Abstract
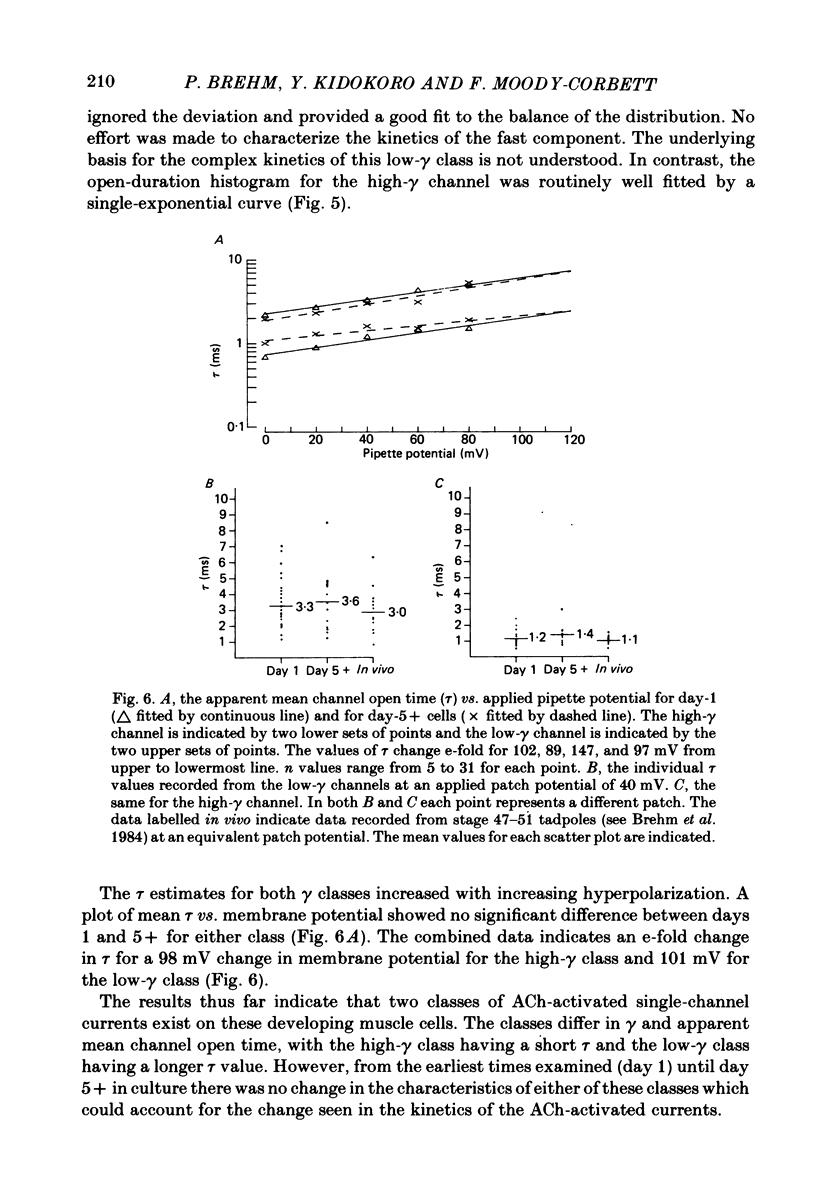
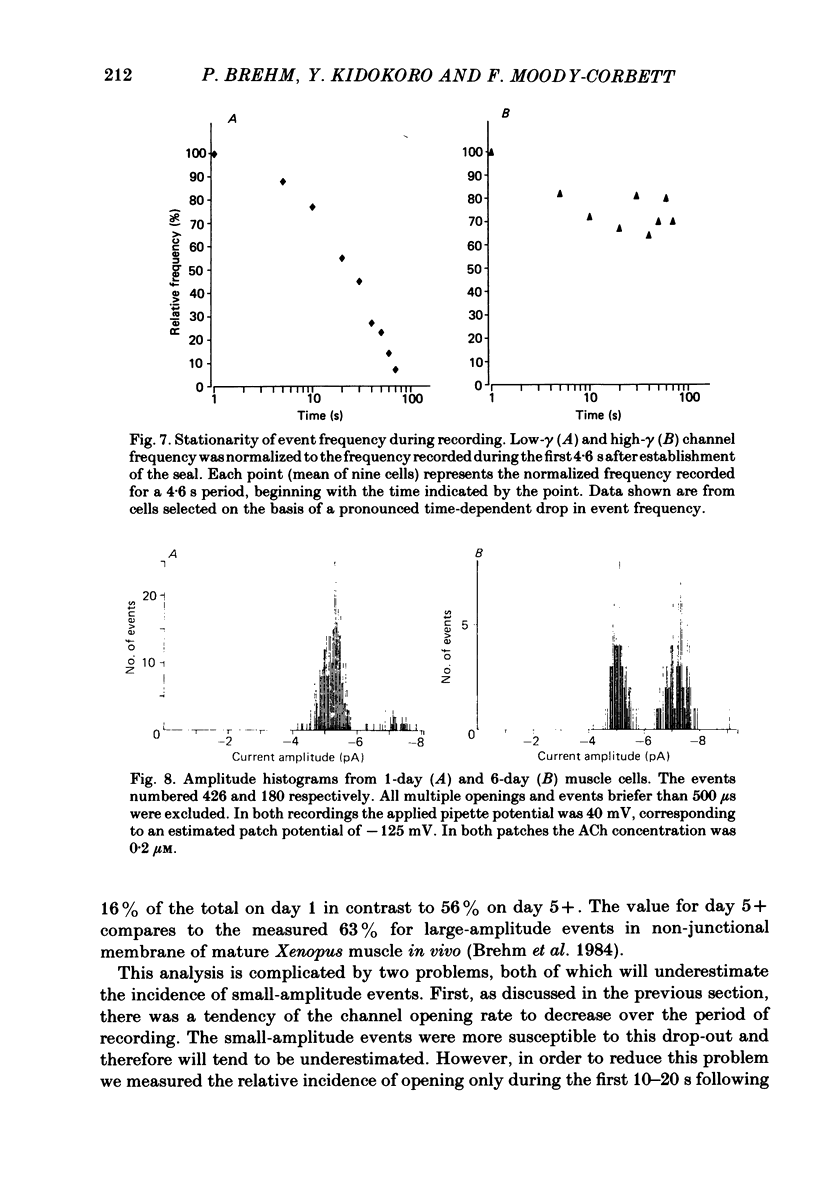
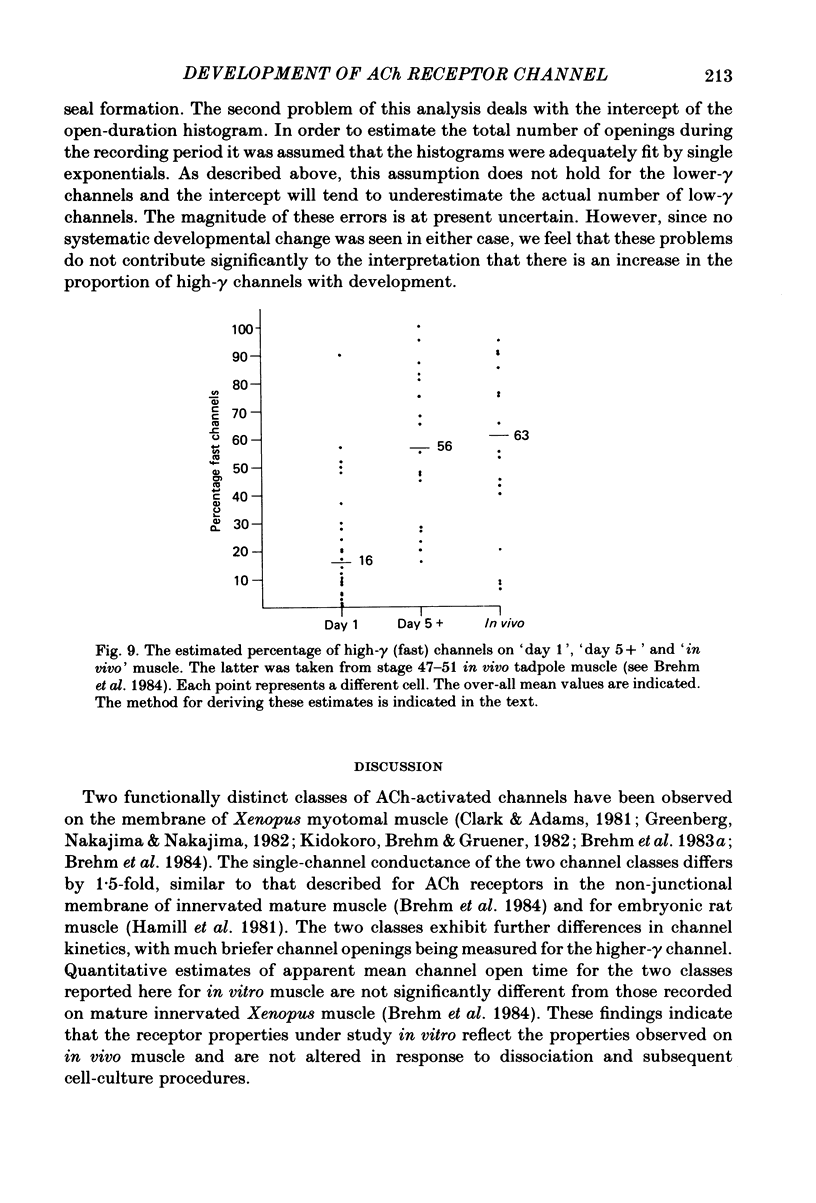
Developmental changes in acetylcholine (ACh) receptor channel function on aneural cultures of embryonic myotomal muscle cells were examined using the patch-clamp technique. At all stages of differentiation two different unitary-event amplitudes were observed, corresponding to high-gamma (single-channel conductance) (64 pS) and low-gamma (46 pS) channel types. No change in conductance occurred for either channel type during the 6-day in vitro period examined. At resting membrane potential (-85 mV) the low-gamma channel exhibited a mean open time of approximately 2 ms which, on the average, was 2-3-fold longer than that measured for the high-gamma channel. Neither the estimated mean channel open time nor the voltage dependence of the open state measured for either channel type changed during development. In recordings with low ACh concentration (0.1-0.25 microM) both high-gamma and low-gamma channel types exhibited non-stationary opening probabilities over the recording period. Usually the opening rate of both channel types decreased with time following seal formation, however, the 'drop-out' rate was faster for the low-gamma channel. A developmental increase in the proportion of high-gamma events occurred between day 1 (16%) and day 5 (56%) in culture, paralleling the time-dependent changes in the channel kinetics based on ACh-activated membrane noise. We conclude that the development of non-junctional muscle membrane is associated with increased expression of high-gamma channels and that this process is primarily responsible for the previously reported developmental alterations in macroscopic ACh receptor channel currents.
Full text
PDF

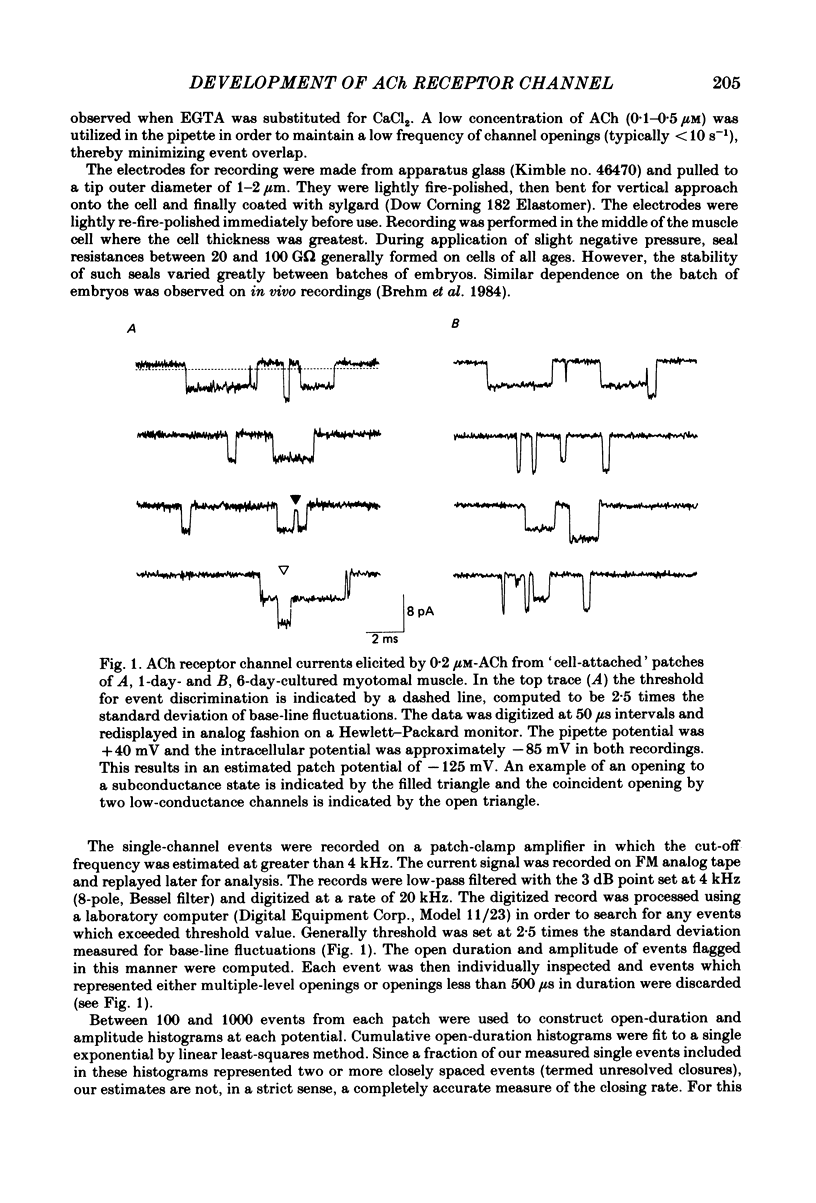

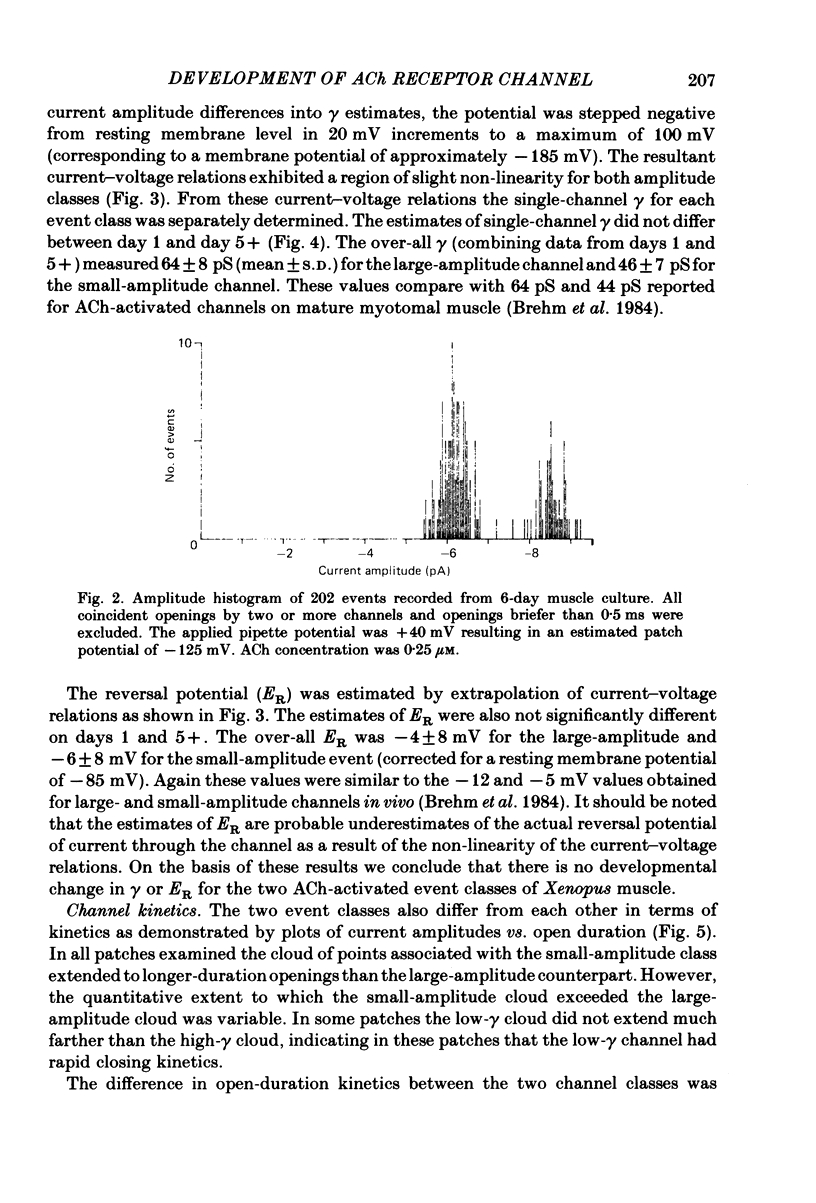
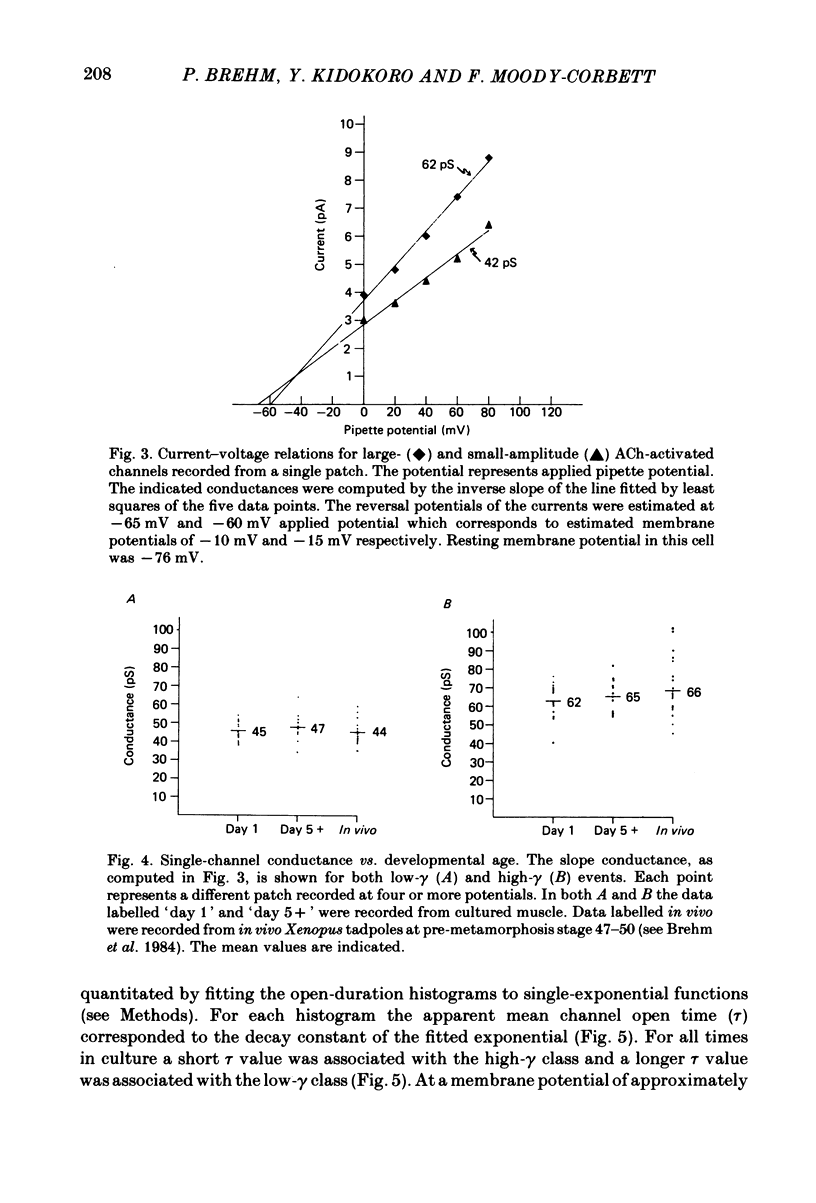
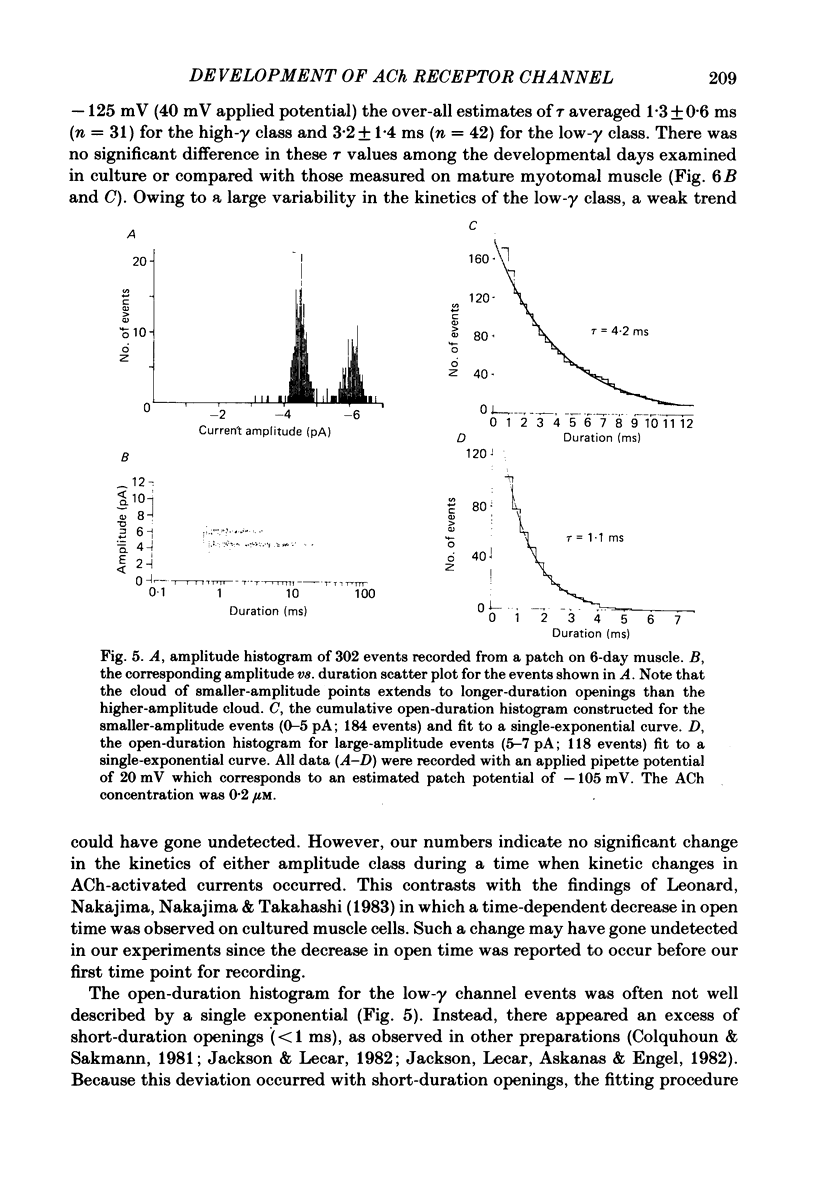





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Steinbach J. H., Kidokoro Y. Channel open time of acetylcholine receptors on Xenopus muscle cells in dissociated cell culture. Dev Biol. 1982 May;91(1):93–102. doi: 10.1016/0012-1606(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Brehm P., Yeh E., Patrick J., Kidokoro Y. Metabolism of acetylcholine receptors on embryonic amphibian muscle. J Neurosci. 1983 Jan;3(1):101–107. doi: 10.1523/JNEUROSCI.03-01-00101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Sakmann B. Gating properties of acetycholine receptor in newly formed neuromuscular synapses. Nature. 1978 Jan 26;271(5643):366–368. doi: 10.1038/271366a0. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Sakmann B. Neurotrophic control of channel properties at neuromuscular synapses of rat muscle. J Physiol. 1983 Apr;337:159–171. doi: 10.1113/jphysiol.1983.sp014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hironaka T., Morimoto S. The resting membrane potential of frog sartorius muscle. J Physiol. 1979 Dec;297(0):1–8. doi: 10.1113/jphysiol.1979.sp013024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Askanas V., Engel W. K. Single cholinergic receptor channel currents in cultured human muscle. J Neurosci. 1982 Oct;2(10):1465–1473. doi: 10.1523/JNEUROSCI.02-10-01465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R. W., Brehm P., Steinbach J. H. Nonjunctional acetylcholine receptor channel open time decreases during development of Xenopus muscle. Nature. 1981 Jan 29;289(5796):411–413. doi: 10.1038/289411a0. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler A., Sakmann B. Receptor stability and channel conversion in the subsynaptic membrane of the developing mammalian neuromuscular junction. Dev Biol. 1980 Nov;80(1):1–17. doi: 10.1016/0012-1606(80)90494-7. [DOI] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]



