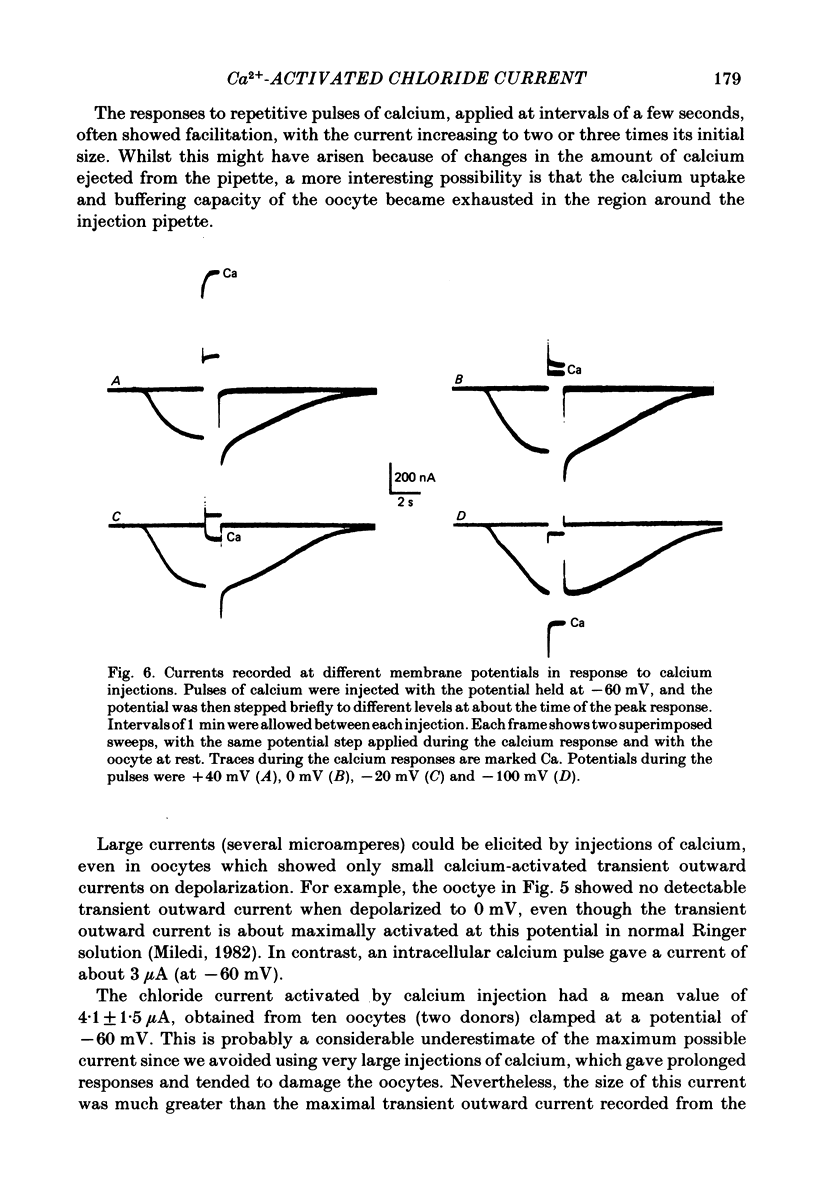
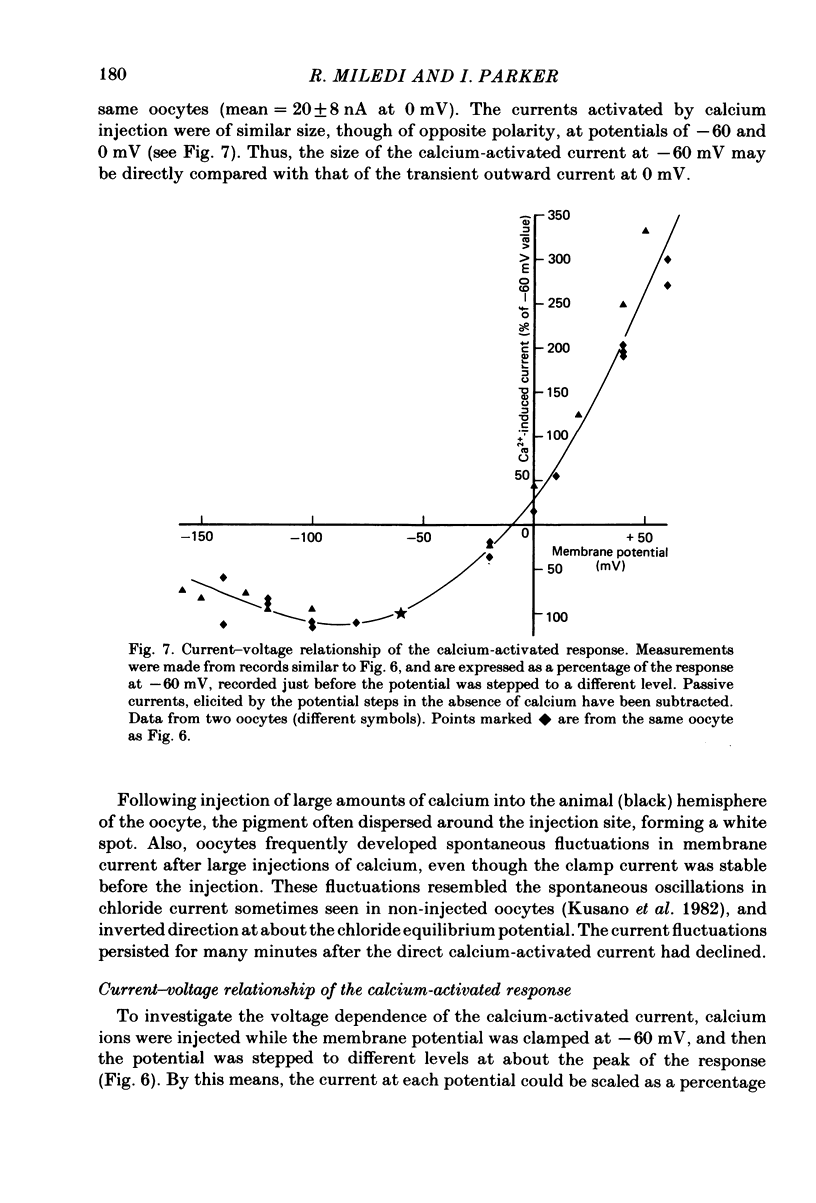
Abstract
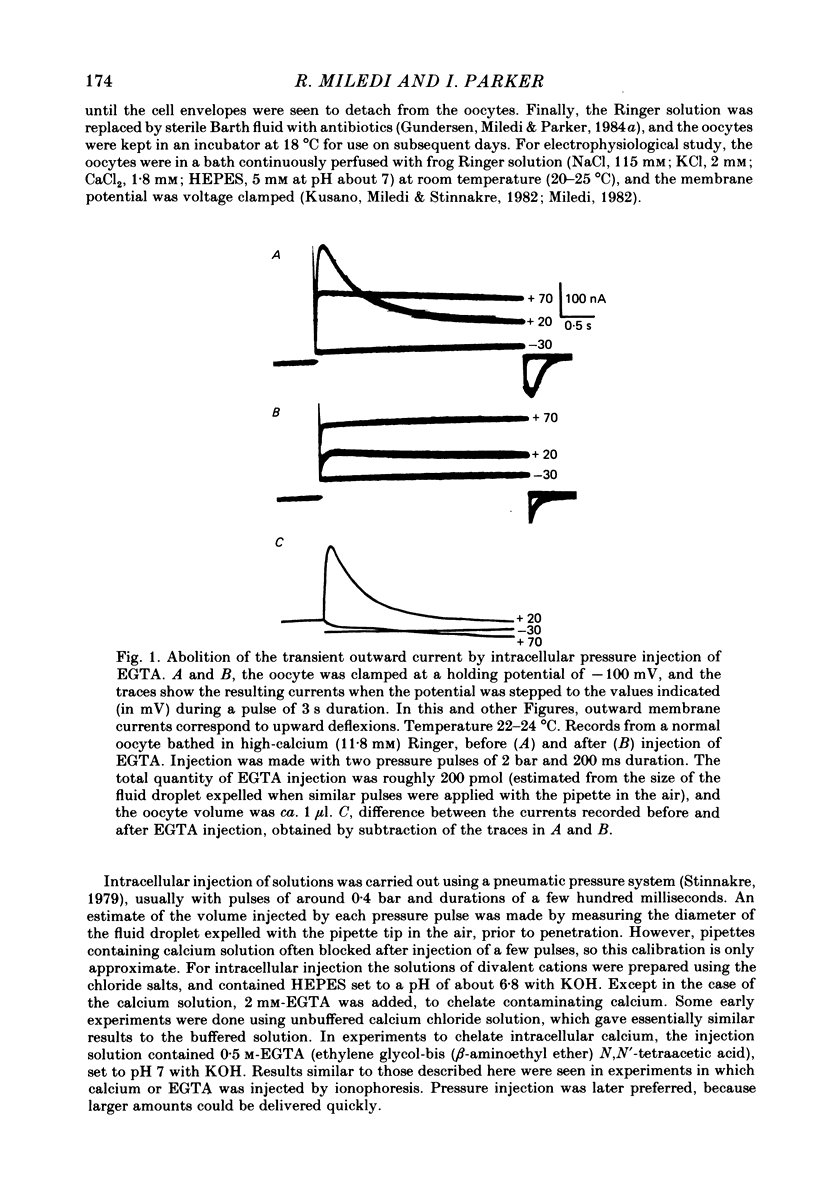
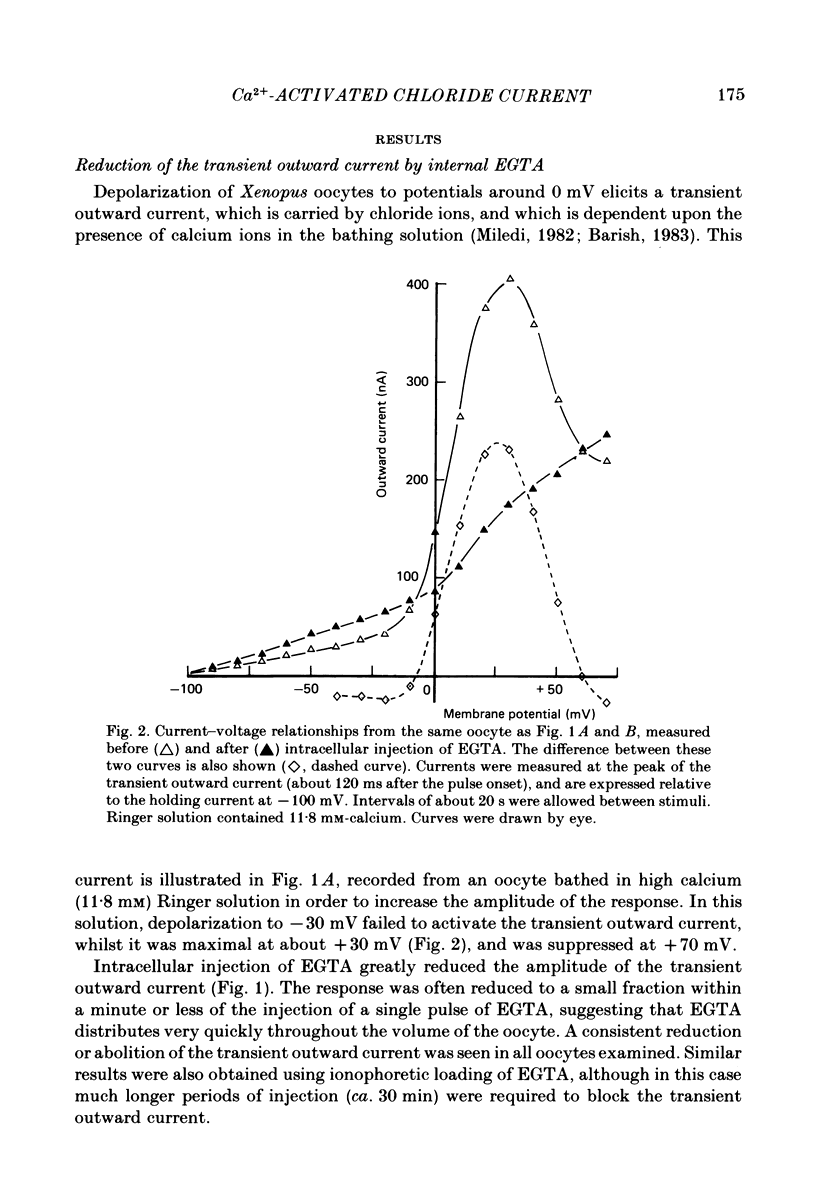
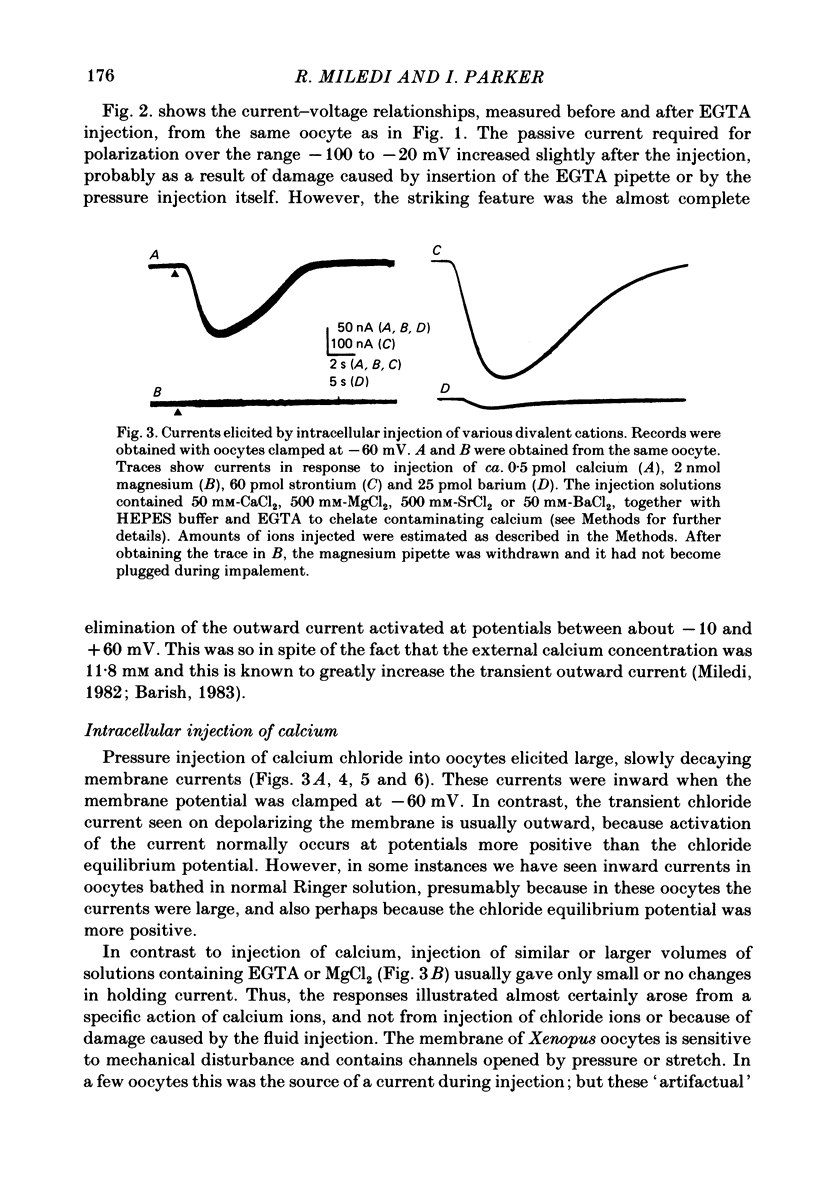
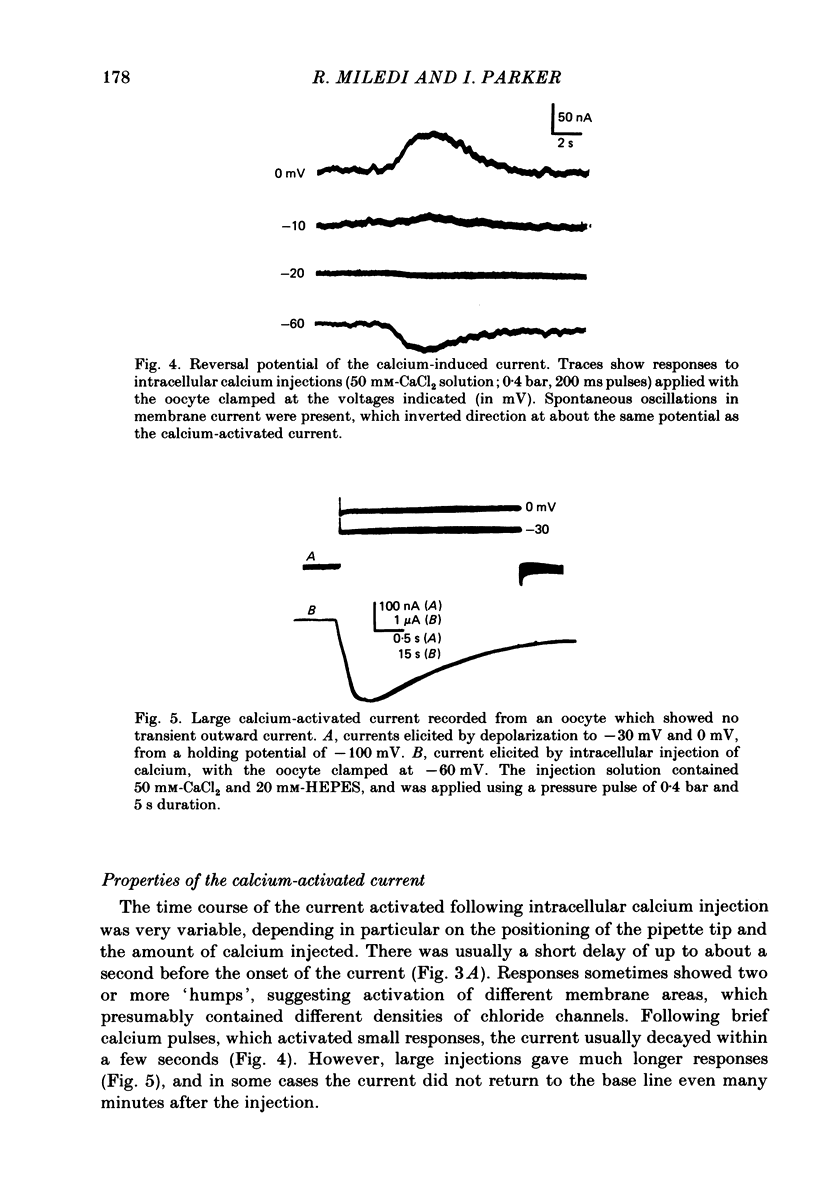
Membrane currents of Xenopus oocytes were studied with the membrane under voltage clamp. Intracellular injection of the calcium-chelating agent EGTA reduced, or abolished, the transient outward chloride current normally activated by membrane depolarization. Intracellular injection of calcium ions evoked large membrane currents, which inverted direction close to the chloride equilibrium potential. Injections of strontium, or barium, were less effective than calcium, while magnesium was ineffective. Large chloride currents could be evoked by calcium injections in oocytes which showed only small or no transient outward currents. The current activated by calcium injection increased with increasing depolarization up to high (ca. +60 mV) positive potentials, even though the transient outward current was suppressed by strong depolarization. The results indicate that the transient outward current depends upon entry of calcium through voltage-gated calcium ion channels and show that the oocyte membrane contains numerous chloride channels which are activated by intracellular calcium. Only a few of these chloride channels are activated by depolarization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross N. L. Initiation of the activation potential by an increase in intracellular calcium in eggs of the frog, Rana pipiens. Dev Biol. 1981 Jul 30;85(2):380–384. doi: 10.1016/0012-1606(81)90269-4. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Robinson K. R. Electrical currents through full-grown and maturing Xenopus oocytes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):837–841. doi: 10.1073/pnas.76.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]


