Abstract
Members of the Trypanosomatidae family comprise a large number of species that are causative agents of important diseases such as sleeping sickness, Chagas' disease and Leishmaniasis. These organisms are also of biological interest since they are able to change the morphology according to the environment where they live, through a process of reversible cell transformation, and possess structures and organelles that are not found in mammalian cells. This review analyses the process of transformation, which takes place during the life cycle of Trypanosoma cruzi in the vertebrate and invertebrate hosts. Special attention is given to the interaction of the parasite with vertebrate cells. In addition, the present knowledge of structures and organelles such as the nucleus, the plasma membrane, the sub-pellicular microtubules, the flagellum, the kinetoplast-mitochondrion complex, the peroxisome (glycosome), the acidocalcisome and the structures and organelles involved in the endocytic pathway, is reviewed from a cell biology perspective. The possible use of available data for the development of new anti parasite drugs is also discussed.
Introduction
Among the protozoa of the Trypanosomatidae family a large number of species represent agents of diseases such as Chagas' disease (American trypanosomiasis), sleeping sickness (African trypanosomiasis) and, leishmaniasis, that are of considerable medical and veterinary importance. Special programs for investigation in this area have been supported by the World Health Organization. Chagas' disease, caused by the protozoan. The most important of these diseases in large areas of South and Central America, is Trypanosoma cruzi which effects affecting about 14 million people, with about 100 million people remaining at risk (Fig. 1).
Figure 1.
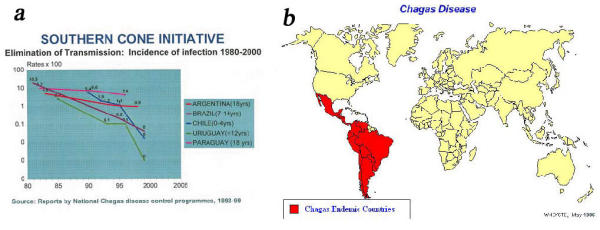
a: Elimination of transmission of Chagas' disease. b: Distribution of Chaga's disease in the world.
In recent years, important health programs have been established to control transmission of the parasites from insect to man and from person to person during blood transfusion. Recent studies using isoenzyme analysis, riboprinting analysis, rRNA promoter activity, sequence of miniexon genes and microsatellite markers have provided clear evidences that T. cruzi is not a single species but corresponds to two highly divergent genetic subgroups, designated as lineages 1 and 2 [1]. Lineages 1 predominate in the domestic cycle while lineage 2 is mainly represented in the sylvatic cycle. Multilocus enzyme electrophoresis and random amplified polymorphic DNA data showed that lineage 2 is further subdivided into five smaller lineages [2]. Both lineages are potentially pathogenic for man. It is important to point out that although at present there is no transmission of Chagas' disease in places like the Rio de Janeiro state, 100 % of the small primitive monkeys and 84 % of the opossums found in a residual florest localized 80 km from the city of Rio de Janeiro are infected with T. cruzi. Therefore, it is important to intensify basic work on the biology of the parasite in order to develop new strategies of disease control. In addition this group of eukaryotic microorganisms possess a significant number of special structures and organelles, such as the paraflagellar rod, the subpellicular microtubules, the glycosome and the kinetoplastmitochondrion complex, among others, which make them very interesting from a cell biology perspective.
The aim of this article is to review some aspects of the cell biology of T. cruzi, emphasizing basic biological processes structures and organelles that may be relevant to the development of new drugs. For those that work with a practical perspective emphasis has been given to the analysis of cell structures and organelles, or even biochemical processes, which significantly differ from vertebrate cells. The basic idea is that a deep knowledge of what is going on in the structures and organelles may open new perspectives for the control of disease through the development of (a) new chemotherapeutic agents, (b) vaccines or (c) more specific diagnostic procedures. In this work I will focus mainly on Trypanosoma cruzi since other important trypanosomatids, such as Trypanosoma brucei andLeishmania, have been covered in recent reviews. The list of potential targets is inevitably selective, and represent a rather personal view.
Developmental forms and cell transformation
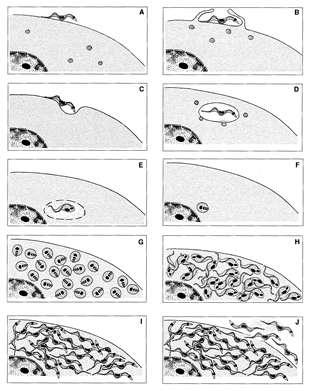
Protozoa of the Trypanosomatidae family show, during their life cycle, several forms that can be easily identified by light microscopy in Giemsa-stained preparations (Fig. 2).
Figure 2.
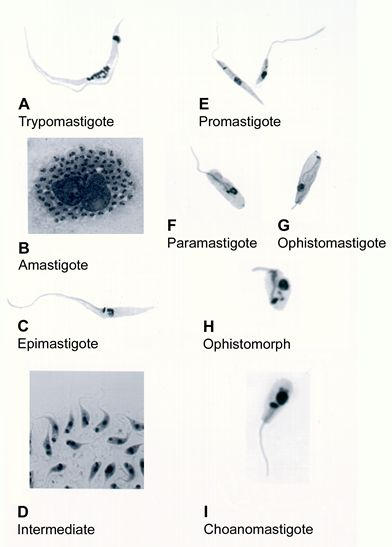
Light microscopy of stained cells showing the developmental stages found in the members of the Trypanosomatidae family.
In the case of T. cruzi the following forms can be identified: (a) Amastigotes, also known as spheromastigote, micromastigote, aflagellate form, or leishmanial form. Studies carried out using amastigote forms obtained from different sources have shown that they are able to divide and are also infective for vertebrate cells [3,4]. (b) Epimastigotes are spindle-shaped, 20–40 μm long with kinetoplast located anterior to the nucleus. These forms are able to divide and are observed at the logarithmic phase of growth in axenic cultures and in the intestine of the invertebrate host. Epimastigotes are also found within vertebrate cells towards the end of the intracellular cycle when the spheromastigotes transform into trypomastigotes or at the beginning of a new cycle the trypomastigotes transform into spheromastigotes [5,6]. (c) Trypomastigotes, these forms have a length of about 25 μm and a diameter of about 2 μm. The kinetoplast is located posterior to the nucleus. They can be observed (i) in the tissue cells and in the blood of the vertebrate host, (ii) in the posterior intestine, in the feces, and in the urine of the invertebrate host, (iii) at the stationary phase of growth in axenic cultures, and (iv) in the liquid phase of cell cultures. These forms are not able to divide.
The biological cycle of T. cruzi starts when the invertebrate host feeds on the vertebrate host by sucking blood (Fig. 3). The invertebrate hosts are Hemiptera and Reduvidae such as Rhodinus prolixus, Triatoma infestans, and Panstrongylus megistus. During feeding, the trypomastigote forms in the blood of the infected vertebrate host are ingested by the insect. It has been assumed that in the stomach of the insect most of the bloodstream trypomastigotes transform into epimastigotes and some rounded forms. In the intestine the epimastigotes divide repeatedly by a process of binary fission and can attach to the intestinal cells by hemidesmosomes [7]. In the rectum a certain proportion of the epimastigotes transform into metacyclic trypomastigotes which are eliminated with the feces and are able to infect the vertebrate host [7-9]. It has been shown that the trypomastigote form can transform into a rounded form possessing a free flagellum. This form, which appears in the stomach, is able to transform into either short epimastigotes, which start a process of multiplication in the intestine, or into long epimastigotes that move to the more posterior region of the digestive tract of the bug. Apparently these long epimastigotes are unable to divide. Some days after the ingestion of infected blood, spheromastigotes, which are able to transform into trypomastigotes, are found in the rectum. Relying on the information available up to the present moment it seems that both, epimastigotes and the spheromastigotes, are able to transform into trypomastigotes. Based on these observations it is possible to develop alternatives for the control of T. cruzi transmission by inhibiting either the adhesion of the parasites to the intestinal cells, or the transformation of noninfective epimastigote into infective trypomastigote forms.
Figure 3.
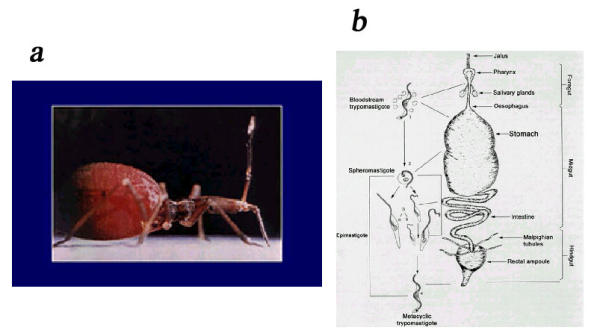
a: View of a triatomine after blood meal (courtesy of P Azambuja and E Garcia). b: Schematic view of the development of Trypanosma cruzi within the invertebrate host.
The trypomastigote forms eliminated in the feces and urine of the invertebrate host are able to penetrate into vertebrate cells where they transform into spheromastigotes (Fig. 4). These divide repeatedly by binary fission and give rise to trypomastigotes that are liberated into the intercellular space, may reach the bloodstream, and can penetrate into other cells initiating a new cycle. Contrary to what is known for the African trypanosomes, the trypomastigote form of T cruzi does not divide in the bloodstream. Two morphological types of bloodstream trypomastigotes have been observed: one is slender, with an elongated nucleus, a subterminal kinetoplast, and a short free flagellum; the other is broad, with an oval nucleus, an almost terminal kinetoplast, and a long free flagellum. The predominance of one form over the other is dependent on the strain of T. cruzi and the time of infection. It has been suggested that the slender forms are mainly responsible for the infection of the vertebrate cells while the broad forms are more able to infect the invertebrate host [Review in [10]].
Figure 4.
Schematic view of the various phases of the interaction of T. cruzi with vertebrate cells.
A better understanding of the life cycle of T. cruzi in vertebrate cells was made possible with by the use of tissue and cell cultures [5,11]. Since T. cruzi is an intracellular parasite in the vertebrate host and the invasion of the host cells plays a fundamental role in the maintenance and the amplification of the infection, several groups have studied in some detail the basic mechanisms involved in the host cell parasite relationship. I will describe here only those aspects considered to be more relevant from the perspective of the present review, analyzing processes such as parasite adhesion, invasion and biogenesis of the parasitophorous vacuole.
The initial process of T. cruzi host cell interaction involves attachment of the parasite to the host cell surface (Figs. 4,5). Videomicroscopic observation of the interaction shows that not all parasites that touch the host cell surface remain attached. Attachment is usually more evident in situations in which the subsequent phase of internalization is interrupted, as occurs in cells previously treated with drugs which interfere with actin filaments [12]. The available data indicate that attachment is due to a process of cell-to-cell recognition with the involvement of sugar residues exposed on the surface of both interacting cells [13]. There is a large body of evidence showing that sialic acid residues are involved in the recognition process [14]. One surface glycoprotein which possesses both neuraminidase and trans-sialidase activity plays a major role in T. cruzi host cell interaction [15]. This molecule belongs to a multigenic family coding for a group of heterogeneous proteins with molecular weights varying from 100 to 200 kDa. These proteins can be found in all developmental forms, however, they are more abundant in trypomastigote forms where they can be found on the parasite surface, attached to the plasma membrane via a GPI anchor sensitive to phospholipase C. Usually these proteins are found only in about 20% of the trypomastigote population and those expressing them correspond to the most invasive forms [16]. These molecules remove sialic acid residues, preferentially linked in a α-2,3 position, transferring it to acceptor molecules, mainly represented by a highly glycosylated protein of 70-200 kDa, known as Ssp3 [17], and of 3550 kDa molecule found in metacyclic trypomastigotes [18]. The trans-sialidase family also includes other glycoproteins which do not possess enzyme activity, but which are also involved on the process of T. cruzi host cell interaction, as is the case of one glycoprotein of 85 kDa known as Tc85 [19]. It has been shown that this molecule possesses adhesive properties to laminin and a carboxyl terminal segment that binds to cytokeratin 18 [20].
Figure 5.
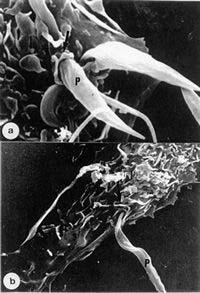
Two different views of the interaction of T. cruzi with macrophages, as seen by scanning electron microscopy.
Studies using monosaccharides, lectins and enzyme treatments have shown that in addition to sialic acid there is evidence that sugars such as galactose, mannose and N-acetylglucosamine exposed both on the parasite and the host cell surface are also involved in the process of parasite-host cell recognition [Review in [13]].
The available data provide evidences that the process of interaction of T. cruzi with host cells can be considered as one involving ligands and receptors [Review in [21]]. Most probably immediately after the initial parasite contact, changes take place in the plasma membrane of both cells. It has been shown that parasite proteases, such as cruzipain, are involved in this process [22]. Indeed inhibition of these enzymes significantly inhibited parasite invasion into the host cell [23].
Morphological observations have shown that the parasite always penetrates the host cell by an endocytic process, in which a parasitophorous vacuole is formed (Fig. 6).
Figure 6.
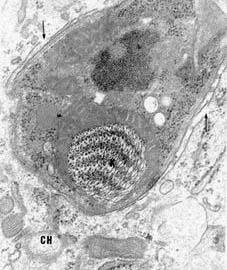
Trypomastigote form of T. cruzi a few hours after penetration into the host cell. Rupture of the membrane lining the parasitophorous vacuole can be seen (arrows).
In some cells, such as macrophages, cell surface projections arise, resembling those typically observed during phagocytosis. In other cells, such processes are not found. However, regardless of the host cell used, drugs interfing with actin polymerization inhibit parasite invasion. In processes involving phagocytic cells, it is clear that following parasite attachment there is a process of protein phosphorylation and dephosphorylation. Previous treatment of the host cell with drugs which inhibit protein kinases significantly inhibit cell infection [24]. These observations are relevant from a therapeutic perspective since the inhibition of the host cell infection by infective forms of T. cruzi is a potential and important drug target. At the beginning of the interaction process there is also a process of Ca2+ release from both the parasite and the host cell [Reviews in [25,26]]. There is also mobilization of host cell lysosomes towards the site of parasite invasion (Review in [21]).
Taken together, the basic data on the biology of T. cruzi opens several possibilities to develop new chemotherapeutic strategies:
First, it is clear that carbohydrate residues are involved in the early steps of the interaction process, which are fundamental for the infection to be established. The blockage of the glycosylation process may be an interesting target, especially in view of recent observations showing, in the case of T. cruzi, the involvement of a unique O-linked-N-acetylglucosamine-containing oligosaccharide in sialoglycoproteins, with the participation of a O-α-GlcNAc transferase [27]. This enzyme is localized in the Golgi complex of the protozoan [28].
Second, Surface-exposed enzymes, such as the trans-sialidase and proteases, also play an important role. The genes encoding these proteins have been cloned and expressed. The proteins themselves are in the process of being characterized and we can expect the development of specific inhibitors for their activity very soon. These inhibitors are potentially interesting from the chemotherapeutic point of view. Indeed, it has been shown that general inhibitors of cysteine proteinases kill T. cruzi[23,29].
Third, it also clear that the phosphorylation of parasite proteins plays a key role on the process of host cell invasion by T. cruzi. Therefore, inhibitors of kinases and phosphatases can be included in the list of potentially interesting drugs to be tested, once the parasite enzymes are characterized and specific inhibitory drugs have been developed.
The available data show that some of the molecules involved in the interaction process elicit an immunological response and antibodies which recognize them can be found in the blood of patients with Chagas' disease or of experimentally infected animals. One portion of the trans-sialidase molecule is particularly interesting and corresponds to what was originally described as Shed Acute Phase Protein-SAPA. These molecules may constitute excellent targets for the development of vaccines, aiming the blockage of cell infection by the parasites, as well as antigens for a more specific diagnosis of Chagas' disease.
Independently of the mechanism used to penetrate into the host cell the parasite resides within a cytoplasmic vacuole lined by a membrane: the parasitophorous vacuole (PV). There are several data showing that some, but not all, molecules found in the plasma membrane of the host cell are internalized and will make up part of the PV [30].
Following parasite penetration, and even during the initial phase of internalization, components of the endolysosomal system of the host cell fuse with the PV discharging their content, rendering it an acidic compartment. In the case of macrophages, and especially if the parasites are opsonized, there is activation of NAD(P)Hoxidase with the production of oxygen-derived products which may kill the parasites.
Subsequent to internalization, the trypomastigote gradually transforms into an intermediate epimastigote and then into an amastigote form. This process is characterized by the shortening of the flagellum and rounding of the cell body. During the trypomastigote-spheromastigote transformation there is an intermediary phase, which is designated as epimastigote, although this form is somewhat different from the epimastigote observed in axenic cultures. Two hours after penetration, a process of gradual lysis of the membrane lining the PV occurs (Fig. 7) [31] due the release of a trypsin-sensitive parasite enzyme known as TcTox [32]. This process depends on the existence of an acidic compartment and the nature of the components of the membrane. After the complete rupture of the PV membrane, the amastigote form establishes direct contact with the host cell cytoplasmic structures and organelles and after 24 to 35 hours starts to divide. The significance of this long lag period in the intracellular life cycle of T. cruzi is not yet completely understood but it is influenced by the growth temperature of the vertebrate cells. Autoradiographic studies indicates that DNA synthesis occurs during the lag period [33]. Using a special cell line of fibroblasts which, due to the loss of hypoxanthineguanine phosphoribosyltransferase, are unable to incorporate guanine into nucleic acids it was shown by autoradiography that parasite RNA synthesis occurs only 2 hours after the penetration of the parasite inside the vertebrate cell [34]. Therefore the synthesis of new RNA does not appear to be required for the process of trypomastigote-spheromastigote transformation. These studies also showed that extracellular trypomastigotes actively synthesize RNA.
Figure 7.
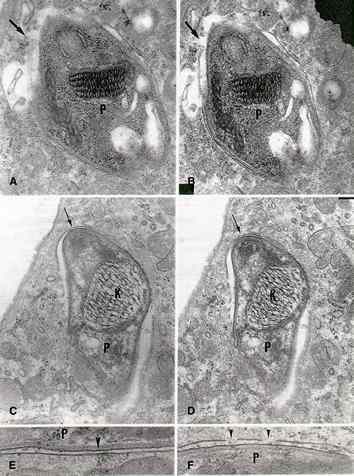
Different views of the membrane lining recem penetrated trypomastigote forms of T. cruzi.
Before division there is a gradual increase in the size of the spheromastigotes. The kinetoplast and the nucleus divide, a new flagellum is formed, and clefts appear in the anterior and posterior regions of the cell which subsequently divides the parasite. The doubling time of the parasite is about 14 hours, but can be influenced by the temperature of cell growth and is dependent on the strain of the parasite used. The process of cytokinesis requires about 25 minutes for completion [Review in [11]]. After several successive divisions the amastigotes gradually transform into trypomastigotes, via an epimastigote intermediate stage. This process takes several hours. During the transformation, changes occur in the general organization of the cell, in the structure of the kinetoplast, and in the flagellum [36]. This latter structure increases in length from 1 to 20 μm. The population of intracellular parasites does not differentiate completely synchronously. Therefore, at a certain time all transitional stages between spheromastigotes and trypomastigotes can be found in one cell. There is little information about the crucial stimuli that trigger the amastigote to trypomastigote transformation process. At the end of the intracellular, cycle the movement of the parasites, which now have long flagella, is intense. This helps rupture the host cell and liberate parasites, which are then able to infect new vertebrate cells.
The nucleus of T. cruzi and other trypanosomatids has a structural organization that appears to be similar to that found in other eukaryotic cells. It is small, measuring about 2.5 μm. Therefore, few data can be obtained with the light microscope about its organization.
In trypomastigotes of T. cruzi, the nucleus is elongate and localized in the central portion of the cell. In spheromastigotes and epimastigotes it has a rounded shape. It has a typical nuclear membrane studded with pores (Fig. 8). Continuity between the outer nuclear membrane and the endoplasmic reticulum is evident. In favorable freeze-fracture preparations, large areas of the inner and the outer nuclear membranes are exposed. In the convex as well as in the concave faces of both membranes, intramembranous particles are randomly distributed. The nuclear pores, as seen in freeze-fracture replicas, have a mean diameter of 80 nm. In interphasic T. cruzi cells, the chromatic material agglomerates into masses at the periphery of the nucleus, below its inner membrane. Occasionally, these masses are also found in the more central region. The chromosomes are difficult to distinguish, because they do not condense at any stage of the life cycle. However, with the advent of the pulsed field electrophoresis, chromosomes have been identified. The genome of T. cruzi consists of 87 MB of DNA distributed among 30–40 chromosomal bands ranging from 0.45 to 4.0 (See http://www.dbbm.fiocruz.br/TcruziDB/). In the center of the nucleus, or situated slightly eccentrically, the nucleolus may be found. During division, changes occur in the organization of the nuclear material. The nucleolus is dispersed during division, reappearing at the final phases of the cell division. At the beginning of the division process of T. cruzi, when the basal body is replicating, the first signs of division can be observed in the nucleus. The chromatin material localized below the inner nuclear membrane and the nucleolus disappear. Both are dispersed over the whole nucleus giving it a homogeneous aspect. Immediately after replication of the basal body, when the kinetoplast shows no morphological signs of division, microtubules appear inside the nucleus of trypanosomatids [36,37]. The nucleus, which has a spherical form, changes into a more oval one with the major axis perpendicular to the direction of the flagellum. Shortly thereafter, kinetoplast divides, the two newly formed structures move to the sides, and the nucleus becomes progressively elongated.
Figure 8.
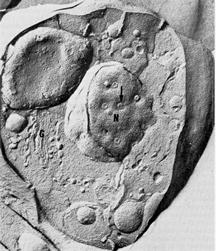
Freeze-fracture view of an epimastigote form of T. cruzi. The nuclear pores are evident.
In all studies made on the nucleus in division, small electron-dense plaques were observed among the intranuclear microtubules (Fig. 9).
Figure 9.
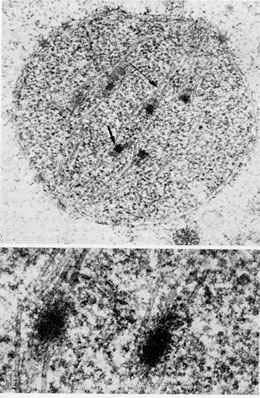
Low and high magnification of a nucleus of an epimastigote form of T. cruzi in process of division. (courtesy of A Solari).
In the case of T. cruzi, the spindle microtubules were seen in connection with the plaques. A more detailed study of the nuclear division of T. cruzi epimastigotes, using serial sections and a three-dimensional reconstruction of each divisional stage, was reported by Solari [37]. He found that the equatorial spindle is formed by about 120 microtubules arranged in two sets of about 60 microtubules, running from each pole to the dense plaques and divided into discrete bundles which reach a single plaque. Gull identified 10 plaques which were not located in one plane but were distributed within a region which covers about 0.4 μm from both sides of the actual equatorial plane [39]. The average dimensions of each plaque were 200 nm long, 120 nm wide, and 70 nm thick. Each plaque had a symmetrical structure formed by transverse bands. Before nuclear elongation occurred the dense plaques split in two halves and began to migrate to the polar regions. At this time, no microtubules were seen between the two halves of each plaque. All microtubules were localized between the plaques and the poles of the nucleus. In the elongated nucleus, it was possible to see 10 half-plaques on each side of the dividing nucleus. The nature and functional role of the dense plaques are not yet clear. It has been suggested that they could represent specialized parts of uncondensed chromosomes [37]. However, there are no data supporting this idea. They could also represent kinetochore-like structures, which would play an important role in the process of separation of the nuclear material into the two new cells. Further cytochemical studies may shed some light on the composition of the electron-dense plaques thus helping to understand their function. As suggested by Solari "if the relationship to chromatin is proved by histochemical or structural evidence, T. cruzi would most probably have ten chromosomes [37]." When the nucleus is elongated it divides by constriction in the middle. When the division is complete the chromatin material and the nucleolus reorganize and assume the position seen in interphase cells and the microtubules disappear. During the whole process of division, the nuclear membrane remains intact. No centrioles have been observed in connection with the microtubules nor have other structures been found which would suggest participation in their formation. The results obtained until now do not permit a precise statement of the functional role of the intranuclear microtubules in the dividing nucleus. It is feasible, however, to attribute to them the same function assumed for metazoan cells, i.e., helping to separate and move the genetic material into the two newly forming nuclei. They might also be engaged in elongating the dividing nucleus.
Recent studies [38] have shown that a nucleolus is not observed in trypomastigote forms and that there is a decrease in the transcription rates by RNA polymerases I and II when epimastigote forms transform into trypomastigote forms.
Studies performed on other cells types, especially neoplastic cells, have opened up the possibility of controlling cell proliferation by inhibiting different steps of the mitotic cycle. This is a potential and yet unexplored area for the chemotherapy of Chagas' disease. It has been shown that the microtubules of trypanosomatids are resistant to classical inhibitors of the microtubules such as colchicine and benzimidazoles. Vinca alkaloids such as vinblastine and vincristine and macrolides (maytansine, ansamitocin and rhizoxin) are effective. In addition, dinitroaline herbicides such as trifluralin and chloralin, which are potent disrupters of microtubules, and taxol, which reduces microtubule instability through promotion of polymerization, have been shown to inhibit growth of trypanosomatids [Reviews in [39,40]) and deserve further investigation.
The cell surface
The cell surface of trypanosomatids can be considered as composed by two components: the plasma membrane and a layer formed by the subpellicular microtubules (Fig. 10).
Figure 10.
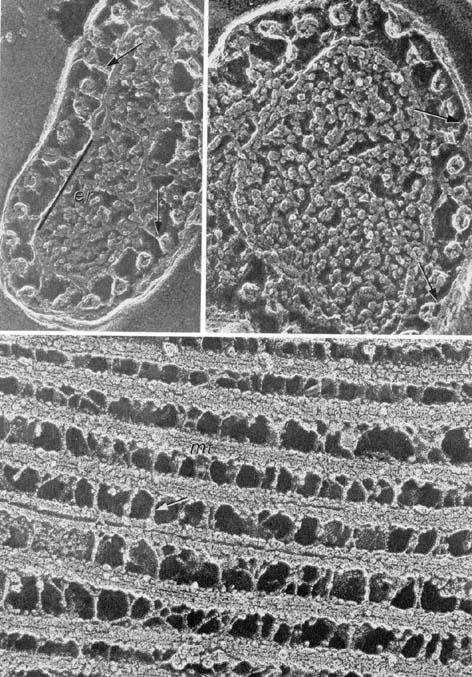
Different views of the association of the sub-pellicular microtubules with each other and with the plasma membrane.
Although it is now well established that in most eukaryotic cells microtubules and microfilaments are associated with the plasma membrane, in no other cell type is such association as strong as in trypanosomatids. Where, these two components of the cell surface remain associated even after lysis of the protozoan and isolation of a membrane-enriched fraction. The structural organization and the biochemical composition of the plasma membrane of T. cruzi has been the subject of several reviews [See [41]]. For the purposes of the present review, it is important to point out that a large number of proteins are associated with the plasma membrane through either a glycosylphosphatidylin ositol anchor, or following attachment of 15-carbon farnesyl or 20-carbon geranyl group to the C-terminal cysteine residues, a process known as protein prenylation. Recent studies have characterized in some detail the biosynthesis of GPI anchor, which involves several biochemical steps. Drugs that inhibit key enzymes of this pathway constitute potential candidates for chemotherapeutic use. Drugs that inhibit enzymes involved in the process of protein prenylation may also be interesting anti-parasite agents. For instance, drugs inhibiting protein farnesyl transferase arrest the growth of T. cruzi [Review in [42]].
The subpellicular microtubules
One of the characteristic features of protozoa Trypanosomatidae is the presence of a layer of microtubules localized below the plasma membrane and designated as subpellicular microtubules. It has been observed that the microtubules are connected to each other and to the plasma membrane by short filaments of still unknown nature (Fig. 10). This association is probably responsible for the rigidity of the cell and the difficulties found in the disruption of the cell by mechanical means. Transverse sections through different regions of the trypomastigote form of T. cruzi, show that the microtubules are regularly spaced, with a distance equalling 44 nm (center to center). Occasionally one or more microtubules may be lacking, especially in the region of attachment of the flagellum to the protozoan body. It was observed that the number of microtubules is related to the diameter of the cell. At the posterior and anterior regions of the protozoan, the number of microtubules is smaller while the largest number is found between the nucleus and the kinetoplast, at the region where the Golgi complex is located. Crosssections of the subpellicular microtubules of T. cruzi show that they are hollow structures. Their wall is composed of 13 protofilaments, has a thickness of about 5 nm and the central portion shows a diameter of about 20 nm.
Important information has recently been obtained on the biochemistry of the subpellicular microtubules of trypanosomatids, especially in T. brucei. Biochemical studies have shown the presence of six tubulins in trypanosomatids: alpha and beta, which constitute the subunits of the microtubule: gamma, which is a minor component responsible for microtubule nucleation: delta, epsilon and zeta tubulins [39]. The possible chemotherapeutic use of drugs that inhibit microtubule polymerization was discussed previously.
The flagellum
All members of the Trypanosomatidae family have a flagellum that emerges from an invagination called the flagellar pocket. In developmental stages such as promastigote, paramastigote, opisthomastigote, and choanomastigote, the flagellum emerges at the anterior tip whereas in epimastigote and trypomastigote forms it emerges somewhere along the side. The proportion of the total flagellar length to that inside the flagellar pocket region varies according to the developmental stage.
The flagellum of T. cruzi has a basic structure which is similar to other flagella, showing a 9 + 2 pattern of axonemal microtubules. In addition, the flagellum also possesses a complex a highly organized filamentous structure known as the paraflagellar rod.
In trypanosomatids the length of the flagellum varies according to their developmental stage. For example, spheromastigotes of T. cruzi have a short flagellum, 1 μm in length (Fig. 11). However, at the end of the intracellular cycle the parasite elongates and the flagellum also grows up to about 20 μm (Fig. 12).
Figure 11.
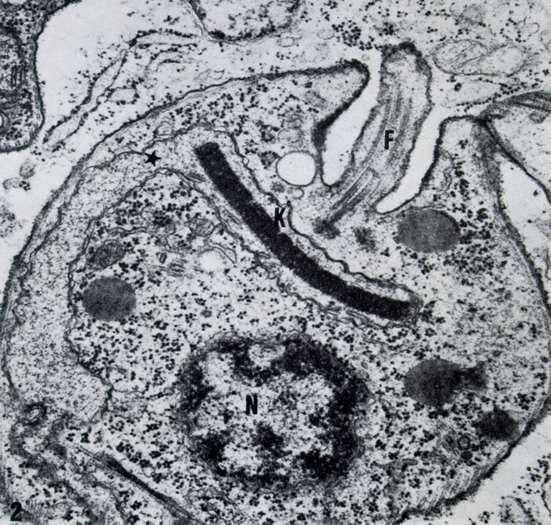
Amastigote form of T. cusi as seen in a thin section. A short flagellum is evident.
Figure 12.
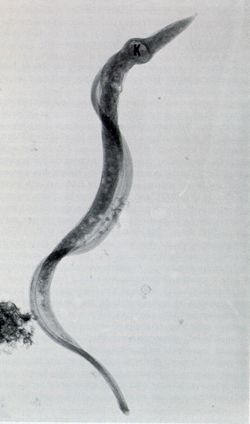
High voltage electron microscopy showing an intact tyrypomastigote form of T. cruzi.
This fact makes the flagellum of T. cruzi a suitable model for the study of the natural growth and shortening of this structure. At the side of the axoneme of the flagellum of trypanosomatids there is a filamentous, lattice-like structure, which has been called paraflagellar or paraxial rod. The flagellum of trypanosomatids is usually attached to the cell body. This attachment occurs only in a determined region of the flagellum of trypanosomatids in which the flagellum emerges from the central portion. However, it is extensive in developmental stages in which it emerges laterally as occurs with epimastigote and trypomastigote forms. Such attachment is made by a junction, which has been considered to be of the desmosome type [Review in [41]]. When the flagellar movement starts, the wave propagates throughout the flagellum and upon reaching the region in which it is attached to the body of the parasite, induces an apparent movement of the body; giving the visual impression of an undulating membrane. The undulating membrane of trypanosomatids does not appear in thin sections as an actual structure, as it does in Trichomonadidae. Observations of thin sections of the attachment zone by electron microscopy show that the junctional complex is formed by a linear series of apposed macular densities, each measuring 25 nm in diameter and formed from an amorphous material spaced at intervals of 90 nm. Freeze-fracture studies indicate that there is a specialization of the T. cruzi flagellar membrane at the region of attachment of the flagellum to the cell body of epimastigote and trypomastigote forms. This appears as clusters of intramembranous particles spaced at more or less regular intervals (Fig. 13).
Figure 13.
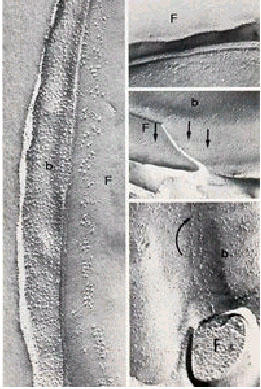
Different views of the attachment of the flagellum to the cell body of T. cruzi as seen in freeze-fracture replicas.
In epimastigotes of T. cruzi, a second specialization was found in both P and E faces of the flagellar membrane. It appeared as a linear array of particles, longitudinally oriented in relation to the main axis of the flagellum. The functional role of this structure was not yet determined. The nature of the connection between the flagellum and the cell body of trypanosomatids is still uncertain. Vickerman [43] observed that "trypanosomes dividing in citrated blood produce a completely free daughter flagellum while the parent flagellum remains attached", suggesting that Ca2+ ions chelated by citrate are necessary for adhesion of the developing flagellum, but not for maintenance of attachment. Results obtained with T. brucei have pointed out the existence of a filamentous network that underlies the region of flagellum-cell body attachment, designated as FAZ (Flagellar Attachment Zone). A set of four specialized microtubules which originate close to the basal bodies, run around the flagellar pocket and then participate in the sub-pellicular array at the region of flagellum-cell body attachment, has been described. These microtubules, which are also components of the FAZ, are much more resistant to high salt treatments than the subpellicular microtubules [Review in [44]]. Immunocytochemical studies have shown that some highly immunogenic parasite proteins are located in the FAZ. In the case of T. cruzi these molecules have a high molecular weight, posses repetitive motifs and have been shown to be among the best antigens used for diganosis of Chagas'disease. Indeed, one of the best diagnostic kits presently available for the diagnosis of Chagas' disease uses one antigen that is part of the FAZ complex.
The paraflagellar rod (PFR), also known as paraflagellar structure, runs alongside the axoneme since emerging from the flagellar pocket (Fig. 14). Three distinct zones can be identified in the PFR: (a) the proximal and distal zones possess 7–10 nm thick filaments intersected at an angle of 100°, which gives the structure a lattice-like aspect. This zone is connected to doublet numbers 4 to 7 of the axoneme through filamentous bridges. (b) The intermediate zone is formed by 5 nm thick filaments which intersect at an angle of 45°. (c) The distal zone, whose size varies according to the species, is basically similar to the proximal one. Biochemical studies carried out in T. brucei have shown that the PFR is made of two major proteins of 68 and 76 kDa and several minor components. Recent studies showed that mutants induced to ablate expression of the major PFR proteins do not show a complete PFR structure. Some of these mutants show a reduced motility, altered flagellar beat pattern or even complete immobilization [Reviews in [44,45]].
Figure 14.
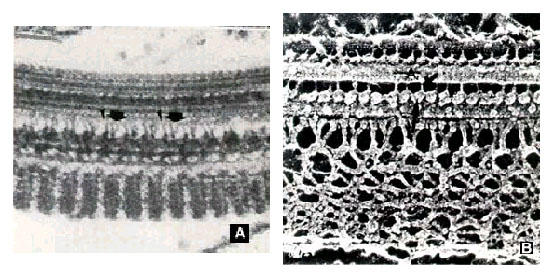
a and b: Two different views of the paraflagellar structure as seen in thin sections and in replicas of quick frozen, freeze-fracture and deep-etched cells.
Kinetoplast/Mitochondrion
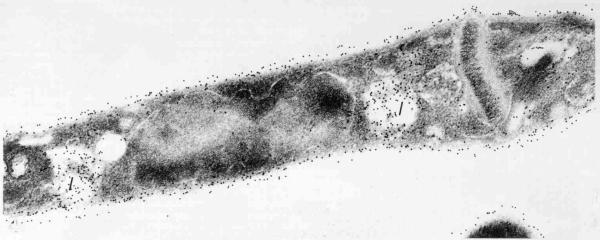
Trypanosoma cruzi, as well as other members of the Trypanosomatidae family, possesses only one mitochondrion that extends throughout the cell body. At a certain portion of the mitochondrion, localized near the basal body, there is a complex array of DNA fibrils in the mitochondrial matrix which forms the structure known as kinetoplast [Review in [46]] (Fig. 11). This structure is diagnostic of the order Kinetoplastida to which the Trypanosomatidae family belongs. The kinetoplast of epimastigote and spheromastigote forms of T. cruzi have a similar morphology. The filamentous material is arranged in a tightly packed row of fibers oriented parallel to the longitudinal axis of the protozoan. The whole structure appears as a slightly concave disk of 1.0 ~μm in length, and a depth of 0.1 μm. There is a space between the kinetoplast and the inner mitochondrial membrane in which mitochondrial cristae can be seen. At least some fibrils of the kinetoplast DNA make contact with the inner mitochondrial membrane. Such contact is observed mainly in the portion of the mitochondrion that faces the basal body (Fig. 15).
Figure 15.
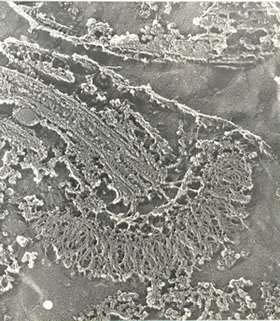
Deep-etchinng view of the kinetoplast and the region of flagellar emergence of an epimastigote form of T. cruzi.
In T. cruzi (both epimastigotes and spheromastigotes) the kinetoplast may have the appearance of a double layer.
The kinetoplast of T. cruzi was studied in detail by Riou and coworkers [47,48]. It is now established that the kinetoplast DNA represents between 20 and 25% of the total DNA of epimastigotes of T. cruzi. Electron microscopy showed that the kinetoplast consists of a network of 20,000 to 30,000 minicircle molecules associated with each other and with long linear molecules. Each minicircle has a length of about 0.45 μm, which corresponds to about 1440 base pairs, and a molecular weight of 0.94 x 106[47]. It has been shown that the diameter of the minicircles corresponds to the width of the kinetoplast, as seen in thin sections. It was suggested that the minicircles, figure eights, and loops of the long molecules are aligned in situ, in package fashion, to form the kDNA quaternary structure. Biochemical studies indicate the existence of proteins associated with the kinetoplast of T. cruzi. The following proteins have been detected in association with the kinetoplast DNA: type II topoisomerase, DNA polymerase P, minicircle originbinding protein, kDNA condensing proteins, kinetoplast-associated protein, a nicking enzyme, mitochondrial heat shock proteins, and a set of kinetoplast binding proteins which could bind both mini and maxicircles [49-55]. Restriction enzyme analysis has shown that the minicircles are heterogeneous in sequence. The digestion of kinetoplast DNA of T. cruzi with restriction endonucleases, followed by the electrophoretic analysis of the fragments on polyacrylamide gradient gels, has been used for the characterization of stocks, strains, and clones of this protozoan [56]. In addition to the minicircles a large circle, which has been designated as a maxicircle, with a diameter of about 10 μm, was observed in the kDNA of epimastigotes of T. cruzi[57]. The information contained in the DNA of a minicircle is small. If transcription and translation were to occur, a small protein would result. Therefore, it is difficult to understand what is the functional role played by the minicircle in the physiology of the trypanosomatids. The maxicircles, however, have a size comparable to that found in the mitochondrial DNA of other eukaryotic cells. Therefore, it is belived that the maxicircles contain genetic information akin to other eukaryotic cells, whose mitochondrial DNAs encode the RNA of mitochondrial ribosomes, a limited group of proteins and a set of tRNAs, containing information for the synthesis of subunits of enzymes associated with the inner mitochondrial membrane such as cytochrome b and ATPase.
The maxicircle and minicircle DNA codes for small guide RNAs (gRNAs) that control the specificity of the process of editing of the mRNA formed [58,59]. The editing process consists in the insertion or deletion of uridine in transcripts of the maxicircle DNA, involving addition of few uridines, as in the case of the mRNA coding for cytochrome oxidase II or addition of many uridines followed by deletion of few uridines as in the case of the mRNA coding for cytochrome oxidase III in T. brucei.
Two major RNAs, sedimenting at 12 and 9 S, and several other lowmolecularweight RNAs were detected in a kinetoplast-enriched fraction isolated from L. tarentolae and other trypanosomatids. The 12 and 9 S RNAs lack a poly(A) tail, hybridize to the maxicircle fraction of kDNA, and their synthesis is blocked by ethidium. It was also observed that both RNAs stimulate leucine incorporation into protein by a wheat germ system [60]. Both RNAs did not hybridize to minicircle fragments. It was suggested that these RNAs are the mitochondrial ribosomal RNAs. Hybridization studies carried on T. brucei indicate that "the maxicircle is the genetically active component in the kDNA network" [61]. These authors point out several facts that indicate that the two major RNA species found initially in L. tarentolae are mitochondrial ribosomal RNAs. They would be the smallest rRNAs known in nature, a fact that could explain the difficulties found in isolating mitochondrial ribosomes from trypanosomatids.
The division of the kinetoplast is co-ordinated with the process of cell division. Division in T. cruzi begins with the replication of the basal body and flagellum, followed by the division of the kinetoplast [38]. As previously explained the kinetoplast of interphasic cells has a length and a width which is characteristic for a given species. In dividing forms the length of the kinetoplast increases until it reaches a certain value when partition occurs in the middle. In a certain percentage of epimastigotes of T. cruzi and T. conorhini the kinetoplast doubles transversally and the new band shifts sideways until it lies next to the original band, after which contriction occurs. In some forms parallel disks are seen looking like an amorphous material. By using electron microscope autoradiography Burton and Dusanic [62] as well as Anderson and Hill [63] showed that the incorporation of 3H-thymidine apparently occurred mainly along the periphery of the kinetoplast of dividing culture forms of T. lewisi and C. fasciculata. However, incorporation of material was occasionally found in other regions of the kinetoplast. Unfortunately, similar studies in which either a statistical analysis of the frequency of labeling patterns were carried out, or synchronized cultures were used, have not been carried out yet. Simpson and Simpson [64] showed by autoradiography of isolated networks of kDNA, after short pulses, that labelling occurred predominantly at two sites of the network rim separated by 180°. After longer pulses labelling of the entire rim was observed. Based on a large number of experiments carried out in several trypanosomatids the picture that emerges is that the minicircle replication takes place at two opposite positions of the network and leads to an increase in the size of the network. Concomitant with the minicircle replication the newly synthesized DNA is distributed through the network by a process of recombination. Replication of the kDNA only takes place during the S phase of the cell cycle. According to the current models of kDNA replication the minicircles do not replicate while linked to the network but only after release due to the action of toposiomerase II [Review in [65]].
Taken together all information presently available on the mitochondrion-kinetoplast complex of trypanosomatids we can conclude that it offers several potential targets for the action of chemotherapeutic agents. First, we must not forget that this complex structure is a mitochondrion whose functionality is vital for the survival of the cell. It has been shown recently that in contrast to the mitochondria of mammalian cells, the mitochondrial membrane of trypanosomatids possess a significant amount of sterols. This fact probably explains the dramatic effect observed in the mitochondria of cells incubated in the presence of drugs which inhibit the biosynthesis or ergosterol (Fig. 16), and which have been shown to be among the most interesting prospects for the chemotherapy of Chagas'disease and leishmaniasis (Review in [66]). Drugs which interfere with one of the several steps of the processes of minicircle and maxicircle replication, inhibitors of topoisomerases, DNA polymerases, RNA-editing, etc, should be analyzed in more detail in the next years.
Figure 16.
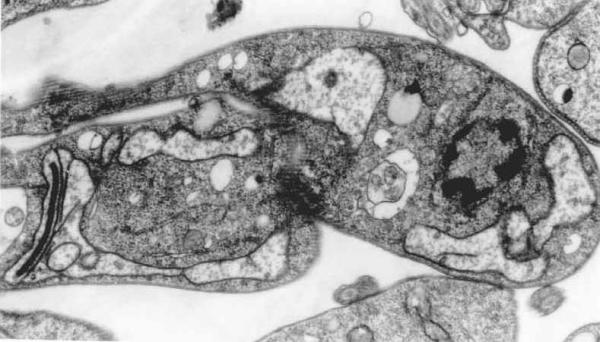
General view of an epimastigote form of T. cruzi incubated in the presence of a drug which inhibits the biosynthesis of ergosterol. An intense swelling of the mitochondrion is observed.
The peroxisome (glycosome in trypanosomatids)
Membranebound cytoplasmic structures resembling those initially described as microbodies, and later as peroxisomes in mammalian cells, have been described in trypanosomatids since the initial studies on their fine structure. Peroxisomes usually appear as spherical organelles with a diameter of about 0.7 μm, randomly distributed throughout the cell (Fig. 17).
Figure 17.
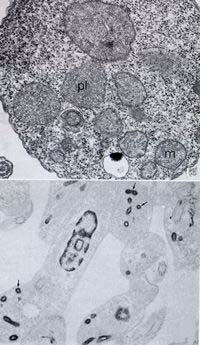
Two views of the glycosomes in trypanosomatids as seen in a conventional thin section and in cells incubated in the presence of ethanolic phosphotungstic acid, which reveals basic proteins.
Usually the glycosome exhibits a homogenous and slightly dense matrix, although a crystalline core can be seen in some protozoa such as T. brucei [Review in [67]]. Peroxisomes have been defined as organelles that are bounded by a single membrane and that contain catalase and H2O2-producing oxidases. Catalase has been used as an enzyme marker, easily localized using the alkaline diaminobenzidine medium to identify an organelle as peroxisome. The application of this approach in trypanosmatids led to the identification of two groups. One includes digenetic trypanosomatids such as T. brucei, T. cruzi and Leishmania where no significant enzyme activity could be detected both by enzyme assay and cytochemistry. The other includes the monogenetic trypanosomatids such as Crithidia, Leptomonas, etc, where the enzyme is easily detected.
A major contribution to the role played by the peroxisomes in trypanosomatids came from the work made by Opperdoes and co-workers initially in T. brucei and then extended to other members of the Trypanosomatidae family. They found, using cell fractionation and biochemical studies, that the glycolytic enzymes involved in the conversion of glucose to 3-phosphoglycerate are located in the peroxisomes [Review in [68]]. Based on these results the term glycosome was suggested to designate the microbodies or peroxisomes of trypanosomatids. An interesting observation was that the glycolytic enzymes isolated from the glycosomes have a higher isoelectric point as than similar enzymes found in the cytosol of mammalian cells. In addition to catalase the peroxisomes of mammalian cells possess more than 50 different enzymes involved in metabolic pathways such as peroxide metabolism, β oxidation of fatty acids, ether phospholipid synthesis, etc. Evidence have been obtained indicating that in addition to the metabolic routes described above, other metabolic pathways such as carbon dioxide fixation, purine salvage, and pyrimidine biosynthesis de novo [Review in [68]], which typically occur in the cytoplasm of other cells, also take place in the glycosome of trypanosomatids.
The glycosome does not possess a genome. Therefore, all proteins found in the glycosome are encoded by nuclear genes, translated on free ribosomes, and posttranslationally imported into the organelle [Review in [69]]. The uptake of proteins into the glycosomes occurs within 5 minutes of protein synthesis. Several types of peroxisomal targeting signals have been identified in mammalian cells. One type is a C-terminal SerLysLeu (SKL) that can also direct heterologous proteins into the glycosome [70,71]. Peroxisomes of several species, including the glycosomes of trypanosomatids, are labelled when antibodies recognizing SKL is used [72]. However, there are many evidences that considerably more variation in the signal is tolerated for efficient glycosomal targeting in trypanosomatids than in peroxisomes of mammalian cells [71]. For instance studies that analysed the target of phosphoglycerate kinase showed that a basic amino acid is not required and that the first amino acid of the COOH-terminal peptide can be serine, alanine or cysteine and the third leucine can be replaced by tyrosine or methionine without much effect on glycosomal targeting [73]. It is clear from the results obtained that SKL-like signals are not the only possible targeting signal. Other entry signals are (a) the 39-amino acid extension at the COOH terminus of the glycosomal phosphoglycerate kinase of Crithidia fasciculata [74,75], (b) a possible Nterminal or internal peptide for the targeting of fructose biphosphate aldolase in T. brucei[76,77]. Flaspohler et al. [78] used a positive genetic selection procedure to isolate a Leishmania mutant with a defect in the import of glycosomal proteins. The mutant, designated as giml (glycosome import mutant) mislocalizes to the cytosol a subset of proteins with the SKL-like signal. Phenotypic changes were observed in the mutant, the most interesting being the decrease in the number of lipid inclusions, which may be related to the role played by the glycosomes in the lipid metabolism. The product of the giml gene shares substantial amino acid sequence similarity to the peroxin 2 family of proteins of the membrane of peroxisomes and which are required for the biogenesis of the organelle. This observation, in association to that showing similarities in the protein target signals, support the view that peroxisomes and glycosomes have a common evolutionary origin.
In my personal view the work carried out on the glycosome constitutes one of the landmarks of the cell biology of parasites. Due to all characteristic features displayed by this organelle and the tremendous amount of work done on its characterization one would expect the development of highly efficient and specific drugs for the chemotherapy of diseases caused by the trypanosomatids. Unfortunately such drugs have not been obtained yet. Further studies are necessary, especially taking into consideration the involvement of the organelles in several biochemical pathways, in addition to its involvement in the glycolytic pathway.
The acidocalcisome
Since the beginning of the 20th Century the microscopists have observed the presence of metachromatic granules, designated as volutin granules, in microorganisms stained with basic dyes. In protozoa this structure was described in 1958 and has received several designations such as reservoir of metabolic products, pigment bodies, osmiophilic granules, polyphosphate granules or volutin granules. Some studies showed that some of the vacuoles contain a linear polymer of orthophosphate residues linked by high-energy phosphoanhydride bonds, forming the so-called polyphosphates, or to contain pyrophosphate (Review in [79]). Biochemical studies have indicated the presence of phosphate-containing granules in several trypanosomatids, however their subcellular localization was not well established. On the other hand, electron microscopic studies have shown the presence of electron-dense structures in the cytoplasm of trypanosomatids (Fig. 9).
The use of X-ray microanalysis, where it is possible to correlate an image seen in the transmission electron microscope with the element composition, showed the presence of Ca, Mg, Na, Zn and Fe in the cytoplasmic electron-dense granules of trypanosomatids [80,81].
A major breakthrough on the study of cytoplasmic vacuoles of trypanosomatids took place in 1994 when Vercesi et al. [82] put together two observations: (a) reports in trypanosomatids showing the existence of an intracellular Ca2+ pool released when cells were treated with nigericin and the cytoplasm became acidic; (b) results from studies on the slime mold Dictiostelium discoideum and mammal cells showing the existence of acidic organelles with an ATP-driven Ca2+/H+ antiport and a vacuolar-type H+ATPase, named as acidosomes. In addition Vercesi et al. [82] found that Ca2+ was released from the intracellular pool not because of acidification of the cytosol by nigericin, but because this drug released the ions from intracellular acidic vacuoles. Based on the fact that the cytoplasmic vacuoles contained a very high Ca2+ concentration and a Ca2+/H+ translocating ATPase activity, the organelle was designated as acidocalcisome [84].
From the morphological point of view the structure now designated as acidocalcisome has been described since the first observations of thin sections of trypanosomatids by electron microscopy. It is a membrane bound structure with an electron-dense content (Fig. 18).
Figure 18.
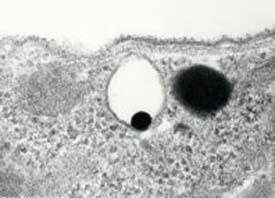
Visualization of an acidocalcisome of T. cruzi as seen in a thin section.
The amount of dense material varies according to the procedures used to process the samples for electron microscopy. In routine procedures part of the dense material may be removed, leaving a thin dense ring below the membrane. Electron-dense product is seen within acidocalcisomes of cells fixed in the presence of potassium pyroantimonate, which precipitates calcium [80]. The best way to have a general view of all acidocalcisomes to observe whole cells allowed to dry on carbon and formvarcoated grids in the transmission electron microscope, especially if it is equipped with an energy filter so that the electron spectroscopic images can be obtained (Fig. 19).
Figure 19.
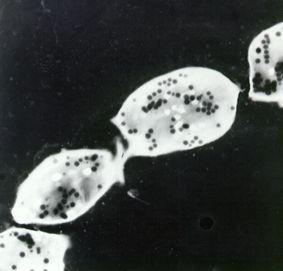
Electron spectroscopic image of whole amastigote forms of T. cruzi. The acidocalcisomes appear as electron-dense structures.
The first indication that the acidocalcisome is an acidic organelle comes from studies where it was shown that the inclusion vacuoles found in procyclic forms of T. brucei became larger when the cells were cultivated in the presence of chloroquine, an acidotropic drug [Review in [79]]. Later on it was shown by fluorescence microscopy that vacuoles of varying size found in T. brucei and T. cruzi were labelled with acridine orange and that the accumulation of this dye was sensitive to bafilomycin A, nigericin, and NH4C1 [Review in [79]]. In the case of epimastigotes of T. cruzi it is important to have in mind that another acidic organelle, the reservosome, also concentrates acridine orange [83]. The exact pH of the acidocalcisome has not been determined yet.
From a biochemical point of view we should consider two basic components of the acidocalcisome: the matrix and the membrane. The matrix of the acidocalcisome has been mainly analyzed in terms of its elemental composition, based mainly on analytical methods associated with electron microscopy. In these experiments the element content is compared between the inner portion of the organelle and other portions of the cell, such as the cytoplasm. The following elements have been shown to be concentrated into the acidocalcisome: P, Mg, Ca, Na and Zn, and very little Cl, K and S (see [80,81]). The low content of S suggests a low content of proteins. Electron energy loss spectroscopy revealed the presence of P and O, suggesting the presence of carbohydrates. Recent studies have shown that the phosphorous observed in acidocalcisomes of T. cruzi is in the form of PPi and shortchain polyphosphates. In addition, PPi seems to be the most abundant high energy phosphate present in T. cruzi[84]. The membrane of the acidocalcisome is about 6 nm thick. It was not yet isolated so that there are few information on its proteins and no information about its lipid content. Physiological studies using permeabilized cells showed the involvement of a bafilomycin A1-sensitive vacuolar H+ATPase in the process of acidification and of a vanadate sensitive Ca2+ATPase in the uptake of Ca2+. These observations were also confirmed for trypanosomatids and Apicomplexa using intact cells loaded with fura2, a fluorescent indicator of Ca2+. The use of an immunochemical (immunobloting, immunoprecipitation and immunocytochemistry) approach showed the presence of the following enzymes in the membrane of the acidocalsiomes of trypanosomatids: (a) a vacuolar H+ATPase; (b) a Ca2+/H+- translocating ATPase whose gene was cloned, sequenced and expressed. Antibodies generated against the protein product of the gene (Tcal) labelled the membrane of the acidocalcisome as well as the plasma membrane of T. cruzi; (c) a vacuolartype proton translocating pyrophosphatase (VH+PPase) was identified and localized in the membrane of the acidocalcisome and in the plasma membrane of trypanosomatids using antibodies recognizing the enzyme found in plants (Review in [81]); (d) evidence has been obtained for the presence of a Na+ as H+ exchanger and a Ca2+/H+ exchanger in the membrane of the acidocalcisome [Review in [79]].
What are the functions played by the acidocalcisome? Docampo and Moreno [79] consider four possibilities that are not exclusive. Indeed, based on the information available it is possible that this organelle is involved in several biological processes. (a) The first possibility is a role in the process of Ca2+ storage to be used in certain moments of the parasite's life cycle. For instance amastigote forms of T. cruzi live in the cytoplasm of the host cell where the concentration of Ca2+ is in the range of 0.1 mM, in contrast to the trypomastigote form which lives in a environment where the concentration is around 2 mM. Since Ca2+ is involved in several signalling processes, including cell transformation, cell interaction, etc., the amastigote form of T. cruzi developed a special way to accumulate this ion in the acidocalcisome since it would not be available in the normal environment where this developmental stage of the parasite lives, thus explaining the presence of a large number of acidocalcisomes in amastigote forms; (b) A second role would be to act as a energy store organelle containing a large amount of inorganic PPi. It is expected that further biochemical studies on this area will led to new information on the bioenergetics of protozoa, opening new perspectives for the development of new parasitic drugs; (c) in view of the presence of a H+ATPase in the membrane of the acidocalciome it can also play some role in the regulation of the cytoplasmic pH; (d) the acidocalcisome can also play some role on the control of the process of osmoregulation, as another organelle known as acidosome does in Dictiostelium discoideum which possesses an elaborated contractile vacuole system.
Recent studies have shown that the acidocalcisome may be an interesting target for the development of new drugs. Indeed drugs currently used in humans for the treatment of bone resorption disorders, as the non-metabolizable pyrophosphate analogs such as pamidronate, alendornates and risedronate, selectively inhibit the proliferation of T. cruzi both in cell cultures and in animals [84]. These drugs may act by inhibiting the pyrophosphate-dependent proton pumps and /or isoprenoid synthesis at the level of farnesyl-pyrophosphate synthase.
The flagellar pocket and the endocytic pathway in T. cruzi
All trypanosomatids possess a region known as the flagellar pocket which appears as a depression found in the anterior region of the cell from where the flagellum emerges [Review in [85]]. It is formed by an invagination of the plasma membrane that establishes a direct continuity with the membrane of the flagellum. Due to the fact that the membranes lining the cell body and the flagellum establishes a physical contact to each other at the point of emergence of the flagellum, the pocket can be considered as a special extracellular compartment, in some way isolated from the extracellular medium. Indeed, the use of cytochemical labels has shown that macromolecules and large particles added to the culture medium may be excluded from the pocket.
There are several lines of evidence showing that the flagellar pocket is a highly specialized region of the surface of trypanosomatids: (a) it is the only region without the layer of microtubules, known as subpellicular microtubules, closely associated with the membrane; (b) the membrane lining the pocket differs both from the membrane lining the cell body and the flagellar membrane in terms of distribution of intramembranous particles, and localization of proteins, including some enzymes [Review in [41]]; (c) there is much morphological and cytochemical evidence showing that the flagellar pocket is the place where intense endocytic and exocytic activity takes place.
The epimastigote stage of members of the Trypanosoma genus belonging to the Schyzotrypanum subgenus, such as Trypanosoma cruzi, Trypanosoma vespertilionis and Trypanosoma dionisii, possess a flagellar pocket which differs in shape form those found in other developmental stages. In the case of epimastigote and amastigote forms of members of the subgenus Schizotrypanum there is a highly specialized structure known as the cytostome-cytopharynx complex. It appears as a funnel-shaped structure formed due to a deep invagination of the plasma membrane, which may reach the nuclear region. The opening of this complex, which is known as the cytostome, may reach a diameter of 0.3 μm and is significantly smaller in the deeper portion of the cytopharinx. The subpellicular microtubules follow the invagination of the plasma membrane. There is a specialized region of the membrane lining the parasite, which starts in the opening of the cytostome and projects towards the flagellar pocket region. Freeze-fracture studies have shown that this area is delimited from the other portions of the plasma membrane by a pallisade-like array of closely associated particles (Fig. 20) [Review in [41]].
Figure 20.
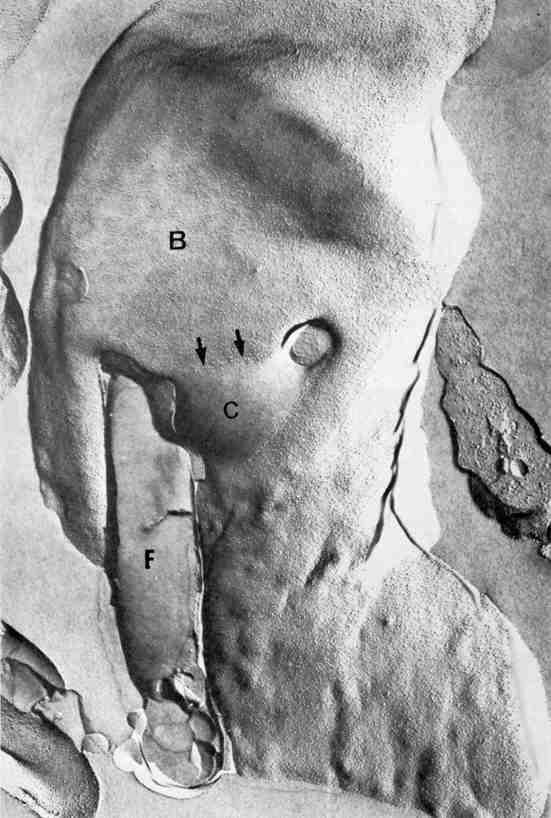
General view of an epimastigote form of T. cruzi as seen in a freeze-fracture replica. The flagellar membrane and the cytostome can be seen.
It has been shown that when epimastigotes are incubated in the presence of gold labelled macromolecules such as transferrin, LDL, etc., they initially bind to the cytostome region and then are internalized via endocytic vesicles, which are formed at the bottom of the cytopharynx (Fig. 21). The LDL incorporated by the protozoan is subsequently processed in the reservosome, cholesterol is directly incorporated into the cell membranes since T. cruzi is not able to synthesize this sterol. It has been shown that in the absence of preformed cholesterol T. cruzi is able to synthesize ergosterol and this information has been used for the development of new anti parasite drugs. Following binding to the cytostome macromolecules are rapidly internalized via the cytopharinx and appear in small endocytic vesicles which bud from the deepst region of this structure. Subsequently, these vesicles fuse to each other to form tubular structures that can be observed in the most central portion of the protozoan. Later on the macromolecules are concentrated in structures known as the reservosome [86] (Fig. 22).
Figure 21.
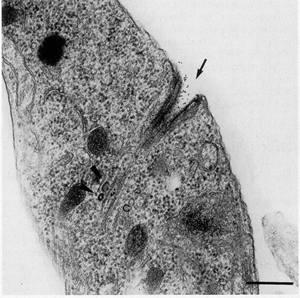
Thin section showing the initial internalization of proteins through the cytostome (arrow).
Figure 22.
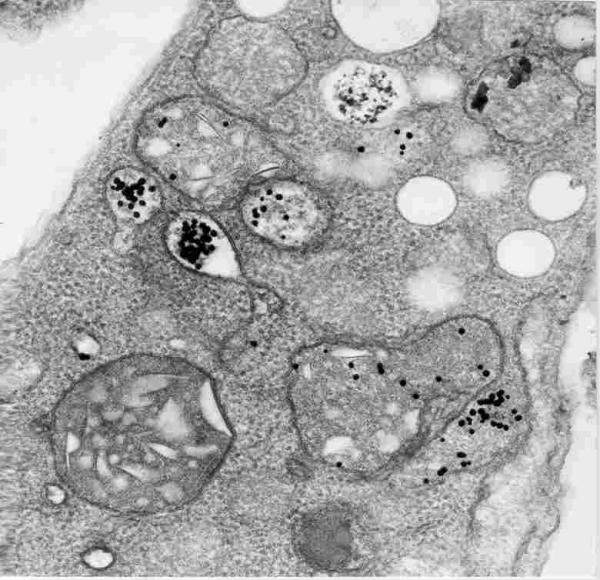
Reservosomes found in the epimastigote form of T. cruzi.
Each epimastigote form possesses several reservosomes, mainly localized in the posterior region of the cell. Although its morphology can vary according to the growth conditions and the parasite strain usually it is a spherical organelle, with a mean diameter of 0.7 μm, surrounded by a unit membrane. The matrix of the reservosome is slightly dense and possesses some inclusions. Cytochemical studies have shown that the matrix is mainly made of proteins and the inclusions contain lipids [83,86]. The organelle was designated as reservosome based on two criteria. First, because all macromolecules ingested by the parasite through an endocytic process, accumulate in the organelle. Second, because it gradually disappears when epimastigotes are incubated in a poor culture medium, conditions which trigger the process of transformation of non infective epimastigote into infective trypomastigote forms.
Most of the endocytic compartments of T. cruzi are acidic, as indicated by labeling with acridine orange, a fluorescent probe widely used to monitor pH gradients across membranes. The determination of the pH of the organelle using the DAMP technique indicated a value of pH 6.0, thus suggesting that the reservosome corresponds to a prelysosomal compartment. No acid phosphatase activity can be systematically detected in the organelle [83].
One characteristic feature of the reservosome in T. cruzi is the accumulation of a large amount of cruzipain, the major cysteine proteinase found in the cell (Fig. 23) [21]. Although glycosylated this protein does not possess mannose-6-phosphate residues. No mannose-6-phosphate receptors could be immunocytochemically detected in T. cruzi [83]. Therefore, another un-characterized intracellular route is used to deliver cruzipain to the endosomal system. Cruzipain has been the subject of intense investigation in the last years mainly due to its biological importance. The multicopy genes encoding this protease are part of a large array of polymorphic sequences located in different chromosomes [87,88]. The genes coding for this cathepsin L-like cysteine proteinase have been cloned and expressed and the crystal and molecular structure of the recombinant protein determined. Enzyme inhibitors, which are peptide analogs, inhibit the proliferation of epimastigote and amastigote forms and arrest the process of transformation of non-infective epimastigotes into infective trypomastigotes [23].
Figure 23.
Immunocytochemical localization of cysteine proteinase in an epimastigote form of T. cruzi. Intense labeling of the cell surface and reservosomes can be observed.
Final comments
After 30 years working with pathogenic protozoa, especially with trypanosmatids, it is clear to me that at present we know much more about these small, intelligent and intriguing organisms. Looking through the most recent literature, certainly influenced by the tremendous advances in the molecular biology area, it is clear that almost all research groups are concentrating their efforts on the further identification of molecules and definition of their functional roles. These studies will provide new important information on the biology of the parasites and probably will identify new potential targets for immunological and chemotherapeutic approaches. However, they will not contribute in short terms to the solution of the problems. It seems to me that it is important, and urgent, to provide incentive for classical studies on metabolic pathways, especially those which are being characterized in fungi and plants, in order to make a more direct approach to the development of new anti parasitic drugs. In some way, I am proposing to go back to the type of studies made many years ago, now significantly facilitated by the new techniques and equipments available. This type of decision will minimize the uncomfortable sensation of the existence of a large, and increasing distance between the dreams of all those which work with parasitic diseases, particularly those working in endemic areas, and the reality we experience daily in endemic areas.
References
- Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999;104:219–232. doi: 10.1016/S0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31:1218–1226. doi: 10.1016/S0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Carvalho TU, De Souza W. Infectivity of amastigotes of Trypanosoma cruzi. Rev Inst Med Trop São Paulo. 1986;28:205–212. doi: 10.1590/s0036-46651986000400001. [DOI] [PubMed] [Google Scholar]
- Ley V, Andrews NW, Robbins ES, Nussenszweig V. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990;168:649–659. doi: 10.1084/jem.171.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H, De Oliveira M. Cultivation of Trypanosoma cruzi in tissue cultures: a four year study. Parasitology. 1948;39:91–94. doi: 10.1017/s0031182000083591. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–481. doi: 10.1016/S0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- Zeledon R. Infection of the insect host by Trypanosoma cruzi. In "Atlas of Chaga's Disease Vectors in the Americas. Editora Fiocruz. 1997;1:271–287. [Google Scholar]
- Kolien AH, Schaub GA. The development of Trypanosoma cruzi in triatomine. Parasitol Today. 2000;16:381–387. doi: 10.1016/S0169-4758(00)01724-5. [DOI] [PubMed] [Google Scholar]
- Garcia ES, Azambuja P. Development and interactions of Trypanosoma cruzi within the insect vector. Parasitol Today. 1991;7:240–244. doi: 10.1016/0169-4758(91)90237-I. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Dvorak JA. New in vitro approach to quantification of Trypanosoma cruzi-vertebrate interactions. American Trypanosomiasis Research. PAHO Scientific Publication. 1976;318:109–120. [Google Scholar]
- Meirelles MN, Araujo Jorge TC, De Souza W. Interaction of Trypanosoma cruzi with macrophages in vitro: dissociation of atachement and internalization phases by low temperature and cytochalasin B. Z Parasitenkd. 1982;68:7–14. doi: 10.1007/BF00926652. [DOI] [PubMed] [Google Scholar]
- Araujo Jorge TC. The Biology of Trypanosoma cruzi-macrophage interaction. Mem Inst Oswaldo Cruz. 1989;84:441–462. doi: 10.1590/s0074-02761989000400001. [DOI] [PubMed] [Google Scholar]
- Ciavaglia MC, Carvalho TU, De Souza W. Interaction of Trypanosoma cruzi with cells with altered glycosylation paterns. Biochem Biophys Res Comm. 1993;193:718–721. doi: 10.1006/bbrc.1993.1684. [DOI] [PubMed] [Google Scholar]
- Schenckman S, Eichinger D, Pereira MEA, Nussenszweig V. Structural and functional properties of Trypanosoma cruzi transialidase. Ann Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- Pereira MEA, Zhang K, Gong YY, Herrera EM, Ming M :Invasive phenotype of Trypanosoma cruzi restricted to a population expressing transialidase. Infect Immun. 1996;64:3884–3892. doi: 10.1128/iai.64.9.3884-3892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenckman S, Ferguson MAJ, Heise N, Cardoso de Almeida ML, Mortara RA, Yoshida N. Mucin-like glycoproteins linked to the membrane by a glycosylphosphatididylinositol are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;59:293–303. doi: 10.1016/0166-6851(93)90227-O. [DOI] [PubMed] [Google Scholar]
- Santori RR, Dorta ML, Juliano L, Juliano MA, Franco da Silveira J, Ruiz RC, Yoshida N. Identification of a domain of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp 82 required for attachment and invasion of mammalian cells. Mol Biochem Parasitol. 1996;78:209–216. doi: 10.1016/S0166-6851(96)02626-6. [DOI] [PubMed] [Google Scholar]
- Colli W, Giordano R, Chammas R, Verga SS, Alves MJ. Trypanosoma cruzi binds to laminin in a carbohydrate-independent way. Braz J Med Biol Res. 1994;27:2318–2320. [PubMed] [Google Scholar]
- Magdesian MH, Giordano R, Ulrich H, Juliano M, Juliano L, Schumacher RI, Colli W, Alves MJ. Infection by Trypanosoma cruzi. Identification of a parasite ligand and its host cell receptor. J Biol Chem. 2001;276:19382–19389. doi: 10.1074/jbc.M011474200. [DOI] [PubMed] [Google Scholar]
- Burleigh BA, Andrews NW. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Ann Rev Microbiol. 1995;49:175–200. doi: 10.1146/annurev.mi.49.100195.001135. [DOI] [PubMed] [Google Scholar]
- Souto-Padron T, Campetella OE, Cazzulo JJ, De Souza W. Cysteine proteinase in Trypannosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. J Cell Sci. 1990;96:485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- Meirelles MN, Juliano M, Carmona L, Silva SG, Costa EM, Murta ACM, Scharfstein J. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest intracellular development of. Trypanosoma cruzi. 1992;52:175–184. doi: 10.1016/0166-6851(92)90050-T. [DOI] [PubMed] [Google Scholar]
- Vieira M, Carvalho TU, Cunha NL, De Souza W. Effect of protein kinase inhibitors on the invasion process of macrophages by Trypanosoma cruzi. Biochem Biophys Res Comm. 1994;203:967–971. doi: 10.1006/bbrc.1994.2276. [DOI] [PubMed] [Google Scholar]
- Burleigh B, Andrews NW. Signaling and host cell invasion by Trypanosoma cruzi. Curr Opin Microbiol. 1998;1:461–465. doi: 10.1016/S1369-5274(98)80066-0. [DOI] [PubMed] [Google Scholar]
- Caler EV, Morty RE, Burleigh BA, Andrews NW. Dual role of signaling pathways leading to Ca 2+ and cyclic AMP elevation in host cell invasion by Trypanosoma cruzi . Infect Immun. 2000;68:6602–6610. doi: 10.1128/IAI.68.12.6602-6610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato JO, Sola-Penna M, Agollos AO, Jones C, Oeltman T, Travassos LR, Mendonça-Previato L. Biosynthesis of O-N-acetylglucosamine-linked glycans in Trypanosoma cruzi: characterization of a novel uridine diphospho-N-acetylglucosamine:polypeptide N-acetyl-glucosaminyl transferase catalysing formation of the α-N-acetylglucosamine (1-O)-threonine linkage. J Biol Chem. 1998;273:14982–14988. doi: 10.1074/jbc.273.24.14982. [DOI] [PubMed] [Google Scholar]
- Morgado-Díaz JA, Nakamura CV, Agrellos AO, Dias WB, Previato JO, Mendonça-Previato L, De Souza W. Isolation and characterization of the Golgi complex of the protozoan Trypanosoma cruzi. Parasitology. 2001;123:33–43. doi: 10.1017/S0031182001007946. [DOI] [PubMed] [Google Scholar]
- Engel JC, Garcia CT, Hsieh I, Doyle PS, McKerrow JH. Upregulation of the secretory pathway in cysteine protease inhibitor-resistant. Trypanosoma cruzi. 2000;113:1345–1354. doi: 10.1242/jcs.113.8.1345. [DOI] [PubMed] [Google Scholar]
- Meirelles MN, De Souza W. The fate of the plasma membrane macrophage enzyme markers during endocytosis of Trypanosoma cruzi. J Submicroscop Cytol. 1986;18:99–107. [PubMed] [Google Scholar]
- Carvalho TU, De Souza W. Early events related with behaviour of Trypanosoma cruzi in na endocytic vacuole in mouse peritoneal macrophages. Cell Struct Funct. 1989;14:383–392. doi: 10.1247/csf.14.383. [DOI] [PubMed] [Google Scholar]
- Andrews N, Abraans CK, Slatin SL, Griffiths G. A Trypanosoma cruzi secreted protein immunologically related to the complement C9: evidence for membrane pore-forming activity. Cell. 1990;61:1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- Crane MJ, Dvorak JA. Trypanosoma cruzi: interaction with vertebrate cells. DNA synthesis and growth of intracellular amastigotes and their relationship to host cell DNA synthesis and growth. J Protozool. 1979;26:599–604. doi: 10.1111/j.1550-7408.1979.tb04203.x. [DOI] [PubMed] [Google Scholar]
- Crane MJ, Dvorak JA. Trypanosoma cruzi: pattern of RNA synthesis following infection of vertebrate cells. J Protozool. 1980;27:336–338. doi: 10.1111/j.1550-7408.1980.tb04273.x. [DOI] [PubMed] [Google Scholar]
- Meyer H, De Souza W. Electron microscopic study of Trypanosoama cruzi periplast in tissue cultures. I. Number and arrangement of the peripheral microtubules in the various forms of the parasite's life cycle. J Protozool. 1976;23:385–390. doi: 10.1111/j.1550-7408.1976.tb03792.x. [DOI] [PubMed] [Google Scholar]
- De Souza W, Meyer H. On the fine structure of the nucleus in Trypanosoma cruzi in tissue culture forms. Spindle fibers in the dividing nucleus. J Protozool. 1974;21:48–52. doi: 10.1111/j.1550-7408.1974.tb03615.x. [DOI] [PubMed] [Google Scholar]
- Solari AJ. The 3-dimensional fine structure of the mitotic spindle in Trypanosoma cruzi. Chromosoma. 1980;78:239–255. doi: 10.1007/BF00328395. [DOI] [PubMed] [Google Scholar]
- Elias MCQB, Marques-Porto R, Freymuller E, Schenckman S. Transcription rate modulation through the Trypanosoma cruzi life cycle occurs in parallel with changes in nuclear organization. Mol Biochem Parasitol. 2001;112:79–90. doi: 10.1016/S0166-6851(00)00349-2. [DOI] [PubMed] [Google Scholar]
- Gull K. The cell biology of parasitism in Trypanosoma brucei. Curr Pharmaceut Des. 2002. [DOI] [PubMed]
- Croft SL, Yardley V. Chemotherapy of Leishmaniasis. Curr Pharmaceut Des. 2002. [DOI] [PubMed]
- De Souza W. Components of the cell surface of Trypanosomatids. Prog Protistol. 1989;3:87–184. [Google Scholar]
- Docampo R. Biphosphonates as chemotherapeutic agents against Trypanosomatid and Apicomplexan parasites. Curr Pharmaceut Des. 2001;7:1157–1164. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesionin trypanosomes. J Cell Sci. 1969;5:163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Gull K. The cytoskeleton of trypanosomatid parasites. Ann Rev Microbiol. 1999;53:629–655. doi: 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- Landfear SM, Ignatushchenko M. The flagellum and the flagellar pocket of trypanosomatids. Mol Biochem Parasitol. 2001;115:1–17. doi: 10.1016/S0166-6851(01)00262-6. [DOI] [PubMed] [Google Scholar]
- Simpson L. The kinetoplast of hemoflagellates. Int Rev Cytol. 1972;32:139–207. [Google Scholar]
- Riou G, Delain E. Electron microscopy of the circular kinetoplastic DMA from Trypanosoma cruzi: occurrence of catenated forms. Proc Natl Acad Sci. 1969;62:210–217. doi: 10.1073/pnas.62.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou G, Yot P. Heterogeneity of the kinetoplast DNA molecules of. Trypanosoma cruzi. 1977;16:2390–2396. doi: 10.1021/bi00630a013. [DOI] [PubMed] [Google Scholar]
- Torri AF, Englund PT. Purification of a mitochondrial DNA polymerase from Crithidia fasciculata. J Biol Chem. 1992;267:4786–4792. [PubMed] [Google Scholar]
- Tzfati Y, Abeliovich H, Kapeller I, Shlomai J. A single-stranded DNA-binding protein from Crithidia fasciculata recognizes the nucleotide sequence at he origin of replication of kinetoplast DNA minicircles. Proc Natl Acad Sci USA. 1992;89:6891–6895. doi: 10.1073/pnas.89.15.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Ray DS. Isolation of proteins associated with kinetoplast DNA networks in vivo. Proc Natl Acad Sci USA. 1993;90:1786–1789. doi: 10.1073/pnas.90.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Rosales JL, Ley V, Diaz C. Cloning and characterization of a gene coding for a protein (KAP) associated with the kinetoplast of epimastigotes and amastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1990;40:233–244. doi: 10.1016/0166-6851(90)90045-N. [DOI] [PubMed] [Google Scholar]
- Li CJ, Hwa KY, Englund PT. A DNase from the trypanosomatid Crithidia fasciculata. Nucleic Acid Res. 1995;23:4426–4433. [PMC free article] [PubMed] [Google Scholar]
- Engman DM, Kirchoff LV, Donelson JE. Molecular cloning of mtp70, a mitochondrial member of the hsp70 family. Mol Cell Biol. 1989;9:5163–5168. doi: 10.1128/mcb.9.11.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro JE, Acosta-Viana K, Guzman-Marin E, Rosado-Barrera ME, Rosales-Encina JL. Stage specific kinetoplast DNA-binding proteins in Trypanosoma cruzi. Acta Trop. 2000;76:139–146. doi: 10.1016/S0001-706X(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Morel C, Chiari E, Camargo EP, Mattei DM, Romanha AJ, Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by restriction endonuclease fingerprinting of kinetoplast DNA minicircles. Proc Natl Acad Sci USA. 1980;77:6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon W, Frasch ACC, Hoeijmakers JHJ, FaseFowler F, Borst P, Brunnel F, Davison J. Maxi-circles and mini-circles in kinetoplast DNA from Trypanosoma cruzi. Biochim Biophys Acta. 1980;607:221–231. doi: 10.1016/0005-2787(80)90075-1. [DOI] [PubMed] [Google Scholar]
- DF Smith, M Parsons, editor. Molecular Biology of Parasitic Protozoa. IRL Press, New York; 1996. RNA editing: post-transcriptional restructuring of the genetic information. pp. 134–158. [Google Scholar]
- Gao G, Kapushog ST, Simpson AM, Thiemann OH, Simpson L. Guided RNAs of the recently isolated LEM 125 strain of Leishmania tarentolae: an unexpected complexity. RNA. 2001;7:1335–1347. doi: 10.1017/S1355838201018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Larsky L. Kinetoplast messenger RNA's. J Cell Biol. 1975;67:402A. [Google Scholar]
- Hoeijmakers JHJ, Snidjers A, Janssen JWG, Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981;5:329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Burton P, Dusanic DG. Fine structure and replication of the kinetoplast of. Trypanosoma lewisi. 1968;39:318–331. doi: 10.1083/jcb.39.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Hill GC. Division and DNA synthesis in the kinetoplast of Crithidia fasciculata. J Cell Sci. 1969;4:611–620. doi: 10.1242/jcs.4.3.611. [DOI] [PubMed] [Google Scholar]
- Simpson AM, Simpson L. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978;14:169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Morris JC, Drew ME, Klingbeil MM, Motyka SA, Saxowsky T, Wang Z, Englund PT. Replication of kinetoplast DNA: an update for the new millenium. Int J Parasitol. 2001;31:453–458. doi: 10.1016/S0020-7519(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Urbina J. Chemotherapy of Chagas disease. Curr Pharmaceut Des. 2002. [DOI] [PubMed]
- Vickerman K, Preston TM. Comparative cell biology of the kinetoplastida flagellates. In: WHR Lumsden, DE Evans, editor. In Biology of Kinetoplastida. New York, Academic Press; 1976. pp. 35–130. [Google Scholar]
- Opperdoes FR. Compartmentation od carbohydrate metabolism in trypanosomes. Ann Rev Microbiol. 1987;41:128–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- Parsons M, Furuya T, Pal S, Kessler P. Biogenesis and function of peroxisomes and glycosomes. Mol Biochem Parasitol. 2001;115:19–28. doi: 10.1016/S0166-6851(01)00261-4. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Keller GA, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989;108:1403–1411. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JM, Cheng QI, Keller GA, Wang CC. In vitro import of firefly luciferase into the glycosomes of Trypanosoma brucei and mutational analysis of the C-terminal targeting signal. Mol Biol Cell. 1992;3:749–759. doi: 10.1091/mbc.3.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GA, Krisans S, Gould SJ, Sommer JN, Wang CC, Schliebs W, Kunau W, Brody S, Subramani S. Evolutionary conservation of a microbody targeting signal that targets proteins to peroxisomes and glycosomes. J Cell Biol. 1991;114:893–904. doi: 10.1083/jcb.114.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S, Hwang HY, Carter D, Stenberg P, Ullman B. Localization and targeting of the Leishmania donovani hypoxanthine-guanine phosphoribosyltransferase to the glycosome. J Biol Chem. 1998;273:1534–1541. doi: 10.1074/jbc.273.3.1534. [DOI] [PubMed] [Google Scholar]
- Blattner J, Swinkels B, Dorsam H, Prospero T, Subramani S. Glycosome assembly in trypanosomes: variations in the acceptable degeneracy of a COOH-terminal microbody targeting signal. J Cell Biol. 1992;119:1129–1136. doi: 10.1083/jcb.119.5.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels BW, Evers R, Borst P. The topogenic signal of the glycosomal (microbody) phosphoglycerate kinase of Crithidia fasciculata resides in a carboxyterminal extension. EMBO J. 1988;7:1159–1165. doi: 10.1002/j.1460-2075.1988.tb02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. Structure and regulated expression of genes encoding fructose biphosphate aldolase in Trypanosoma brucei. EMBO J. 1985;4:2997–3003. doi: 10.1002/j.1460-2075.1985.tb04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. Import of fructose biphosphate aldolase into the glycosomes of Trypanosoma brucei. J Cell Biol. 1987;105:2649–2653. doi: 10.1083/jcb.105.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaspohler JA, Rickoll WL, Beverley SM, Parsons M. Functional identification of a Leishmania gene related to the peroxin 2 gene reveals common ancestry of glycosomes and peroxisomes. Mol Cell Biol. 1997;17:1093–1101. doi: 10.1128/mcb.17.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Moreno SNJ. Acidocalcisome: a novel Ca2+ storage compartment in trypanosomatids and apicomplexan parasites. Parasitol Today. 1999;15:443–448. doi: 10.1016/S0169-4758(99)01531-8. [DOI] [PubMed] [Google Scholar]
- Miranda K, Benchimol M, Docampo R, De Souza W. The fine structure of acidocalcisomes of Trypanosoma cruzi. Parasitol Res. 2000;86:373–384. doi: 10.1007/s004360050682. [DOI] [PubMed] [Google Scholar]
- Scott DA, Docampo R, Dvorak JÁ, Shi S, Leapman RD. In situ compositional analysis of acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 1997;272:28020–28029. doi: 10.1074/jbc.272.44.28020. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Moreno SNJ, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, De Souza W. Identification of a large prelysosomal compartment in the pathogenic protozoan Trypanosoma cruzi. J Cell Sci. 1992;102:157–167. doi: 10.1242/jcs.102.1.157. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Moreno B, Vieerkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SNJ, Docampo R. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J Biol Chem. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- Webster P, Russel DG. The flagellar pocket of trypanosomatids. Parasitol Today. 1993;9:201–206. doi: 10.1016/0169-4758(93)90008-4. [DOI] [PubMed] [Google Scholar]
- Soares MJ, De Souza W. Cytoplasmic organelles of trypanosomatids: a cytochemical and stereological study. J Submicroscop Cytol Pathol. 1988;20:349–361. [PubMed] [Google Scholar]
- Lima AP, Tessier DC, Thomas DY, Scharfstein J, Storer AC, Vernet T. Identification of new cysteine protease gene isoforms in Trypanosoma cruzi. Mol Biochem Parasitol. 1994;67:333–338. doi: 10.1016/0166-6851(94)00144-8. [DOI] [PubMed] [Google Scholar]
- Campetella O, Henriksson J, Aslund L, Frasch AC, Petterson U. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol Biochem Parasitol. 1992;50:225–234. doi: 10.1016/0166-6851(92)90219-A. [DOI] [PubMed] [Google Scholar]