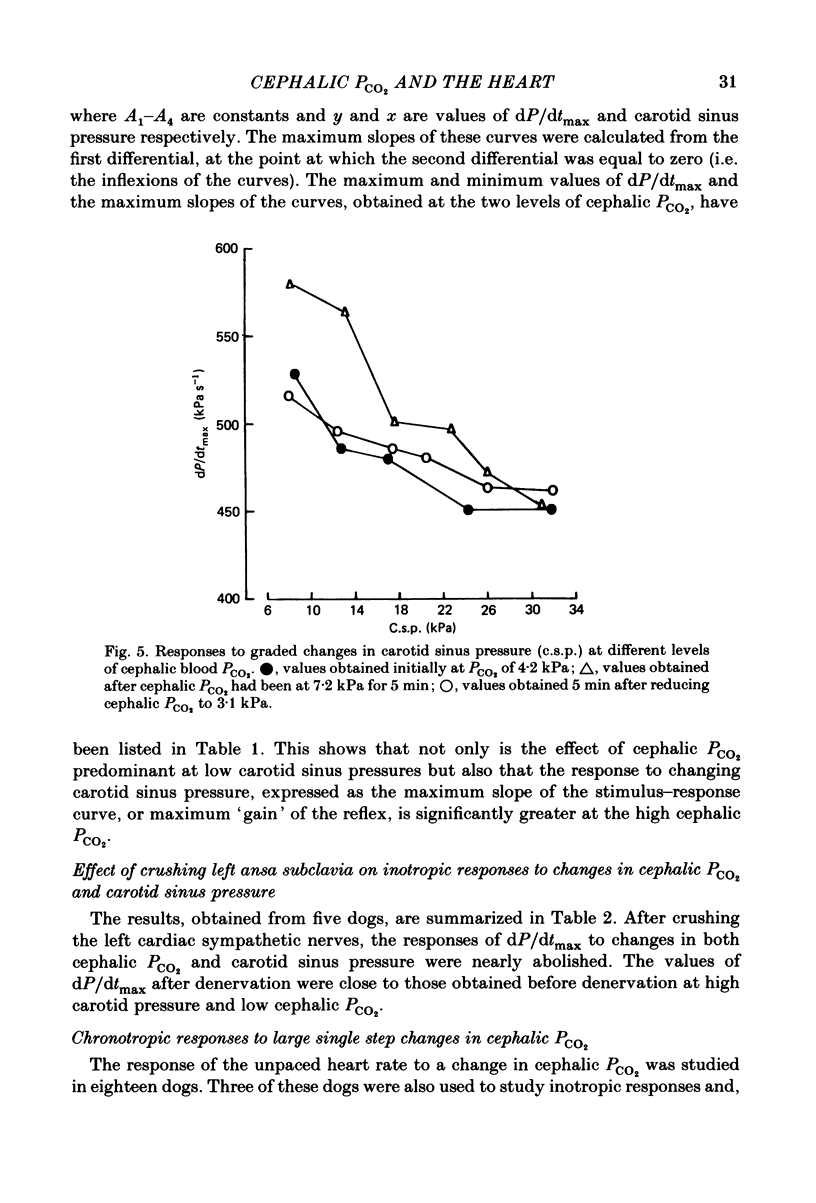
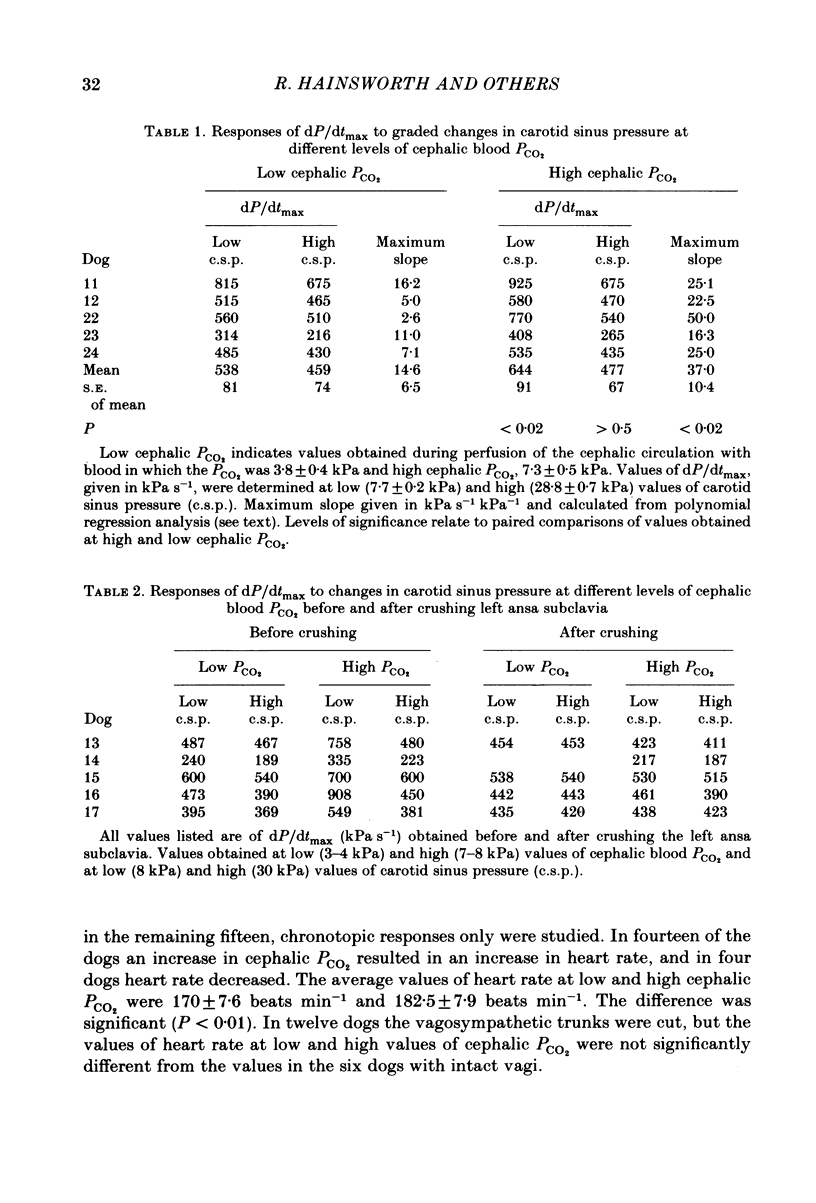
Abstract
Experiments were performed on anaesthetized dogs to determine the effects of moderate changes in PCO2 in the cephalic circulation on the inotropic state of the heart and on the reflex inotropic responses from changes in carotid sinus pressure. The cephalic circulation was perfused, through the brachiocephalic and left subclavian arteries, with blood taken from the superior vena cava and equilibrated with various gas mixtures in a gas exchange unit. The carotid sinus regions were vascularly isolated and perfused with arterial blood at controlled pressures. Cardiac inotropic responses were assessed from the maximum rate of change of left ventricular pressure (dP/dtmax) with heart rate and mean aortic pressure held constant. An increase in cephalic blood PCO2 resulted in an increase in dP/dtmax and an increase in the unpaced heart rate. Small, graded changes in cephalic PCO2 resulted in graded responses of dP/dtmax. A change in carotid sinus pressure resulted in a significantly greater response of dP/dtmax when cephalic PCO2 was high. After interruption of the left cardiac sympathetic nerves, the responses of dP/dtmax to changes in cephalic PCO2 and carotid sinus pressure were nearly abolished. These results indicate that the tension of carbon dioxide in the cephalic circulation is likely to be of importance in the control of the inotropic state of the heart. They also imply that, in studies of cardiovascular reflex responses, it is important to control the carbon dioxide tension in the arterial blood.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtel R. A., Downing S. E. Ventricular responses to hypoxemia following chemoreceptor denervation and adrenalectomy. Am Heart J. 1972 Sep;84(3):377–386. doi: 10.1016/0002-8703(72)90371-7. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S. The reflex effects of intralaryngeal carbon dioxide on the pattern of breathing. J Physiol. 1973 Jan;228(1):181–191. doi: 10.1113/jphysiol.1973.sp010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S., Widdicombe J. G. Reflex effects of laryngeal irritation on the pattern of breathing and total lung resistance. J Physiol. 1972 Jul;224(2):501–513. doi: 10.1113/jphysiol.1972.sp009908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Howe A. Thoracic chemoreceptors in the dog. A histological and electrophysiological study of the location, innervation and blood supply of the aortic bodies. Circ Res. 1970 Feb;26(2):235–247. doi: 10.1161/01.res.26.2.235. [DOI] [PubMed] [Google Scholar]
- DeGeest H., Levy M. N., Zieske H. Reflex effects of cephalic hypoxia, hypercapnia, and ischemia upon ventricular contractility. Circ Res. 1965 Oct;17(4):349–358. doi: 10.1161/01.res.17.4.349. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. Vasodepressor effects obtained by drugs acting on the ventral surface of the brain stem. J Physiol. 1976 Jun;258(2):337–355. doi: 10.1113/jphysiol.1976.sp011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnival C. M., Linden R. J., Snow H. M. Inotropic changes in the left ventricle: the effect of changes in heart rate, aortic pressure and end-diastolic pressure. J Physiol. 1970 Dec;211(2):359–387. doi: 10.1113/jphysiol.1970.sp009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillilan L. A. Extra- and intra-cranial blood supply to brains of dog and cat. Am J Anat. 1976 Jul;146(3):237–253. doi: 10.1002/aja.1001460303. [DOI] [PubMed] [Google Scholar]
- Hainsworth R., Karim F. Inotropic responses of the left ventricle to changes in aortic arch pressure in anaesthetized dogs. J Physiol. 1972 May;223(1):213–228. doi: 10.1113/jphysiol.1972.sp009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R., Karim F. Left ventricular inotropic and peripheral vasomotor responses from independent changes in pressure in the carotid sinuses and cerebral arteries in anaesthetized dogs. J Physiol. 1973 Jan;228(1):139–155. doi: 10.1113/jphysiol.1973.sp010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R., Karim F., Sofola O. A. Left ventricular inotropic responses to stimulation of carotid body chemoreceptors in anaesthetized dogs. J Physiol. 1979 Feb;287:455–466. doi: 10.1113/jphysiol.1979.sp012670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. E., De Burgh Daly M. Reflex respiratory and cardiovascular effects of stimulation of receptors in the nose of the dog. J Physiol. 1972 Feb;220(3):673–696. doi: 10.1113/jphysiol.1972.sp009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy F., Hanna B. D., Polosa C. CO2-dependent component of the neurogenic vascular tone in the cat. Pflugers Arch. 1978 May 18;374(2):187–191. doi: 10.1007/BF00581300. [DOI] [PubMed] [Google Scholar]
- Lioy F., Hanna B. D., Polosa C. Cardiovascular control by medullary surface chemoreceptors. J Auton Nerv Syst. 1981 Feb;3(1):1–7. doi: 10.1016/0165-1838(81)90025-4. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., Neil J. J., Loewy A. D. Effects of kainic acid applied to the ventral surface of the medulla oblongata on vasomotor tone, the baroreceptor reflex and hypothalamic autonomic responses. Brain Res. 1982 Apr 22;238(1):65–76. doi: 10.1016/0006-8993(82)90771-5. [DOI] [PubMed] [Google Scholar]
- NEAL J. J., Jr, HALPERN W., REEVES T. J. Velocity and acceleration of pressure change in heart and arteries. J Appl Physiol. 1960 Jul;15:747–749. doi: 10.1152/jappl.1960.15.4.747. [DOI] [PubMed] [Google Scholar]
- RANDALL W. C., ROHSE W. G. The augmentor action of the sympathetic cardiac nerves. Circ Res. 1956 Jul;4(4):470–475. doi: 10.1161/01.res.4.4.470. [DOI] [PubMed] [Google Scholar]
- Sampson S. R., Hainsworth R. Responses of aortic body chemoreceptors of the cat to physiological stimuli. Am J Physiol. 1972 Apr;222(4):953–958. doi: 10.1152/ajplegacy.1972.222.4.953. [DOI] [PubMed] [Google Scholar]
- Schlaefke M. E., See W. R., Loeschcke H. H. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol. 1970 Sep;10(2):198–212. doi: 10.1016/0034-5687(70)90083-6. [DOI] [PubMed] [Google Scholar]
- Szulczyk P., Trzebski A. The local effect of pH changes in the cerebrospinal fluid on the ventrolateral areas of medulla oblongata and on the spinal cord surface on the activity of cardiac and vertebral sympathetic nerves. Acta Physiol Pol. 1976 Jan-Feb;27(1):9–17. [PubMed] [Google Scholar]
- Wellens D. L., Wouters L. J., De Reese R. J., Beirnaert P., Reneman R. S. The cerebral blood distribution in dogs and cats. An anatomical and functional study. Brain Res. 1975 Mar 28;86(3):429–438. doi: 10.1016/0006-8993(75)90893-8. [DOI] [PubMed] [Google Scholar]
- Wennergren G., Oberg B. Cardiovascular effects elicited from the ventral surface of medulla oblongata in the cat. Pflugers Arch. 1980 Sep;387(2):189–195. doi: 10.1007/BF00584271. [DOI] [PubMed] [Google Scholar]
- van den Bos G. C., Drake A. J., Noble M. I. The effect of carbon dioxide upon myocardial contractile performance, blood flow and oxygen consumption. J Physiol. 1979 Feb;287:149–161. doi: 10.1113/jphysiol.1979.sp012651. [DOI] [PMC free article] [PubMed] [Google Scholar]


