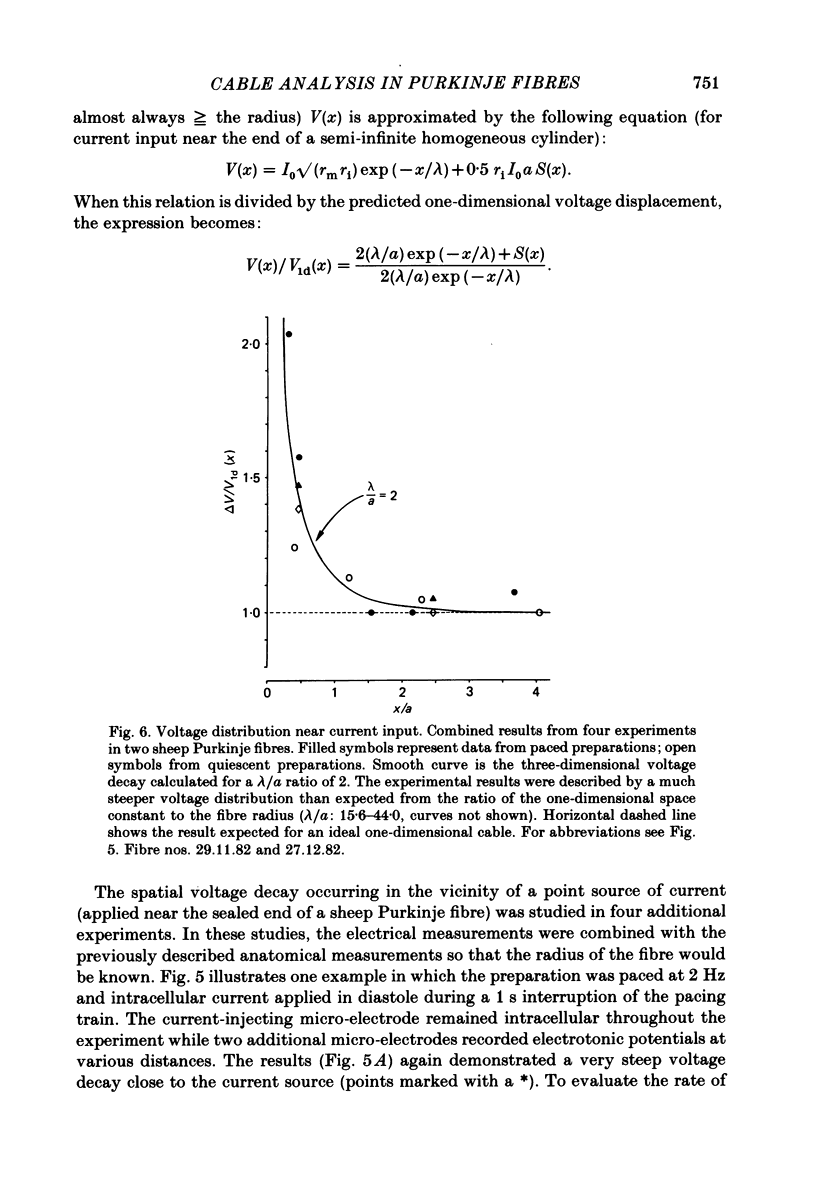
Abstract
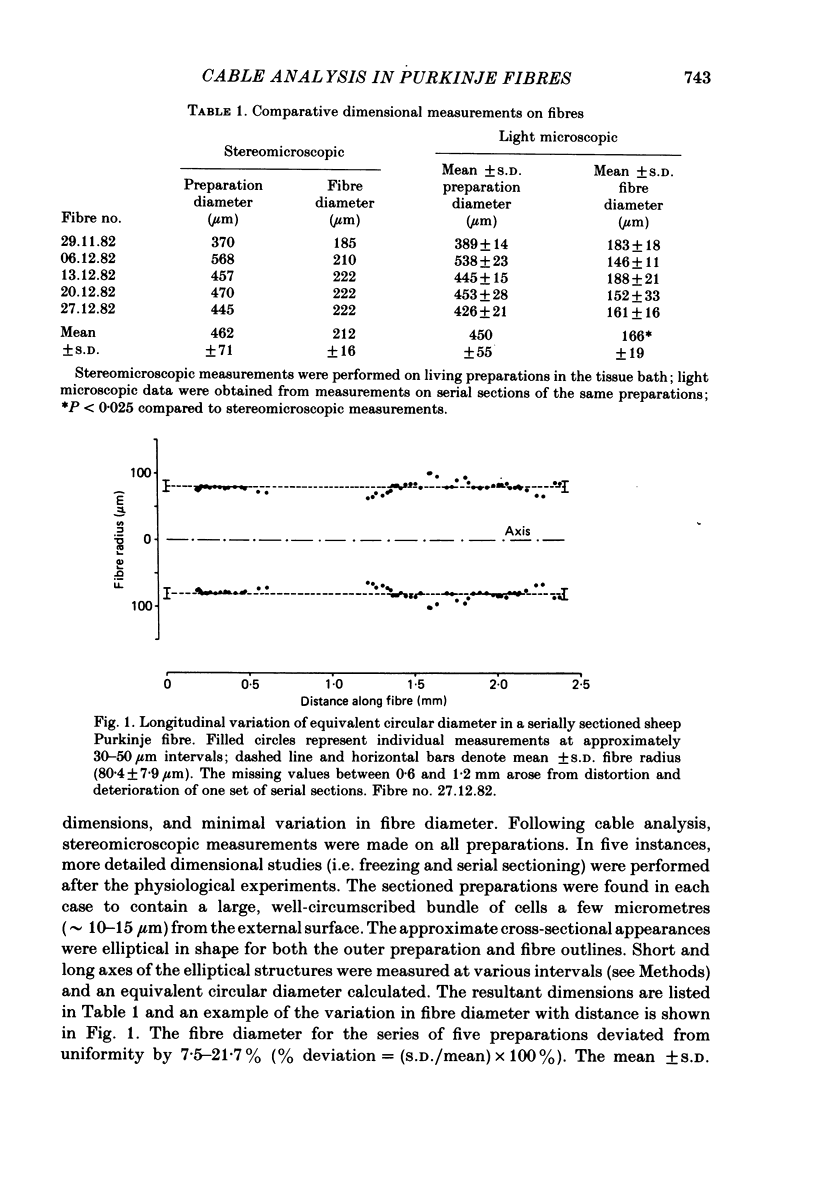
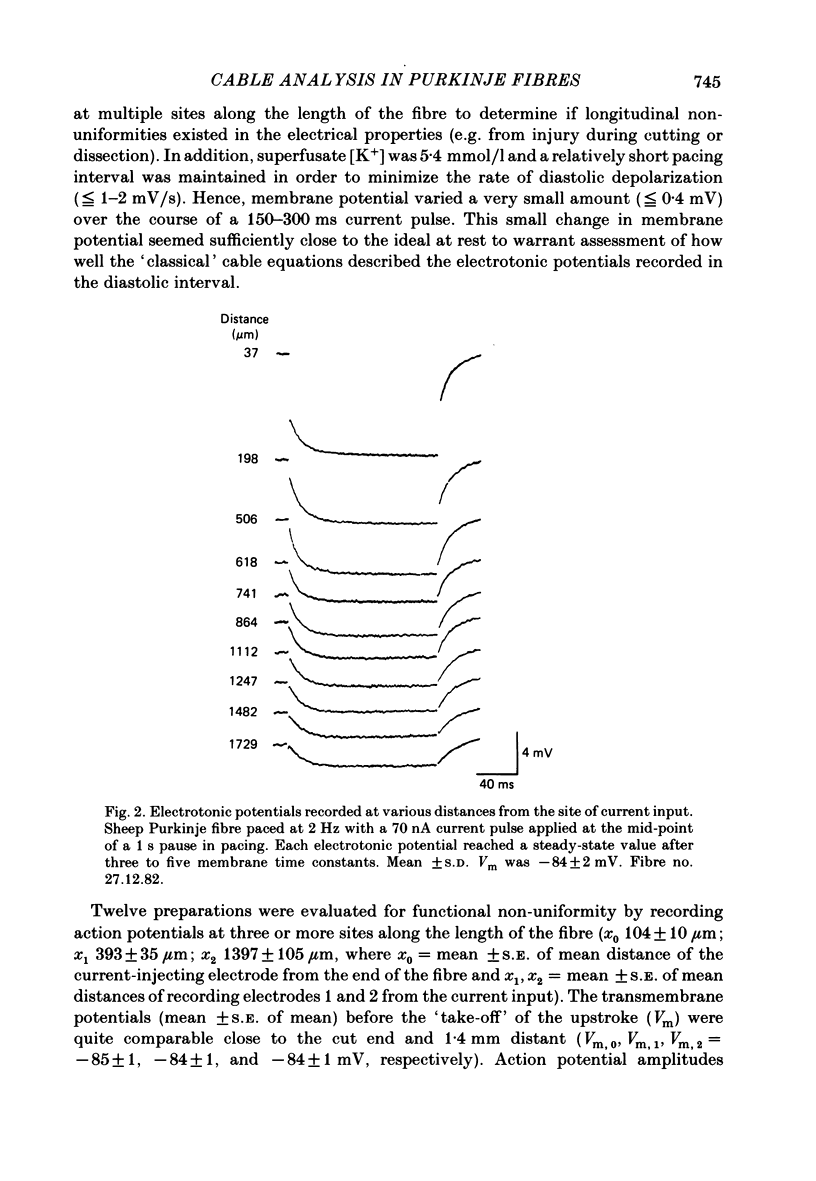
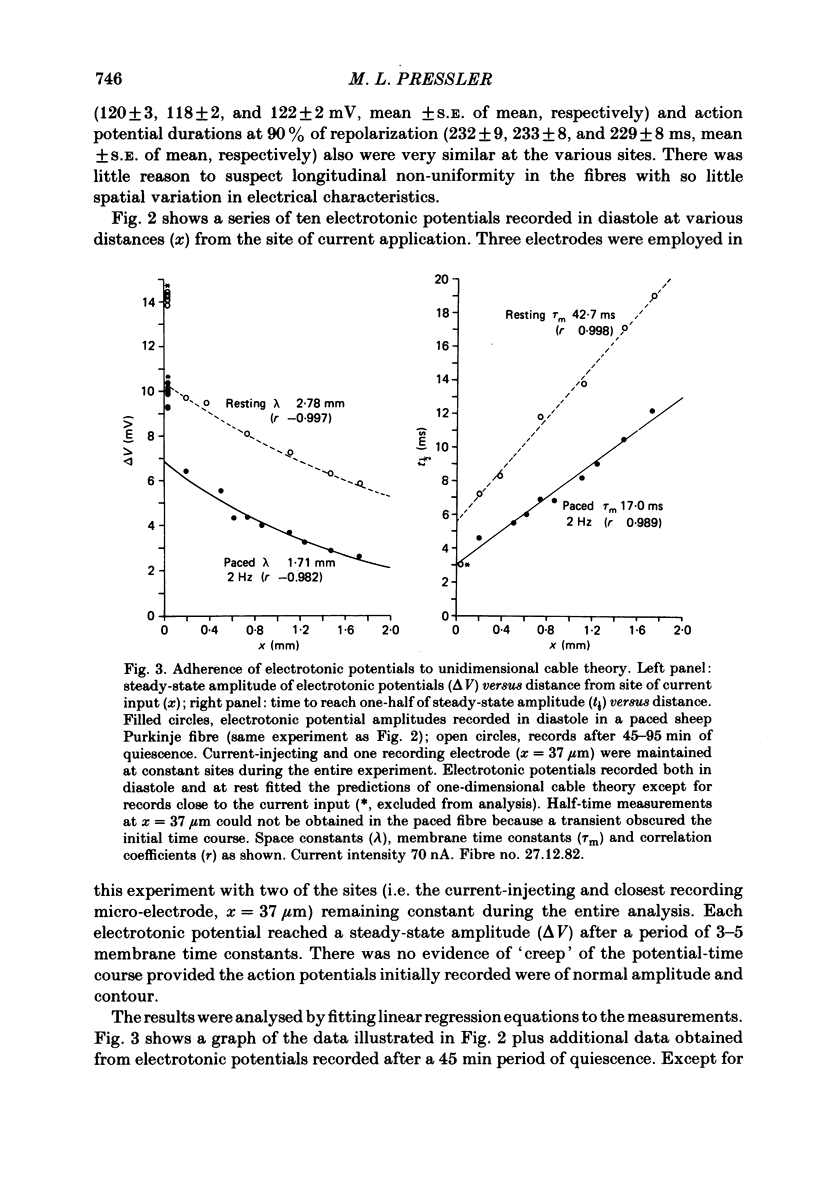
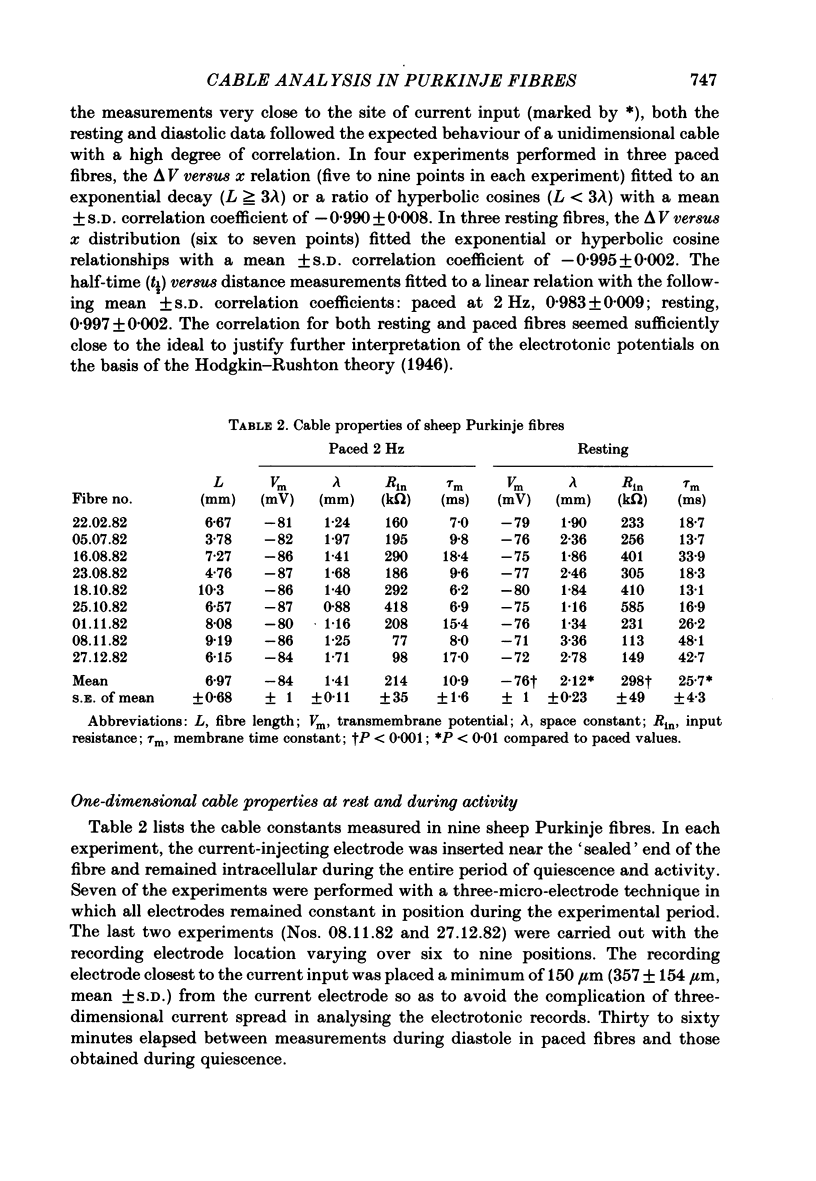
Cable properties of sheep cardiac Purkinje fibres were studied under resting and paced conditions. Standard micro-electrode techniques were used to apply intracellular current pulses and record the resultant voltage changes at various distances from the current input. In a parallel set of experiments, fibre dimensions were measured after freezing and serial sectioning. Fibres selected on the basis of a cylindrical appearance had approximately uniform cross-sectional diameters which varied +/- 12% along their length. Electrotonic potentials recorded at rest and in diastole (under conditions that minimized diastolic depolarization) adhered quite closely to the behaviour expected for a unidimensional cable provided voltages were recorded greater than or equal to one fibre diameter from the current source. The unidimensional space constant, input resistance, and membrane time constant were significantly larger during quiescence than in diastole. These differences were accounted for by a 90% increase in membrane resistance at rest. There was no significant change in internal longitudinal resistance nor membrane capacitance associated with activity. The voltage distribution close to the current input (i.e. within one fibre diameter) strongly deviated from the theoretical three-dimensional voltage decay expected for a homogeneous cylinder. This finding suggests that the transverse resistance to current flow is much greater than the longitudinal resistance. The anisotropic behaviour within the cardiac Purkinje fibre may explain several previous observations: (i) the lack of a relationship between conduction velocity and fibre diameter; and (ii) the much shorter liminal length for excitation in Purkinje fibres than for point-stimulated squid axons.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Bredikis J., Bukauskas F., Veteikis R. Decreased intercellular coupling after prolonged rapid stimulation in rabbit atrial muscle. Circ Res. 1981 Sep;49(3):815–820. doi: 10.1161/01.res.49.3.815. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. E. Chloride ions and the membrane potential of Purkinje fibres. J Physiol. 1961 Apr;156:375–388. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Cohen I., Falk R., Kline R. Membrane currents following activity in canine cardiac Purkinje fibers. Biophys J. 1981 Feb;33(2):281–288. doi: 10.1016/S0006-3495(81)84890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A. Membrane capacity of the cardiac Purkinje fibre. J Physiol. 1966 Jan;182(2):255–267. doi: 10.1113/jphysiol.1966.sp007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Schoenberg M. Strength-duration curves in cardiac Purkinje fibres: effects of liminal length and charge distribution. J Physiol. 1972 Nov;226(3):593–618. doi: 10.1113/jphysiol.1972.sp009999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. A note on conduction velocity. J Physiol. 1954 Jul 28;125(1):221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. Linear analysis of membrane conductance and capacitance in cardiac Purkinje fibres. J Physiol. 1974 Dec;243(3):661–694. doi: 10.1113/jphysiol.1974.sp010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. P., Cohen I., Falk R., Kupersmith J. Activity-dependent extracellular K+ fluctuations in canine Purkinje fibres. Nature. 1980 Jul 3;286(5768):68–71. doi: 10.1038/286068a0. [DOI] [PubMed] [Google Scholar]
- Kline R. P., Morad M. Potassium efflux in heart muscle during activity: extracellular accumulation and its implications. J Physiol. 1978 Jul;280:537–558. doi: 10.1113/jphysiol.1978.sp012400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootsey J. M., Johnson E. A., Lieberman M. The cylindrical cell with a time-variant membrane resistance. Measuring passive parameters. Biophys J. 1977 Feb;17(2):145–154. doi: 10.1016/S0006-3495(77)85632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado M. G., Sheu S. S., Fozzard H. A. Changes in intracellular Ca2+ activity with stimulation in sheep cardiac Purkinje strands. Am J Physiol. 1982 Jul;243(1):H133–H137. doi: 10.1152/ajpheart.1982.243.1.H133. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kootsey J. M., Johnson E. A., Sawanobori T. Low conduction in cardiac muscle. Biophysical model. Biophys J. 1973 Jan;13(1):37–55. doi: 10.1016/s0006-3495(73)85968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Sawanobori T., Kootsey J. M., Johnson E. A. A synthetic strand of cardiac muscle: its passive electrical properties. J Gen Physiol. 1975 Apr;65(4):527–550. doi: 10.1085/jgp.65.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The relation of Rushton's 'liminal length' for excitation to the resting and active conductances of excitable cells. J Physiol. 1972 Oct;226(2):573–591. doi: 10.1113/jphysiol.1972.sp009998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler M. L., Elharrar V., Bailey J. C. Effects of extracellular calcium ions, verapamil, and lanthanum on active and passive properties of canine cardiac purkinje fibers. Circ Res. 1982 Nov;51(5):637–651. doi: 10.1161/01.res.51.5.637. [DOI] [PubMed] [Google Scholar]
- Sachs F. Electrophysiological properties of tissue cultured heart cells grown in a linear array. J Membr Biol. 1976 Sep 17;28(4):373–399. doi: 10.1007/BF01869706. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Dominguez G., Fozzard H. A. Effect of diameter on membrane capacity and conductance of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Apr;65(4):441–458. doi: 10.1085/jgp.65.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Kootsey J. M., Sloan J. D. Active modulation of electrical coupling between cardiac cells of the dog. A mechanism for transient and steady state variations in conduction velocity. Circ Res. 1982 Sep;51(3):347–362. doi: 10.1161/01.res.51.3.347. [DOI] [PubMed] [Google Scholar]
- Vassalle M. Electrogenic suppression of automaticity in sheep and dog purkinje fibers. Circ Res. 1970 Sep;27(3):361–377. doi: 10.1161/01.res.27.3.361. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]


